1. Introduction
The word "functional" indicates that a prepared material is potentially useful in a given application (Thomas, 2010). In this regard, a functional material can be conceived from the desired application, where the properties of the material are tailored for achieving the desired functionality. In addition, functional materials have one or more properties that can be significantly changed according to an external stimulus. Consequently, these materials can be applied in quite diverse applications, such as electronics, catalysts, storage of greenhouse gases, etc.
Zeolites are interesting and important raw materials for preparing a variety of target materials. From a traditional point of view, they are aluminosilicate oxide. Due to an array of interconnected nanopores that are open to the exterior, they possess a nanoporous structure. These pores can be uni-, bi-, or tridirectional, leading to more or less hindered diffusion. In the case of tridirectional zeolites (in particular, faujasite X and Y), the shape of these micropores is not cylindrical, but consists of internal voids, called "supercages", connected via smaller diameter cavities (Jong, 2009). Furthermore, it is known that the pore size could favor the preferential diffusion of product and reactants inside the zeolite channels, this grants the capacity of zeolites for having unique catalytic activities, which match specific chemical processes (Moliner et al., 2015).
Zeolites themselves are adsorbents, ion exchangers, and catalysts. Ion exchange capacity and selective adsorption are two of the main intrinsic properties of zeolites. The availability of natural minerals, as well as methods for the synthesis of their analogs, and modification of both natural and synthetic materials, developed in sufficient detail, allows to obtain a variety of substances with predetermined properties, which can be tailored for achieving the desired functionality and for specific purposes. In order to design modern materials, these properties must be tuned to obtain a functional material. This means that in some way, the zeolite of interest is modified to obtain the desired improved properties. The specified modifications can be achieved through controlled changes in the chemical composition of the zeolite surface. Zeolites and related materials (including a wide range of microporous and mesoporous materials with ordered pore structure) have been one of the areas in the field of materials and catalysis with the largest impact on science, technology, and industrial processes (Čejka et al., 2012).
In this work, a comprehensive literature of revision is presented, where the use of zeolites as initial structures for the preparation of functional materials is discussed. In difference to other reviews (Caro et al., 2000; Weckhuysen & Yu, 2015; Rangnekar et al., 2015; Thomas, 2010), this is an updated revision, where the main objective is to show current trends, namely: structural and textural features, sensors, and human health related applications.
2. Structural and textural features of zeolites
Zeolites are nanoporous crystalline materials with pores ranging from 3 to 7Å. In addition, these materials are typically composed by corner sharing ENT#091;SiO4ENT#093; and/or ENT#091;AlO4ENT#093; tetrahedral via a common oxygen atom giving a macromolecule, and for some structures the Al or Si can be replaced by other tri or tetravalent elements as Fe, Ga, Ti, etc. These nanoporous compounds have well-ordered channels and cavities of molecular dimensions. Nowadays, 242 fully ordered and 11 partially disordered structures have been reported and accepted by the International Zeolite Association (IZA) (Baerlocher & McCusker, 2017). The zeolites are chemically represented by the formula M2/nO·Al2O3·γSiO2·wH2O, where γ is 2-200, n is the valence of cation, and w represents the water contained in the void spaces of zeolites. The cations in the structure are mobile and are susceptible to cation exchange, while the water molecules can be removed of the void space in a reversible way, generally by the application of heat, leaving intact the zeolitic structure (Flanigen et al., 2010).
The genesis of a zeolite is by the assembly of a tetrahedral element known as a basic building unit (BBU), which is arranged in different secondary building units (SBU) and after as composed building units (CBU) (Xu et al., 2009), as can be seen in the schematic representation of Figure 1. In the same way, crystallization of zeolites can be driven by selecting an adequate zeolite precursor (this can be a zeolite previously synthesized), which share a common SBU with the target zeolite; this gives us the opportunity to create zeolite by a template-free route with better textural properties (Li et al., 2018). The functionality of zeolites is due to their typical properties such as strong acidity, shape selectivity and loading. In this sense, some zeolites can be synthesized with a broad framework composition as the MFI case that can grow with a Si/Al ratio from 2.55 to infinity.
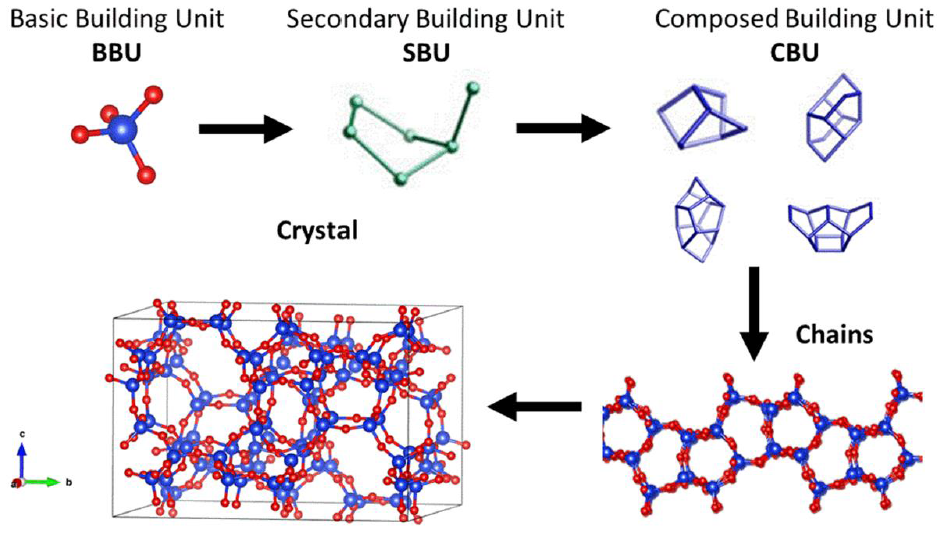
Figure 1 Schematic representation of construction components of a MFI structure (from IZA database) (Baerlocher & McCusker, (2017)).
For an aluminosilicate composition, the 4+ charge of silicon in the framework can be easily neutralized by 2- charge of the oxygen coordinated to two tetrahedral sites. In this sense, the inclusion of a trivalent element as Al, Fe and Ga in the zeolite framework creates an unbalanced charge 1- in the structure, which is located in the oxygen bridges. In other words, one negative charge is located in the tetrahedron, which has aluminum, and it must be compensated to neutralize the charge by an extra framework cation - this can be alkali metals, alkaline earth metals and transition metals. The Si/Al ratio is limited by Löwenstein’s rule (Antúnez-García et al., 2021; Loewenstein, 1954), which dictates that two tetrahedral sites containing aluminum linkages (Al-O-Al) are forbidden; this means that Si/Al ratio less than 1 is not possible. In this sense, the Si/Al ratio represents an idea of the ion exchange capacity of a particular material because a low Si/Al ratio constitutes a bigger ion exchange capacity when compared with a higher Si/Al ratio. As a consequence, a number of possibilities arise for applications of zeolites, where every cation brings additional properties to the system. In this way, Na, K, Li, H as monovalent cations can freely interact with a single site of the framework, while Fe, Cu, Pd, Co, etc; must interact with more than a single site to be linked to the structure. In this sense, exchanging the zeolite with hydrogen protons can give to the material acidic properties, which are pretty helpful in a plethora of applications, namely: as catalysis of organic molecules (García-Trenco & Martínez, 2012; Tempelman et al., 2015), sensing of gasses (Pullano et al., 2020), fuel cell (Ahmad et al., 2006; Han et al., 2012), etc. Furthermore, purely siliceous frameworks expose a neutral framework, and it is a well-known fact that its interaction with water is null given its hydrophobic character, so that takes a special relevance when the application is with organic molecules competing with water adsorption and sieving.
On the other hand, due to their porous nature and well- defined channels, zeolites can expose a lot of void space which, in addition to their external properties, can provide of a large internal surface area, micropore and pore volume. These characteristics are known as textural properties. The porous structure of zeolites, and principally their well-defined porous structure, makes them very attractive to adsorption and shape-selectivity of molecules. Textural features are given by the particular array and connection of the tetrahedra; in the case of the connection of channels, textural features are affected by windows for each particular framework. In this sense, every zeolite structure has its proper textural properties, and they are classified in dependence of the window size as small-pore, medium-pore, and big-pore zeolites. Small-pore zeolites generally have pore size of 4 Å formed 8 tetrahedral sites, whose representative structures are LTA and SOD. In the same way, medium-pore zeolites, whose more representative framework is MFI, have 10 tetrahedra sites opening windows with diameters of 5.5 Å. Similarly, large-pore zeolites such as MOR, FAU and BEA have pore opening of 12 tetrahedra sites with diameters of 7.5 Å. On the other hand, zeolites with pores bigger than 12 tetrahedra are known as extra-large pore zeolites (Xu et al., 2009). All these properties and features make them very attractive for different applications such as adsorption (Regmi & Boyer, 2020; Wu et al., 2021), molecular sieves (Dakhchoune et al., 2020; Li et al., 2020) and shape selecting catalysis (Fang et al., 2021).
3. Catalysts
As previously mentioned, zeolites hold different characteristics such as well-dimensioned pores, channels, and cavities with nanometric size, which turn them an interesting option for adsorption applications. In addition, the introduction of trivalent cations into the framework by isomorphous substitution endows exchange properties to the zeolite. This turn zeolites in valuable materials for exchanging cationic species and catalysis. Moreover, the zeolite’s hydrophobicity and hydrophilicity can be tuned by the aluminum content and therewith, the polarity of molecules can be selected. Aluminum in zeolite framework introduces a negative charge in the framework, which requires a cation for balancing the negative charge; such a chemical polarity can be compensated by Na+ or K+ ions depending on of the synthesis conditions or particular application. There are applications of catalysts, particularly for organics molecules, where a site in zeolites is a very appreciated, this is known as an acid site. Zeolites can present two types of acid sites, Brønsted acid site (BAS) and Lewis acid sites (LAS) as shown in Figure 2. The first one can be formed by the exchange of sodium zeolite with ammonium ion (NH4 +), which can be easily decomposed by thermal treatment at high temperature to ammonia; this leaves a hydrogen proton H+ anchored in the framework that balances the negative charge. On the other hand, the LAS can be produced by dehydration of zeolite at high temperatures (near to 873 K) and under evacuation.
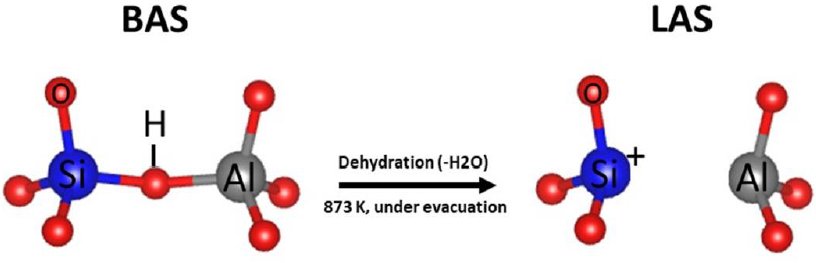
Figure 2 Representation of acid Bronsted acid sites and Lewis acid sites in zeolites (from IZA database) (Baerlocher & McCusker, n.d.).
The composition of the active phase of the catalyst affects the choice of process conditions and the structure of the resulting reaction products. Zeolites are characterized by polyfunctional catalytic properties and a variety of catalytic processes occurring on them. The activity of zeolites is determined by the following main factors: the composition of the framework (SiO2/Al2O3 ratio), the structure of the selected zeolite lattice, the nature and degree of exchange of extra framework cations in it, as well as the correspondence of the size of the molecules of the reacting substances and reaction products with the size of the zeolite windows (Limlamthong & Yip, 2020). Dehydration occurs at the acid sites of zeolites, and oxidative transformations occur at isolated transition metal cations and their associates or clusters with the bridging oxygen of the zeolite framework. Metal clusters and zeolite acid sites mutually influence each other, making it possible to fine-tune the catalytic properties of the resulting zeolite catalyst.
When manufacturing a functional material, the initial properties must be tailored to the specific task or application. In this sense, hierarchical zeolites can be chemically tuned for specific applications such as catalysts. The use of the influence of crystallite sizes, as well as the synthesis of laminar and pillared materials, has become widespread. Methods of isomorphic substitution of tetrahedral atoms in a crystal structure are being developed to regulate acidity.
One of the most important and widespread industrial applications of zeolites in catalysis is for catalytic cracking (FCC) (Scherzer & Gruia, 1996). Faujasite Y zeolite remains as one of the most used catalysts for FCC (Lipin et al., 2020; Vermeiren & Gilson, 2009). In this sense, many catalytic reactions of organic molecules are responsible for the extended use of zeolites such as cracking (Bao et al., 2020), isomerization (Kostyniuk et al., 2021; Lu, Lyu et al., 2020), alkylation (Cheng et al., 2021; Gjyli et al., 2019), catalytic dewaxing (Gerasimov et al., 2019), hydrotreatment (Anderson et al., 1993; Yang et al., 2021; Yocupicio et al., 2019; Zhang et al., 2018; Zhou et al., 2018), biomass valorization (Kumar et al., 2019), among others.
Catalytic cracking, similar to isomerization and alkylation, requires acidic sites on catalysts to activate C-H and C-C bonds (Prakash, 1994). FCC mainly produces light alkanes, high octane gasoline and aromatic middle distillates (Rigutto, 2010). FCC process is catalyzed by acidic sites, while hydrocracking (HDC) is catalyzed by acid-metallic sites, so zeolites occupy an important niche in cracking and HDC. (Rabo & Schoonover, 2001; Weber et al., 1992). The main purpose of catalytic cracking is to reduce the molecular weight of crude oil and tune the H/C ratio. Catalytic cracking is generally achieved by putting in contact the hydrocarbons with acidic or metallic-acidic catalysts at around 400-600°C. This reaction involves the apparition of carbocation intermediates and the mechanism is by β scission of carbenium ions (Prakash, 1994) and produces fuels; FCC mainly produce gasoline, normally C5-C11 fractions, while HCC middle distillates like diesel and kerosene (Vermeiren & Gilson, 2009). The most important catalyst used in HCC process is USY zeolite which is a steam-dealuminated faujasite Y zeolite with a proper mesopore system very appropriate to allow the diffusion of big molecules (van Donk et al., 2003). In the same way, isomerization involves the participation of carbonium and carbenium ions in the reaction mechanism, while that alkylation reaction is considered as the alkylation of carbenium ion by the protonation of alkene (Prakash, 1994).
Many of those processes are achieved by catalytic reforming; this process can convert low octane naphtha to aromatic, high octane gasoline or into BTX (benzene, toluene, and mix) in the presence of hydrogen of xylene isomers).The desirable reactions are: 1) paraffins dehydrocyclization to aromatics and hydrogen; 2) alkylcyclopentanes to cyclohexanes isomerization; 3) cyclohexanes to aromatics and hydrogen dehydrogenation; 4) linear paraffins to isoparaffins isomerization (Perego et al., 2010). Reforming catalysts contain both metallic and acid functionality. In this sense, Pt is known as the best catalyst and typically is promoted by Re, Sn or Ir (Antos & Aitani, 2004). Many zeolites have been used in combination metals as catalytic systems to catalyze naphtha reforming. In this sense, the most important zeolite is potassium-exchanged L zeolite, which has been shown to increase aromatics activity (Corma, 1993). In the same way, ZSM-5 zeolite is used as a reforming catalyst for the Mobil M- Forming process, where channel size can allow the access to singly branched paraffin sans aromatics (Bonacci & Patterson, 1981; Perego et al., 2010).
On the other hand, some other reactions have been successfully performed as methanol to gasoline (MTG) (del Campo et al., 2018; Kianfar et al., 2020), and methanol to olefines (MTO) (Lu, Huang et al., 2020; Yoshioka et al., 2015), although they are still subject of research and improvement and represent some of the applications to organic reactions not related to hydrocarbons conversion. In this regard, the MTO reaction was first achieved by a ZSM-5 zeolite which produced long-chain, branched paraffin and aromatics but failed in produce light olefins (ethylene/propylene). It was overcome by the use of a SAPO-34 (CHA), which resulted in light olefins production, this was explained based on the differences in the pore size (CHA-8MR and MFI-10MR) (Yilmaz & Müller, 2009). Also, within the range of reactions in which zeolites have been used is the selective catalytic reduction (SCR) reaction of NO with ammonia, urea or propene, which is known as an inorganic reaction. For this last reaction, an appropriate metal center -capable of conducting an oxidation-reduction process- must be included in the system by exchanged cation; Fe, Cu and V are still the most used metals in zeolites for SCR reaction (Baran et al., 2020; Sánchez-López et al., 2019; Shan et al., 2020). In the same way, one of the reactions where zeolites are applied, which is known as the "holy grail" due to its complexity, is the methane to methanol reaction. In this reaction, the best results have been obtained by zeolites as MFI and mordenite exchanged by copper and/or iron (Parfenov et al., 2014; Tomkins et al., 2017) to comply with the oxidants role. In this reaction, α-Fe is considered too be the most important site to produce the most active catalytic site α-O (Smeets et al., 2010). The higher activity reported for those systems, until now, had been for the Cu-MOR catalyst with a production of mol of methanol by mol of copper (molCH3OH/molCu) equal to 0.47 (Pappas et al., 2021).
In the same way, there are a lot of applications in catalysis of fine chemistry, where zeolites are considered as good catalysts, but, unlike petrochemical applications, fine chemistry works on a small scale. The principal reasons to use zeolites in the synthesis of fine chemicals are their features: well-known porosity, narrow pore size, exchange capacity and the possibility of introducing acid, base or redox sites in the framework or in exchange positions. There exist two bigger areas where research is focusing, those catalyzed by acid sites and those accomplished by redox reactions. Some of the principal reactions are briefly discussed in the next paragraphs.
Friedel-Craft acylation is one of the most explored reactions using zeolites as acidic catalysts. This is a very useful reaction to produce pharmaceutical commodities (2-acetyl-6- methoxynaphthalene, precursor for naproxen), fragrances (benzophenone and 2-acetylnaphthalene), and fine chemicals (2-methyl-6-acylnaphtalenes) (Bauer et al., 2008; Čejka & Morris, 2017). Such a reaction can be achieved by using a proper acylation agent such as carboxylic acid, chlorides, and anhydrides. One of the first works reported the high activity of zeolites in Friedel-Craft acylation with carboxylic acids of toluene and xylene, particularly using a faujasite Y zeolite (Chiche et al., 1986). Most of the recent research use beta zeolite (BEA) (Aleixo et al., 2019; Bai et al., 2012; Rao et al., 2019). However, the synthesis of zeolites with new porous properties has been attracting the attention (Bai et al., 2014; Kim et al., 2015; Martins et al., 2021).
Aromatics alkylation is not only used to produce cumene and ethylbenzene, but also in the synthesis of fine chemicals. The best example of this reaction is the synthesis of 2- methylnaphthalene (2-MN) and 2,6-methylnaphthalenes (2,6- DMN); the importance of these chemical compounds lies in the fact that they are important feedstocks for the pharmaceutical and chemical industry (vitamin K from 2-MN and polyester resins from 2,6 DMN) (Güleç et al., 2019; Millini et al., 2003). Different zeolites like MOR, BEA, AFI, IFR, CON and CFI were tested for this purpose, but MOR displayed the best performance in this reaction (Sugi, 2010). In the same way, alkylation of toluene with α-methylbenzyl alcohol has been reported, this over large and medium pore size protonic zeolites as HMFI, HMOR, HBEA and HFAU (Kwak & Kim, 1999). In such a report, it was found that the selectivity to 1-phenyl- 1-tolyl ethane (PTE), which is the target product was obtained with the following selectivity HMOR20 > HBEA25 > HMFI30 > HY5, where the number is related to the Si/Al ratio
Reactions of carbonyl compounds are used for the synthesis of some flavors, pharmaceuticals, fragrances and other compounds by means of acetalization of aldehydes and ketones (Bauer et al., 2008). The synthesis of phenylacetaldehyde glycerol and vanillin propylene glycol acetal has been achieved by the use of different aromatic aldehydes and ketones to produce fragrances. For the acetalization of phenylacetaldehyde and vanillin by glycerol and propylene glycol, it was showed that the hydrophobic properties of catalysts are important, since those zeolites with higher Si/Al ratio displayed the best activity (Climent et al., 2004).
Since epoxides are intermediates for the synthesis of some alcohols and ethers, they are very important substances for the fragrance industry. Terpenes are one of the biggest groups used in production of fragrances by epoxidation. In this reaction, limonene, geraniol, pinene, camphene, etc; constitute the most explored compounds. Most of the research is focused on the use of titanosilicates, principally zeolites with isomorphous substitution or impregnated titanium (Přech, Eliášová, et al., 2015; Přech, Vitvarová, et al., 2015; van der Waal et al., 1998; Wróblewska, 2014). However, other metals such as Co, Ni, Cu, Cr, Zn and Fe have been considered (Patil et al., 2007; Tang et al., 2012). Ti-BEA system has been used in the epoxidation of some terpenes, but it fails to catalyze pinenes (van der Waal et al., 1998), however cobalt exchanged in MFI and BEA zeolites displayed very high activity and selectivity in the epoxidation of α-pinene (Tang et al., 2012). An improvement in the catalytic activity was observed for those materials with MFI structure, which were synthesized with better textural properties (Přech, Eliášová, et al., 2015); as a result, it was observed that the conversion increased linearly with the external surface area and mesopore volume of the catalyst (Přech et al., 2021), which shows that textural properties represent quite important features of the catalytic system to improve their performance in the conversion of bigger molecules.
Another of the applications where zeolites have potential applications is for biomass transformation. One of the biggest is the conversion of oil and fats. In this sense, soybean, rapeseed, sunflower oil, jatropha, etc., have been researched (Buzetzki et al., 2011; Ishihara et al., 2021; Luz Martínez et al., 2011; Teixeira et al., 2017). Biofuels can be obtained by different catalytic processes like gasification, pyrolysis, esterification and catalytic cracking (Emori et al., 2017; Galli et al., 2018; Prinsen et al., 2018) with transesterification being the most common route (Kusuma et al., 2013; Leclercq et al., 2001). However, catalytic cracking has been considered as a rising alternative to the production of vegetable oil to produce gas oil gasoline, diesel and kerosene (Hua et al., 2008), which are free of heteroatoms like sulfur or nitrogen. Cracking of rapeseed oil by different zeolites like NaY, NH4Y, clinoptilolite and ZSM-5 zeolites produced a 10% of gaseous fraction and a 90% of liquid condensate which contained mainly paraffins, olefins and fatty acids (Buzetzki et al., 2011). Cracking of rubber seed by USY zeolite generated similar composition of fuel to that of petroleum fuel nut with low acid values, good cold- flow properties, and high calorific values (Li et al., 2014). When USY was used for cracking of Jatropha Curcas oil, it was increased the production of gasoline and diesel; this was attributed to the mesoporosity since the increasing of mesopore volume directed the selectivity to olefins, while that decrease of mesopore volume increased the selectivity to high octane gasoline and decreased the coke (Zheng, Huo et al., 2017).
Pyrolysis of polymers biomass like lignin can be achieved in the presence of acidic materials like zeolites. This route can represent a cheap and efficient approach to conversion of liquid pyrolysis oil and producing fuels and some chemicals. A lot of zeolites have been used for pyrolysis biomass like SAPO- 34, MCM-22, FAU Y, ZSM-5 and beta, being the last two the most studied and researched (Cai et al., 2020; Ma & van Bokhoven, 2012; Mahadevan et al., 2016; Zheng, Jiang et al., 2017). In this sense, ZSM-5 as a catalyst for biomass produces preferentially BTX (benzene, toluene, xylene) and naphthalene, which are very demanded for the petrochemical industry (Cai et al., 2020; Naqvi & Naqvi, 2018). In a pioneering work, when ZSM-5 was used in the pyrolysis of oil, the oxygen content was reduced from 33% to 13% for vapors treated in the ZSM-5 zeolite, while at the same time the formation of aromatic hydrocarbons was promoted and the concentration of oxygen was decreased (Horne et al., 1995). Gallium has been used in ZSM-5 to catalyze pyrolysis of pine wood; the catalytic system increased the selectivity to monoaromatic hydrocarbons (MAHs) like benzene, toluene and p-xylenes, while selectivity to polyaromatic hydrocarbons (PAHs), like naphthalene, was decreased; this was attributed to the enhanced hydrogenation and the decreased pore size (Li et al., 2015). When textural features of ZSM-5 zeolite are improved, the accessibility for molecules is increased. The high presence of strong acid sites in ZSM-5 favors the occurrence of undesired reactions such as those provoking the deactivation by coke deposition. Besides, ZSM-5 promotes decarbonylation that produces loss of mass and energy yield (Hernando et al., 2018). On the other hand, the introduction of Lewis acid sites by particles or species in extra framework positions can lead to the increase of bio-oil yield with a decrease of severe cracking reactions, coke decrease and an increase of deoxygenation degree (Hernando et al., 2018).
4. Adsorbents
As mentioned in the Introduction, zeolites are excellent adsorbents because of their structure. Due to the difference in pore diameter, each of the more than 240 currently known crystal structures of zeolites (Baerlocher & McCusker, 2017) can be used to separate different gas mixtures. This is possible because in the mixture, each molecule has its own size value. This is the reason why zeolites are known as "molecular sieves". However, as early as the mid-twentieth century, it was discovered that the same zeolite structure could be functionalized to produce selective adsorbents through ion exchange (Breck, 1984). The most famous example of such functionalization is the preparation of LTA zeolite in the K+, Na+ and Ca2+ forms. These materials were commercialized under the trademarks "3A", "4A" and "5A", where the number represents the limiting diameter of the molecules adsorbed by this material, i.e., 3, 4 and 5 angstroms, respectively. This feature of LTA zeolite is currently actively used in the creation of technologically important devices (Wenten et al., 2017). Membranes based on LTA zeolite were synthesized and then subjected to ion exchange in potassium chloride solutions, resulting in material "3A" (Shirazian & Ashrafizadeh, 2015). Such materials selectively filter water vapors from natural gas. Dehydration of the gas prior to transportation through tubes at elevated pressure prevents the formation of clathrate hydrates, which could otherwise clog gas pipelines. Another important area where the production of functionalized adsorbents is of paramount importance is the reduction of the level of carbon dioxide in the atmosphere. CO2 emissions are one of the main drivers of climate change. The world economy is associated with obtaining energy from coal, oil and gas burning. CO2 emissions have doubled over the past 40 years; according to the Global Carbon Budget (Friedlingstein et al., 2019), China, the USA, India, Russia and Japan are the largest emitters of CO2: these five countries account for 57% of the world's anthropogenic CO2 emissions into the atmosphere. In this regard, new solid sorbents for physical sorption, including zeolites, to capture CO2 after combustion are being actively developed (Ben-Mansour et al., 2016; Shelyapina et al., 2020). Zeolites, due to their high stability, adsorption capacity, adsorption selectivity and fast kinetics of the CO2 adsorption/desorption process, are very attractive for CO2 post-combustion adsorption. The adsorption characteristics of zeolites are influenced by pore size, basicity, electric field strength due to the presence of ion-exchange cations, etc. (Regufe et al., 2019). The high selectivity of zeolites is primarily due to the fact that their pore diameter is similar to the kinetic one of adsorbed molecules. As a result, they exhibit a kinetically enhanced CO2 selectivity over CH4 and N2. Ion exchange treatment of zeolites strongly affects their adsorption properties. Replacing some cations with others that significantly differ in size from the original ones, one can play with the pore inlets and, accordingly, regulate the diffusion rate of adsorbed molecules, and, consequently, regulate both the adsorption/desorption rate and selectivity. For example, partial substitution of Na+ for Li+ in ZSM-25 zeolites increases CO2 uptake at low pressure by several percent and the adsorption rate by an order of magnitude (Zhao et al., 2018).
The creation of mesoporosity in the crystal structure is another key for improving the adsorption properties of zeolites in relation to CO2. For example, mesoporous LTA zeolites exhibit faster CO2 adsorption kinetics than microporous ones (at the same Si/Al ratios and particle size) (Chen & Ahn, 2014). Hierarchical porous ZSM-5 exhibits better CO2 adsorption capacity (Liu et al., 2017). In fact, hierarchical zeolites are considered very promising for CO2 capture: a microporous framework is responsible for selectivity, and mesoporous channels provide the rapid mass transfer.
The main disadvantage of zeolites as CO2 adsorbents is that they initially have a higher affinity for polar H2O molecules; the presence of water vapor negatively affects the adsorption capacity of zeolites in relation to CO2. Pre dehumidification prior to CO2 capture significantly increases energy consumption. An alternative for the functionalization of the adsorbent is to protect the zeolite particles with a hydrophobic shell, for example by impregnation with an amine. In humid conditions, the amino groups in the shell will react with H2O and CO2 to form carbonates or bicarbonates, which effectively fix the water molecules, preventing their diffusion into the zeolite core (Liu et al., 2016).
Another interesting application of zeolites as adsorbents includes the selective adsorption of olefins. Selective adsorption of linear α-olefins is reported by Yang et al. (2020). In such a work, the adsorption of 1-hexene/n-hexane (C6), 1- octene/n-octane (C8), and 1-decene/n-decane (C10) by zeolite adsorbents were studied. As a result, it was observed that a modified 5A zeolite exhibited better adsorption capabilities than standard 5A zeolite, this effect was observed for C6 and C8 olefins. Adsorbents for olefin/paraffin separations based on AgY zeolite were proposed by Padin et al. (1999). As it is reported, the separation/purification is done after the selective adsorption of olefins and dienes by π-complexation. It was found out that for the process of butene purification by removal of butadiene, the AgY zeolite offers a better performance than 5A zeolite and NaY.
As it is well-known, due to their similar physical properties, the separation of ethylene from ethane is a complex task. In this regard, the research aims to find an additional route to the traditional cryogenic distillation. Martins et al. explored the use of zeolite 13X as an adsorbent to produce ethylene from ethane/ethylene mixtures (Martins et al., 2016).
5. Sensors
Zeolites are materials that can be used for developing sensors. This is possible due to the capacity of zeolites to selectively adsorb chemical species. For zeolites, this selectivity is due to the characteristic shape of cavities and pore distribution in each one of the reported frameworks, and also, due to their chemical/electronic affinity to the desired analyte (Murrieta-Rico, Mercorelli, et al., 2015; Murrieta-Rico, Petranovskii, et al., 2015). As it has been mentioned, zeolitic affinity can be "tuned" for achieving the capacity for adsorbing a new and specific chemical species. However, by itself, this mechanism is not enough for acting as a sensor. In this regard, the zeolite needs to be used for modifying an appropriate transducer, where the changes that occur in the zeolite during adsorption can be converted into a measurable parameter (Murrieta-Rico, Mercorelli, et al., 2015).
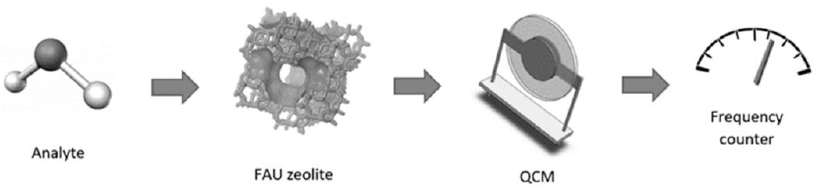
Traditional approaches include the use of zeolites to functionalize quartz crystal microbalances (QCMs), surface acoustic wave (SAW) sensors, or microcantilevers (MCLs) (Murrieta-Rico, Mercorelli, et al., 2015). In these cases, the basic principle of operation is based in the mass variation that occurs when the adsorption/desorption processes take place. This means that when the zeolite interacts with the desired analyte, the latter is adsorbed in the zeolite’s surface. For the QCMs, SAW sensors, and MCLs, the most common operating mode is based on the measurement of their proper frequency, which is shifted because of mass loading. In other words, when a guest molecule is adsorbed on the zeolite surface, there is a variation shift in the frequency generated by the sensor. The Figure 3 shows a schematic representation of the principal elements in sensor operation based on a QCM modified with FAU zeolite.
Each zeolite has an intrinsic adsorption capacity. This implies that the amount and kind of molecules that a specific zeolite can adsorb are limited. Examples of the use of non- functionalized zeolites for modifying QCMs include water detection using LTA, BEA, FAU zeolites (Mintova & Bein, 2001; Murrieta-Rico et al., 2021), and adsorption of hydrocarbons using BEA zeolite; other examples of the use of pure zeolite over the QCM are reported by Sasaki et al. (2002), where zeolite A, silicalite-1 and sodalite were used for detecting NO, SO2 and H2O in He.
With the aim to enhance detection capacity, zeolites can be modified. This process allows the interaction of the zeolite with the desired compound. Some examples include ZSM-5 modified with Ag+ for detecting acetone vapor (Huang et al., 2004) and dimethyl methylphosphonate (Xie et al., 2005), ZSM- 5 modified with Cu to detect dimethyl methylphosphonate (Ji et al., 2012). There are plenty of examples where zeolites are used for modifying QCM, but this approach has been proven to work better when the desired chemical is in a gaseous phase. However, most of the open problems regarding the zeolite modified QCM, are related to the instrumentation circuits that are used in the experiments. In this regard, novel approaches the estimation of dynamic frequency shifts are required (Murrieta-Rico et al., 2017, 2021).
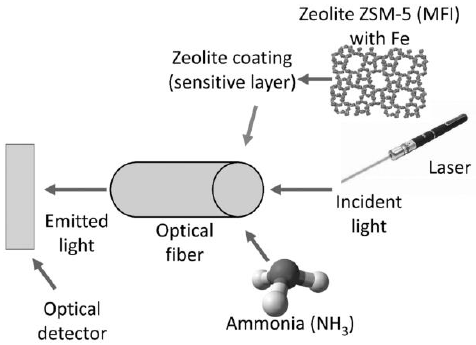
In addition to QCM, there are other approaches for using the sensing capabilities of zeolites. For example, optical sensors are generated after the use of zeolites in the functionalization of optical fibers. In these sensors, the tip of an optical fiber is functionalized with a zeolite capable of interacting with an analyte of interest. On the functionalized tip, a light source generates a signal that travels through the fiber. Finally, the light is emitted by the fiber and this signal is detected by an optical detector, the general mechanism of this process is presented in Figure 4. This principle of operation is especially suitable for detection in liquid media. A comprehensive review of this kind of sensor is presented by Murrieta-Rico et al. (2019).
6. Health applications
6.1. Antibacterial activity
The zeolite is very effective in promoting hemostasis by the concentration of platelets and clotting factors to absorb blood at the site of injury rapidly. Functionalization with organic compounds provides greater adhesion and improves blood absorbance of the zeolite. The hydrogel beads formed by chitosan and zeolite developed in the study by Fathi et al. offer a potential platform for an affordable, accessible and effective hemostatic agent for use in the treatment of traumatic bleeding (Fathi et al., 2018). Other research was to develop compounds zeolite / polymer loaded with antibiotics for hemostatic materials. The microspheres contained three pore sizes, zeolite nanopores, micrometer-sized pores between zeolite particles, and empty nuclei that were tens of microns in size. The combination of zeolite, natural polymers and antibiotics makes it possible to use their characteristics to the maximum to create multifunctional hemostatic materials. (Zhang et al., 2011). These excellent properties have allowed zeolites to interact with different molecules for specific applications. With their porous structure, zeolites can transfer atoms or molecules from their crystals to the environment and vice versa (Taaca & Vasquez, 2017). It was observed that zeolites incorporated with metallic ions are materials with quite interesting antibacterial properties, because they inhibit the proliferation of bacteria and fungi, such is the case of the study carried out by Sánchez et al. (2017) where they functionalized ZSM-5 type zeolite with silver ions and evaluated it by putting it in contact with bacteria such as Escherichia coli, Pseudomonas aeruginosa and the Candida Albicans fungus. The results showed that functionalized zeolite inhibits the proliferation of bacteria and fungi. The copper oxide-zeolite nanocomposite was prepared and investigated, using different weight percentages of zeolite- loaded copper oxide nanoparticles to obtain the optimal copper oxide distribution for antibacterial activities (Alswat et al., 2017). Antibiotics can improve efficiency and increase the antimicrobial effect of the drugs, loading antibiotics within zeolites due to the ordered micropores, such as gentamicin with ZSM-5 zeolite that prevents biofilm formation against Staphylococcus epidermidis (Guo et al., 2014).
Zeolite powders can adhere to surfaces such as stainless steel. The zeolite coating, which contains silver and zinc ions, acts by inhibiting bacterial enzymes, interfering with electron transport, and binding to DNA, increasing the stability of the double helix. Zinc inhibits nutrient absorption and interferes with proton transfer. As reported by Rusin et al. (2003), it can reduce the water-based bacteria that is very common in domestic water sources, such as Legionella pneumophila on stainless steel surfaces. Another similar study by Cowan et al. (2003) tested the efficacy of a zeolite matrix containing silver and zinc ions used as a coating for stainless steel against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Listeria monocytogenes. There was a 99.997% reduction in viable counts compared to uncoated stainless steel. S. aureus like E. coli were virtually eliminated from zeolite-silver-zinc coated surfaces.
6.2. Antiviral activity
Antiviral properties of powders of modified zeolite with metal ions have been evaluated using the human coronavirus 229E, virus infects FIP (FIPV) and the feline calicivirus F-9. It was observed that zeolites containing silver and silver/copper resulted in a significant decrease in the activity of coronavirus 229E and FIPV. Silver/copper zeolite reduced the viruses tested, suggesting that it may be effective against related pathogens of concern ENT#091;i.e., SARS coronavirus, other coronaviruses, human norovirus (calicivirus)ENT#093; (Bright et al., 2008). It is well known that the resistance of viruses to physicochemical agents increases when viruses are contained in certain materials such as feces or mucous secretions It has been clearly demonstrated that the high and rapid inactivating effect of Cu2+ zeolite cotton textile on influenza A (H5N1) virus is not affected by presence of FBS and feces (Imai et al., 2012). Zeolites have been the attention of many researchers due to the modification of the interface, necessary for virus detection. Zeolites provide a biocompatible environment for various biomolecules such as DNA, but they are not conductive. Narag et al. prepared a multi-walled zeolite nanocrystalline and carbon nanotube-based diagnostic genosensor for the detection of polymerase chain amplified hepatitis B virus DNA in the blood of hepatitis B patients. The sensor is capable of improved detection of hepatitis B virus DNA in patients with 99% accuracy (Narang et al., 2016). Zeolite is very suitable for virus adsorption, as reported by Cormier and Janes (2016) investigated the ability of zeolite to adsorb hepatitis A virus in artificial seawater. The zeolite they used is an overcharged material and absorbs negatively and positively charged particles that are suspended in water. Zeolite was able to concentrate Hepatitis A virus from artificial seawater with ~99% efficiency in less than 5 minutes and was more efficient in seawater than freshwater.
6.3. Antitumor treatment
Cancer is one of the leading causes of death in the world and therefore attracts the attention of researchers in finding new therapies for efficient treatment, avoiding adverse side effects. Research by Karimi et al. (2019) focuses on developing a suitable delivery system for curcumin ENT#091;1,7-bis(4-hydroxy-3- methoxyphenyl)-1,6-heptadiene-3,5-dioneENT#093; active ingredient that is isolated from the herb Curcuma longa, which has antioxidant and anti-inflammatory activities, anti-tumor, anti- cancer, antimicrobial and anti-HIV activities. The studies are focused on developing a suitable administration system. Nanocomposites developed using zeolite Y and ZSM-5 with polyethylene glycol as charge carriers and release of curcumin, showed release levels of curcumin higher in zeolite Y compared to ZSM-5.
Zeolites and their antitumor effect are relatively rare and are limited to the application of natural clinoptilolite zeolite, and its potential role as adjuvant chemotherapy applied after initial cancer treatment, especially to suppress secondary tumor formation (Pavelić et al., 2001). Micronized clinoptilolite interferes with lipid peroxidation in the liver of cancer-bearing mice, resulting in a decrease in tumor size, an improvement in general health, and a prolonged lifespan. Zeolite-A micronized with silver ions shows efficacy against the lung carcinoma cell line (A549), followed by the human Caucasian breast adenocarcinoma (MCF7), and then the human hepatocellular carcinoma cell line (HePG2) (Youssef et al., 2015). The use of zeolites as precious metal support has been studied by El-Bahy et al. (2019). In his research, the antitumor activity of NaY zeolite coated with platinum, palladium and copper nanoparticles was evaluated against hepatocarcinoma. The results showed that the copper nanoparticles loaded in zeolite reduced viability by 87%. Platinum and palladium nanoparticles loaded on zeolite show that surviving cells remain around 39-40% after 24 hours. Zeolites may have an impact on the function of anticancer agents.
6.4. Activity in drug delivery systems
At the end of the current decade, it has become evident for most of the planet's inhabitants that we are exposed to the emergence of new pathogens as potential threats. Also, bacteria and fungi are capable of developing resistance to the new generation of antibiotics (Amarasiri et al., 2020; Laukkanen, 2020). The use of nanoparticles with theragnostic applications promises to combat diseases and overcome difficulties to which conventional medicine is limited (Elechiguerra et al., 2005, p. 1; Figueroa et al., 2019; Ghaffari et al., 2019; Koudelka et al., 2015; Maduray & Parboosing, 2020; Nanotechnology versus Coronavirus, 2020). However, in most cases, a coating or support is required to serve as a vehicle to introduce them into the human body. Since there is a large variety of biocompatible zeolites that have a micropore structure of regular size (but can also artificially maintain a mesoporous structure), this makes them ideal for both encapsulating within and dispersing nanoparticles on their surface (Alswat et al., 2017; Bacariza et al., 2020; Chai et al., 2018; Goodarzi et al., 2018; He et al., 2020; Ryoo et al., 2020; Tauanov et al., 2018; Xu et al., 2020; Yoon et al., 2017). These characteristics also make them capable of supporting drugs or genes for controlled release (Abdelhamid, Dowaidar, Hällbrink & Langel, 2020; Abdelhamid, Dowaidar, & Langel, 2020; Abdelhamid et al., 2019; Alsaiari et al., 2018; Anfray et al., 2020; Kehr, 2018; Khatamian et al., 2017; Kocaaga et al., 2019; Poddar et al., 2019; Servatan et al., 2020).
Recent studies have shown that the potential of zeolites in medical applications is due to their structural properties and stability in biological environments. Zeolites have also been explored as suitable hosts for encapsulation of drug molecules, in search of efficient drug delivery systems (Krajišnik et al., 2019, p. 2). Both zeolites and drugs have been administered simultaneously to patients without losing the individual pharmacological effect of the drugs (Vilaça et al., 2013). The adsorption capacity of zeolitic materials depends, in general, on the hydrophobic character of the structure, which increases with decreasing aluminum content, so, the loading of hydrophobic drugs can be expected to be greater in zeolites that are highly dealuminated. Horcajada et al. (2006) reported on the adsorption and in vitro administration of ibuprofen, an anti-inflammatory drug used as a model molecule, in a series of zeolites Y in H-form (structure type code of the International Association of Zeolites FAU), noting that in zeolites with higher aluminum content, ibuprofen binds more strongly to the surface of the zeolite. In addition, synthetic zeolites, such as zeolite X and a zeolite product obtained from a co-crystallization of zeolite X and zeolite A, have been studied to verify their ability to encapsulate and release ketoprofen, an anti-inflammatory drug. Under different pH conditions to mimic gastrointestinal fluids, ketoprofen begins to leave zeolite at pH 5.0, which makes these materials particularly interesting for the treatment of inflammatory pathologies of the gastrointestinal tract (Rimoli et al., 2008).
6.5. Development of personal protective equipment for controlling spread of diseases
Channels and inner cavities of zeolites have the capacity to host chemical species. This mechanism allows controlling the interaction of the host with a medium. For example, if particles with oligodynamic properties are trapped inside the zeolitic framework (Yocupicio-Gaxiola et al., 2021), then the duration of the biocide effect can be extended. In addition, the use of zeolites as the host can improve the adherence to the textile material of the nanoparticle with biocide effects (Imai et al., 2012); a generalized idea of this process is shown in Figure 5. As it has been discussed, this mechanism of "controlled release" can be used in plenty of applications, such as antibacterial/antiviral materials, antitumor treatment, drug delivery, etc.
7. Other applications of zeolites as functional materials
Strategies to accelerate the translation of these porous materials from bench to bedside along with commonly overlooked physiological and pharmacological factors of zeolite nanoparticles are discussed and debated elsewhere (Yocupicio-Gaxiola et al., 2021). Furthermore, for zeolite nanoparticles, it is a matter of crucial importance, in terms of biosafety and nanotoxicology, to appreciate the zeolite-bio interface once the zeolite nanoparticles are exposed to the biomacromolecules in biological media (Derakhshankhah et al., 2020). Zeolites are promising for environment protection, detoxication of animal and human organisms, improvement of the nutrition status and immunity of farm animals, separation of various biomolecules and cells, construction of biosensors and detection of biomarkers of various diseases, controlled drug and gene delivery, radical scavenging, and particularly tissue engineering and biomaterial coating. As components of scaffolds for bone tissue engineering, zeolites can deliver oxygen to cells, can stimulate osteogenic cell differentiation, and can inhibit bone resorption. Zeolites can also act as oxygen reservoirs and can improve cell performance in vascular and skin tissue engineering and wound healing. When deposited on metallic materials for bone implantation, zeolite films showed anticorrosion effects, and improved the osseointegration of these implants (Bacakova et al., 2018).
7.1. Water treatment
The use of zeolites as a matrix to support groups of semiconductors and metal and nanoparticles has increased in recent years, due to the reduced size of the cavities and ordered channels of the zeolite, which also limits the growth of grains, stabilizing their size in nanometric dimensions (Kuznicki et al., 2007). Zeolites are considered promising hosts and stabilizers due to their unique characteristics such as having 1.3 nm diameter cavities, which helps to avoid agglomeration of nanoparticles due to the effect of Van der Waals forces or other interactions. Because of this zeolite can be useful for the removal of contaminants that could cause fatal effects on human health, such as the case of lead (Pb) and arsenic (As) that ingested in water cause various diseases such as cancer kidney, skin lungs, gastrointestinal diseases, bone marrow disorders, cardiovascular diseases, among others.
Alswata et al. (2017) synthesized a nanocomposite zeolite-zinc oxide nanoparticles by the co-precipitation method with a high adsorption capacity and could be used as an efficient and low-cost adsorbent for the removal of heavy metals from water. The elimination of arsenic in solution by zeolites can also be carried out with the use of metallic nanoparticles on the surface of the zeolite and eliminate up to 95.6% of arsenic, as reported by Salem Attia et al. (2014) where they synthesized iron oxide coated zeolite. The colorants used in the textile printing and dyeing processes have become an important environmental problem, which requires adequate and efficient solutions. Rhodamine is one of the most toxic dyes, it can cause mutagenicity and toxicity in cells, besides being a dangerous carcinogenic organic compound for humans. TiO2-chabazite semiconductor compounds are a good alternative to decompose rhodamine molecules by photocatalysis. Zeolite (chabazite) improved semiconductor photocatalysis to remove some of the molecules of rhodamine, which helps the penetration of light to enhance the generation of pairs of electrons and holes (Ramírez-Aparicio et al., 2016).
7.2. Agriculture and food production
There has been growing interest among farmers to use nanotechnology to develop fertilizers in the use of nutrients. The sustainable use for the synthesis of mineral or organic nanofertilizers requires a reflection on the mechanism, as well as the destination of the nutrients and their interaction with the soil-plant systems. The use of zinc oxide nanoparticles as well as zeolite significantly increases the mineral nitrogen content in the soil (Aziz et al., 2019).
Permeability, selective ion (e.g., K+, Ca+, and NH4) exchange capacity, and reversible water absorption/desorption are characteristics of zeolites that make them very attractive in agricultural production. For example, for maize plants (Zea Mays L.), it has been seen that the soil amelioration with zeolites improves the size, the cob weight, and the green forage yield (Aainaa et al., 2018; Kojić et al., 2012; Latifah et al., 2017; Şahin et al., 2020). Similar benefits have been observed for other plants such as tomato (de Bruijn et al., 2020; Valente et al., 1982), lettuce (Aguiar et al., 2020; Gületal., 2005), greenbeans (Türkmen & Kütük, 2017), rice (Wu et al., 2016), wheat (Agegnehu et al., 2014), pears (Şen et al., 2020), and apples (Milosevic & Milosevic, 2009) among others. The remediation of highly toxic soils for cultivation (Hu et al., 2018; Palansooriya et al., 2020; Radziemska et al., 2019; Yamada et al., 2007) is another area in which zeolites have also played an important role in food production. Additionally, zeolites have separately been shown to be effective as insecticides (Bohinc et al., 2020; De Smedt et al., 2016; El-Bakry et al., 2019) or as preservatives (Tzia & Zorpas, 2012) of farm products, characteristics that also have a high impact on food production.
Zeolite helps the infiltration and retention of water in the soil due to its very porous properties and the capillary suction it exerts. It acts as a natural wetting agent, is an excellent amendment for non-wetting sands, and aids in the distribution of water through the soil. This can significantly improve the water retention of sandy soils and increase porosity in clay soils. Improving the water-holding capacity of soils could result in higher crop production in drought-prone areas. Zeolite will further enhance the soil's ability to retain nutrients and produce better yields (Prasad et al., 2014).
7.3. Herbicides, fungicides, and pesticides
Natural zeolites have been considered as possible sorbents for herbicides, fungicides and pesticides with the function of slow- release carriers and therefore retarder of water contamination. In these organic compounds, the structure of the zeolite, sorption is due to polar chemical bonds with the extended external surface of the zeolite (Colella, 2007). Zeolites have also been evaluated for the protection of durable products against post-harvest infestations. Unlike diatomaceous earth, which has been extensively evaluated against insects in stored products, there are generally few studies available on the insecticidal potential of zeolite against such insects (Athanassiou et al., 2005). For example, Roumbos et al. investigated the insecticidal effect of three formulations of natural zeolites against adult confused flour beetle, Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae), the toothed grain beetle, Oryzaephilus surinamensis (Coleoptera: Silvanidae) and Sitophilus oryzae. Roumbos et al. reported that Oryzaephilus surinamensis was the most susceptible to the zeolite formulations tested, while Tribolium confusum was the most tolerant (Rumbos et al., 2016). In the same, Andric et al. investigated the potential insecticidal natural zeolites and diatomaceous earth against S. oryzae and Tribolium castaneum, as a result, it was reported a higher mortality for S. oryzae and Tribolium castaneum; this was found after a long exposure period and 7 days of recovery in wheat treated with natural zeolite in the rate most high and diatomaceous earth rates 0.50-1.0 g/kg. Progeny reduction of over 90% was achieved after 14 and 21 days of contact of both beetle pests with diatomaceous earth-treated wheat. Satisfactory effects can also be achieved with lower concentrations of zeolite. As reported by Bohinc et al. (2020) in their study, they tested the efficacy of natural and synthetic zeolites, achieving a high mortality of the corn weevil (Sitophilus zeamais Motschulsky), influenced by relatively high values of temperature and low relative humidity. It has been established that various types of zeolites can be used against a wide range of insect species. In this regard, zeolites should be considered slow-acting insecticides. This means that exposed insects, even when deteriorated, may have some time to lay eggs before death and lead to the production of progeny. A combination of zeolites with an agent that causes a higher "death rate" (small doses of conventional insecticides) can be carried out, to mitigate the progeny production capacity of the parental individuals (Eroglu et al., 2019).
8. Conclusions, perspectives, and final remarks
This paper discusses the uses of zeolites that can be tailored to specific applications. In this regard, zeolites are functional materials. Their applications can be increased if these materials are modified for specific applications. In this sense, the versatility of zeolites allows, through modifications on their chemical synthesis, to change the volume of inner channels, the pore size, the chemical selectivity, etc. In addition, the creation of lamellar zeolites is an active line of research, because these kinds of materials offer a high exposed surface. This implies that a bigger functionalized surface is available for diverse applications, such as catalysts, sensors, etc.











 text new page (beta)
text new page (beta)