1. Introduction
Foot infections are a damaging and costly health problem that varies from local fungal infections of the foot nails to a necrotizing limb (Adams & Deitch, 2001). This condition is frequent in people who have athlete’s foot, peripheral arterial disease and diabetes mellitus (UcKay et al., 2014). Previous reports suggest that the worldwide incidence of diabetes is growing rapidly, expecting to increase from approximately 280 million adults today to over 400 million adults by 2030 (Shaw et al., 2010). Foot infections are a common complication in diabetic patients and contributes to the apparition of diabetic foot ulcers (Chatzistergos et al., 2015). Around 15-25% of people with diabetes will develop diabetic foot ulceration. This represents the major cause of hospitalization and limbs amputation among diabetic patients, from all the amputation cases, 85% was preceded by microbial infection and foot ulceration (Boyko et al., 1999). The susceptibility for foot infections in diabetes mellitus is increased by the presence of neuropathy, vascular insufficiency and diminution of immune cells function (Bader, 2008; Demirseren et al., 2014). These conditions result in loss of protective sensation for pain which impairs the sensitivity for skin injuries, e.g., blisters, abrasions, and increased plantar pressure by allowing the penetration of foreign materials that may cause infection and foot ulceration (Anisha et al., 2013).
Dermatophytosis is recognized as a common foot infection in the keratin layer of the skin that is caused by Trichophyton, Epidermophyton and Microsporum. These fungi affect about 20-25% of the population in the world; mainly immunocompromised people (i.e., subjected to organ transplantation), patients with diabetes mellitus or cancer. The severity of these fungal infections is explained by their association with local or systemic secondary bacterial infections (Bhatia & Sharma, 2015).
It is well known that footwear can be a promoter of foot infection by their contribution to skin injuries and to provide a microenvironment suitable for the proliferation of microorganisms. The use of occlusive footwear can cause the development of hyperhidrosis, smelly feet, and sometimes a burning sensation when walking. This can cause lesions in the plantar zone of the foot and infections in the corneum stratum by Micrococcus and Corynebacterium bacteria. These bacteria promote major skin injuries and keratolysis due to the production of proteinases and sulfur compounds (Fernández-Crehuet & Ruiz-Villaverde, 2015).
Therefore, preventive strategies to avoid foot infections are needed in addition to therapeutic approaches. Special footwear manufactured with soft materials to reduce skin injuries and foot pressure has been developed; however, after use, low satisfaction by users was reported because of the lack
of protection against foot infections (Otter et al., 2015). In this sense the materials for footwear manufacturing must be improved to provide suitable materials that avoid foot infections and to increase their value to the footwear industry.
Nanotechnology offers promising solutions in biomedical applications. AgNPs (AgNPs) are the most studied nanomaterial because of their potent microbicide activity (Ge et al., 2014). It has been suggested that the antimicrobial properties of AgNPs are explained by the interaction between the positive charge of AgNPs with the negatively charged microbial cell wall, which limits metabolic and reproduction processes in the microorganisms. Therefore, AgNPs have been used worldwide in medical, cosmetic, veterinary, and food products to prevent infections. For example, it has been reported that AgNPs can inhibit the growth of bacteria linked to foot infections and that had developed multidrug resistance such as Bacillus circulans, Acinetobacter baumannii, Pseudomonas aeruginosa and Staphylococcus aureus (Veraldi et al., 2014).
Considering the antimicrobial properties of AgNPs, the aim of this study was to develop leather coated with AgNPs and to investigate their influence on its texture properties. Additionally, the antibacterial and antifungal activity of AgNPs on the coated leather was evaluated against Trichophyton mentagrophytes, Pseudomonas mendocina and Pseudomonas syringae, microorganisms responsible for foot infections (Agner et al., 2000; Gani et al., 2019; Woodfolk, 2005).
2.1. Chemicals
All reagents used for microbiology tests were purchased from Sigma-Aldrich (Saint LousieMO) and J.T. Baker (Radnor, PA). The leather porcine-derived was obtained from a company located in Leon, Guanajuato, Mexico.
2.2. Silver nanoparticle suspension
The silver nanoparticle solution named Argovit was kindly donated by Professor Dr. Vasily Burmistrov from the Scientific and Production Center Vector-Vita (Russia). Argovit is a preparation of highly dispersed AgNPs with an overall concentration of 200 mg/mL (20%) of PVP-coated AgNPs in water. The content of metallic silver in Argovit preparation is 12 mg/mL, stabilized with 188 mg/mL of PVP. AgNPs dilutions were calculated according to metallic silver content in Argovit preparation.
Several dissolutions of Argovit were prepared in distilled water to obtain the following concentrations according with the metallic silver content in each preparation: 0.0025, 0.005, 0.025, 0.05, 0.15 and 0.25%.
2.3. Preparation of AgNPs coated leather
Porcine leather was cut off to take pieces of 225 cm2. Then, the square pieces were sprinkled with 7 mL of dissolutions of AgNPs to obtain the leather-coated with different concentrations of AgNPs as mentioned above (2.2). Subsequently, the leather was dried for 20 minutes at room temperature.
2.4. Quantification of metallic silver on AgNPs coated-leather
After drying, the leather coated with AgNPs was prepared to assess the concentration of metallic silver on the leather by inductively coupled plasma/atomic emission spectrometry (ICP-AES) (Inductively coupled plasma-atomic emission spectrometry, Model ICP-Iris intrepid according to EPA Method 6010). The leather quadrates were reduced to small pieces between 2 and 4 mm. Subsequently, 1 gr of sample was digested in 10 mL of HNO3 concentrated at 140 °C for 15 minutes. After 3 hours, the samples were filtered and filled to 100 mL with distilled water, then the ICP-AES analysis was performed.
2.5. Texture analysis of the leather coated with AgNPs
Texture and softness change after the application of AgNPs were evaluated with a TA.XTPlus Texture Analyzer (Texture Technologies Corp., Hamilton, MA). The test was performed with a speed of 40 mm/s and load handling of 4 and 30 Kg, with a minimum speed of 0.01 mm/s, to determine the final smoothness of the leather. Initially, the electrode, which was used as a point of penetration in different areas of the leather, was calibrated, then, five repetitions per sample were performed, and softness was reported as mm of penetration.
2.6. Antibacterial and antifungal analysis
For microbiological assays, the leather was cut off to obtain 1 cm2 pieces, then, the antimicrobial activity of AgNPs impregnated on the leather was tested against P. mendocina and P. syringae bacteria and the fungi T. mentagrophytes. These specimens were obtained from the American Type Culture Collection (Manassas, VA).
Microorganisms were cultured in a nutrient agar medium and maintained at 4 °C before their use. Fungi T. mentagrophytes was propagated under aseptic conditions in potato dextrose broth (PDB) growth media for 5 to 7 days (Figure 1A). Then, fungi mycelium was removed from the broth for its weighing (Figure 1B) and following deposition (one gram of mycelium) on the leather coated with AgNPs (Figure 1C) to investigate the spreading of fungus on the leather after 20 days of incubation at 37 °C. Thus, T. mentagrophytes susceptibility was determined through the final mycelium weight (after incubation time) on AgNPs-coated leather. A negative control for T. mentagrophytes (leather without treatment) was considered.
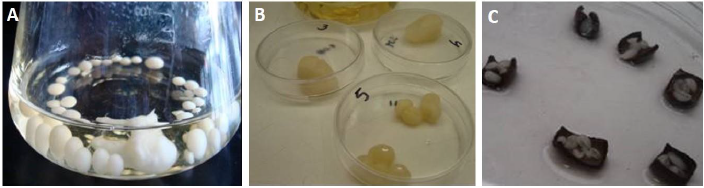
Figure 1 A) T. mentagrophytes mycelium broth; B) fungi mycelium taken from the broth for its weighing for experiment; C) deposition of fungi mycelium on pieces of leather coated with AgNPs.
The spread of both genres of Pseudomonas was performed in a potato dextrose agar (PDA) medium at 37 °C for 24 hours. The antibacterial activity of leather coated with Argovit AgNPs was evaluated by a zone of inhibition (ZoI) test. Small pieces of leather coated with AgNPs (dried and not dried after coating) were placed on agar plates spread with each bacteria culture in potato dextrose agar (PDA). Next, each Petri plates (containing bacteria inoculum and AgNPs-coated leather) according to method (Hudzicki, 2009) were dried, and the ZoI was measured to assess the bacterial sensitivity to leather coated with AgNPs.
3. Results and discussion
Foot infections are a public health problem because they are difficult to eradicate and they lead to other complications like diabetic foot ulcers. The conventional treatments for foot infections include aggressive surgical debridement and antibiotic therapy (Bader & Brooks, 2012). However, some bacterial species have developed multidrug resistance to several antibiotics, making this a relevant public health problem. For this reason, it is necessary to develop a non-surgical approach to prevent foot infections.
Protective footwear and socks made of fabricated leather and textiles with antibacterial properties are available on the market; however, after the use of these commercial products, the satisfaction by the users is low because of the poor protection against foot infections caused by bacterial resistance to antibiotics. Additionally, some of these commercially available products have chemical compounds that could be toxic to humans and may lead to bacterial resistance as well.
Frequently, leather used for footwear manufacturing is derived from animal skins. This favors the spread of dermatophyte microorganisms, i.e., bacteria and fungi. Consequently, in healthy individuals the spread of microorganisms leads to athlete’s foot with high sweating, odor, itching and sometimes infection. In diabetic people, the presence of these microorganisms can cause serious infections and finally limbs amputation. Thus, in this study, we investigated the bacterial and fungal growth inhibition of microorganism responsible for foot infections by leather coated with AgNPs used for footwear fabrication, besides analyzing the effect of AgNPs on the texture of leather.
3.1. Preparation of leather coated with AgNPs
In order to develop leather footwear material coated with AgNPs, we tried a simple method based on sprinkling AgNPs directly on the material and let it dried. Thus, the ICP-AES results showed that the metallic silver content in the leather was not proportional to the AgNPs concentration used (Table 1).
Table 1 Characterization of AgNPs-coated leather. The data shows the content of metallic silver in the leather after the treatment with increasing concentrations of AgNPs. Data were obtained by ICP-AES.
| Concentration of AgNPs (%) | Concentration of AgNPs (mg/mL) | Metallic silver concentration (mg/cm2) |
| 0 | 0 | 0.006 |
| 0.0025 | 0.0015 | 1.260 |
| 0.005 | 0.003 | 2.810 |
| 0.05 | 0.03 | 2.840 |
| 0.25 | 0.15 | 2.300 |
3.2. Effect of the AgNPs on the texture of leather
Textural changes on the leather were evaluated, after the application of AgNPs, through a texturometer. The data represent the penetration distance in the leather. Table 2, shows a decrease of leather softness when coated with AgNPs, and it was inversely proportional to the concentration of AgNPs.
Table 2 Texture characterization of leather coated with AgNPs. The data show an arbitrary value associated with the softness of the material.
| Concentration of AgNPs (%) | Softness |
| 0 | 4.05 |
| 0.0025 | 4.00 |
| 0.005 | 3.90 |
| 0.05 | 3.60 |
| 0.25 | 3.20 |
After the analysis of the content of metallic AgNPs, it was evident that the sprinkling strategy used does not allow a homogenous impregnation of AgNPs in the leather. This method must be optimized to use a sharp sprinkler system. The textural properties were also affected by the application of AgNPs. However, it is possible that, after homogenous impregnation of AgNPs in the leather, its use for manufacturing footwear with suitable softness, not so different from the raw material, is possible.
3.3. Antibacterial and antifungal activity of AgNPs impregnated in leather
Leather sprinkled with AgNPs was used in the ZoI assays to investigate their antimicrobial properties against major bacterium and fungi present in footwear. The results from the ZoI assays showed that the antibacterial effect of AgNPs-coated leather against P. mendocina and P. syringae was evident when the content of silver metallic nanoparticles was 0.05%, higher concentrations of AgNPs did not increase the ZoI (Table 3).
Table 3 Antibacterial effect of leather that was left to dry after the application of AgNPs. Horizontal and vertical diameters of the ZoI in Petri dish after 24 hours of exposure to leather coated with AgNPs. The 0-0 data represents the entire bacterial growth in the Petri dish.
| Concentration of AgNPs (%) | P. mendocina ZoI (cm) | P. syringae ZoI (cm) |
| 0 | 0-0 | 0-0 |
| 0.0025 | 0-0 | 0-0 |
| 0.005 | 0-0 | 0-0 |
| 0.05 | 0.2 | 0.1 |
| 0.025 | 0.2 | 0.1 |
The diameter of the ZoI was also measured for antibacterial activities against P. mendocina and P. syringae bacterium (Table 4). Horizontal and vertical diameters of the ZoI revealed that the inhibition of bacterial growth was evident at 0.05% of metallic silver content; also, a larger inhibition zone was detected when bacteria were exposed to leather coated with 0.25% of metallic AgNPs.
Table 4 Antibacterial effect of leather to which AgNPs were freshly applied (without drying). Horizontal and vertical diameters of the ZoI in Petri dish after 24 hours of exposure to leather coated with AgNPs. The 0-0 data represents total bacterial growth in the entire Petri dish.
| Concentration of AgNPs (%) | P. mendocina ZoI (cm) | P. syringae ZoI (cm) |
| 0 | 0 - 0 | 0 - 0 |
| 0. 0025 | 0 - 0 | 0 - 0 |
| 0. 005 | 0.9 - 1.1 | 1.1 - 1.1 |
| 0. 05 | 1.9 - 2.0 | 1.4 - 1.4 |
| 0. 25 | 2.0 - 1.9 | 1.7 - 2.6 |
The antibacterial effect of AgNPs-coated leather could be affected by the difussion of AgNPs in a solid surface like the fibers of the leather, which could reduce their availability, and therefore, their activity as a microbicide. However, this was not a limitiation since both results of ZoI tests against P. mendocina and P. syringae revealed a clear inhibition of bacterial growth. The diameter of ZoI reached up to 2.6 cm after 24 hours of bacterial exposure to AgNPs-coated leather. When both bacterial genres were compared, P. syringae resulted to be more sensitive to the antimicrobial effect of AgNPs than P. mendocina, as demonstrated by the diameter size obtained from the ZoI test (Table 4).
In the case of the antifungal activity of the AgNPs-coated leather against T. mentagrophytes, it was observed that a silver metallic content of 0.5% decreased fungal growth; nevertheless, this amount of metallic silver was did not eliminate fungal growth, as it is shown by mycelium weight (Table 5).
Table 5 Analysis of the proliferation of fungi T. mentagrophytes on the leather coated with increasing concentrations of metallic AgNPs (AgNPs). The + symbol represents growth intensity.
| Concentration of AgNPs (%) | T. mentagrophytes growth intensity | Features | Mycelium weight | |
| Humid weight (g) | Dry weight (mg) | |||
| 0 | +++++ | Fast growth | 5.1 | 253 |
| 0. 0025 | +++ | Similar to the first day of the culture | 7.0 | 264 |
| 0. 005 | +++ | Aerial growth | 18.2 | 441 |
| 0. 05 | ++ | Weak growth | 7.2 | 253 |
| 0. 25 | + | Hyaline mycelium | 7.7 | 289 |
The antifungal property of AgNPs against T. mentagrophytes was not demonstrated using the metallic AgNPs content herein tested. This can be explained by the diffusion limitation of silver ions through the fungal cell wall. However, after mycelium weighting of control and treated groups, it was evident that the higher concentrations of metallic AgNPs (0.05 and 0.25%) produced in an amount similar to the fungal dry weight. This may suggest that the activity of AgNPs-coated leather is fungistatic rather than fungicidal. The action mechanisms of silver ions with fungi have been reported by the interaction of Ag+ with the NH+ groups or with the positive-charged groups present in lysine residues in the mycelium. This may explain the electrostatic forces interaction favoring the fungistatic activity of Ag+ (Thakkar et al., 2010). It has been reported that besides AgNPs, other metals like Au, Cd, and magnetite had an intracellular interaction with bacterial proteins producing bacterial growth inhibition. Nevertheless, in the case of fungi, this interaction is extracellular, limiting the ability of AgNPs to interact with vital intracellular mechanisms (Thakkar et al., 2010).
Due to the complexity of fungal and bacterial infections in extreme cases of dermatoses, it is necessary to combine dermal, oral, and even laser treatment (Gupta & Paquet, 2014). This proposal becomes a treatment complement to inhibit the growth and spread of dermatophytes, especially on the sole and forefoot. This work presents a technology that can be used for covering the lining of the footwear and act as added value to prevent and control bacterial infections in high-risk users.
To extend the study of the possibility of using AgNPs in the shoe industry, some further experiments are necessary: repetitive application of fungi on the same pieces of leather; influence of temperature, humidity and time of leather contact with fungi and comparison with spore germination under the same conditions, among others.
4. Conclusions
In this study we demonstrated that leather coated with a content of 0.5% of metallic AgNPs exhibited a promising antibacterial activity against P. mendocina and P. syringae. Nevertheless, higher amounts of metallic silver than 0.25% are needed to abolish the growth of T. mentagrophytes. The textural properties of the leather coated with AgNPs was negatively affected causing a decrease of its softness. Due to the promising antibacterial properties, leather coated with AgNPs is a suitable strategy to develop antibacterial material for footwear manufacturing. This strategy will control the growth of bacteria known to cause skin infections.
Prospective studies are needed to demonstrate the beneficial impact of nanotechnology in the footwear industry and the beneficial applications in footwear for diabetic patients.











 text new page (beta)
text new page (beta)


