1. Introduction
The possibility of modifying the porous structure and increasing the diameter of the pores of the SBA-15 with the use of expanding agents that dissolve in the infernal (hydrophobic) zone of the micelles represents great advantages in the design of new catalytic materials (Can, Akga, Yilmaz, & Uner, 2006). There is the possibility of modifying its porous structure and increasing the diameter of the pores of the solid with the use of expanding agents that dissolve in the infernal (hydrophobic) zone of the micelles, which represents great advantages in the design of new catalytic materials (Can et al., 2006). To increase the pore size there are publications that point to various methods such as the addition of salts, the use of co-surfactants, controlling the hydrothermal treatment, mo difying the synthesis temperature, etc. However, the textural properties are affected. There is a wide variety of organic auxiliarles, among these, are paraffin, aromatic com-pounds, and alcohols (Raman, Anderson,& Brinker, 1996).
An organic agent that has been studied to increase the pore size and obtain a porous structure with structural uniformity is trimethylbenzene (TMB). It is only in 1998 that we have the first publication where they use an organic expanding agent 1,3,5-trimethylbenzene (TMB) which is a non-polar reagent that acts on the hydrophobic core of the micelles and expands them, thus increasing the pore size of the final product between 5 and 30 nm (Arellano, Zurita, Sazo, de Navarro, & López, 2008; Stucky et al., 2007; Wang, Noguchi, Takahashi, & Ohtsuka, 2001; Zhao et al., 1998). It was found that this agent provides a linear increase in the pore dimensions as its concentration increases. However, it was also shown that the final arrangement of the porous structure may be less orderly than when this type of expander is not used (Comerford, Farmer, Macquarrie, & Breeden, 2012: Esparza, 2007).
Achieving this type of materials with larger pore sizes allows them to be used as catalyst supports. Mesoporous silicas have recently been studied in heterogeneous reactions as supports for metallic oxides such as cobalt. highlighting the effects of parameters such as catalyst loading, peroxymonosulfate concentration and the reaction temperature in the degradation of phenol (Saputra et al., 2012; Shukla, Sun, Wang, Ang, & Tadé, 2011). In recent years it has been shown that industrial processes genérate organic waste that is toxic to aquatic life, pollute rivers and groundwater. Phenol, when reacted with the chlorine used in most countries for the treatment of drinking water, forms phenyl-polychlorinated compounds that are more toxic and more resistant to biodegradation than phenol itself. The Phenol and phenolic compounds are harmful from the human health; they can cause tissue detachment, necrosis, digestive delay, kidneys and liver damage. Furthermore, if the discharge is at very low concentrations, they are highly dangerous to aquatic life and transfer a particularly unpleasant smell and taste. Therefore, the complete elimination of phenol in wastewater is necessary before it is discharged into the natural water streams. The process of catalytic oxidation via humid has been shown to be efficient in the destruction of this pollutant by reducing the conditions of pressure and reaction temperature in the presence of the catalyst (Chindris et al., 2010; Massa. Ivorra, Haure, Cabello, & Fenoglio, 2007).
The effective elimination of this pollutant becomes a task to be carried out due to the increasingly stringent environmental laws and regulations. The phenolic compounds occupy a prominent place in the list of pollutants of the environmental protection agency (EPA) of the United States of North America, due to the high toxicity, the high chemical demand of oxygen and the low biodegradability of these organic compounds. For this reason, oxidation processes have emerged as interesting alternatives for the destruction of organic pollutants in industrial wastewater (Dohnal & Fenclova, 1995: Esplugas, Giménez, Contreras, Pascual, & Rodríguez. 2002; Gogate & Pandit, 2004). In this work, we investígate the performance of cobalt supported in the mesoporous material SBA-15 and the effect of TMB as an organic expanding agent, in order to expand the pore size and obtain catalytic supports with improved surface and structural properties for the phenol degradation.
2. Materials and methods
2.1 Catalysts preparation
The synthesis of SBA-15 was performed varying the previously reported in the literature (Flodstrón, & Alfredsson, 2003; Zhao et al., 1998). A typical synthesis procedure of SBA-15 has 2.5% weight of Pluronic 123 (1.92 g), 45 g of water, 30 g of 4M HC1 and 4 g of Tetraethyl-orthosilicate (TEOS) as a source of silicon (Zhao et al., 1998).
To synthesize 5 grams of SBA-15, 9.6 g of Pluronic 123 were dissolved in 225 mL of distilled water at room temperature and 150 mL of 0.5 M HNO3 were added. The solution was then heated to 35 °C and stirred at 600 rpm for 4 hours. Then 20 grams of TEOS were added as a silicon source, stirring at 900 rpm for one minute, after that, it was left in modérate agitation (600 rpm) for 24 hours. Then, the solution was placed in a stove at 80 °C for 72 hours for hydrotreating. Finally, the solid obtained was washed with abundant water until neutral pH, dried at 80 °C for one hour and then calcined at 500 °C for 6 hours (with a ramp of 2 °C/min). The support achieved was impregnated with the precursor of Co (NO3)2 • 6H20 (20% w) through a technique of impregnation to volume, after this, it was left to dry at room temperature and calcined at 350 °C for 6 hours with a heating ramp at 2 °C/ minute, the catalyst was designated Co/SBA-15.
In order to increase the pore size, the information reported in the literature was slightly changed (Stucky et al., 2007) and consisted of the following: 4.0 g of Pluronic was dissolved in 30 mL of distilled water and 120 g. HNO3 0.5 M was added (Stucky et al., 2007 used 2M HC1) in a polypropylene canister.
It was stirred at room temperature until the Pluronic was dissolved. Subsequently, the solution was heated to 35 ° C and stirred at 600 rpm. After 2 hours 3.0 g of TMB was added and 2 hours later 8.5 g of TEOS. It was stirred vigorously for one minute at 900 rpm, then left in modérate agitation (600 rpm) for 24 hours. It was placed in a hermetically sealed jar in an oven at 80 °C for 72 h (Stucky et al., 2007). The product was washed with abundant distilled water until obtaining a neutral pH, filtered, dried at room temperature and calcined in air at 500 °C for 6 hours with a heating ramp of 2 °C/min.
To obtain the Co20/SBA-15 and Co20/SBA-15Tmb catalysts, the impregnation was carried out with 20 % by weight of precursor salt (cobalt nitrate) by the impregnation method at pore volume, it was left at room temperature and it was calcined at a temperature of 350 ° C for 6 hours with a heating ramp of 2 °C/min.
2.2 Characterization techniques
The cobalt content was obtained by atomic absorption in a Spectrometer Analyst model 700 AA. The measurements of nitrogen adsorption were carried out by means of a static volumetric procedure in a Micromeritics Tristar II 3020. The measurements were made at the normal boiling temperature of N2 (77 K at atmospheric pressure).
The calculation of the specific surface (Sbet) was made based on the BET method (Brunauer-Emmett-Teller). The total pore volume was determined from the amount adsorbed at a relative pressure of approximately 0.99. The pore size distribution was determined using the Barrett-Joyner-Halenda method (BJH). The degree of ordering of the mesoporous structure was estimated by X-ray diffraction (XRD) in a Model D8 Advance equipment of the Bruker brand using the monochromatic CuKα radiation. The infrared analysis was performed on a Perkin Elmer Precise equipment (Spectrum One FT-IR Spectrometer). The catalysts were studied by scanning electrón microscopy with an Analytical Scanning Electron microscope model JSM-6010LA and by transmission electrón microscopy with Tecnai G2F30 microscope, field emission cannon (Schottky-FEG), with an acceleration voltage of 300 kV. The local chemical analysis by energy dispersive X-ray spectroscopy (EDXS) was performed in an X-ray spectroscope by energy dispersión that is attached to the microscope using STEM-EDX. The materials were identified as: SBA-15, SBA-15tmd; Co20/SBA-15 and Co20/SBA-15Tmd, where: 20 = % Co and TMB = 1,3,5, Trimethylbenzene.
2.3 Phenol degradation
For the oxidation reaction of the phenol, a high-pressure reactor (Parr Instruments Co Ltd, IL), 300 mL of phenol of a 500 ppm solution and 300 mg of catalyst were used. The system was heated to the temperature reaction and pressurized with nitrogen and with continuous stirring of 750 rpm, time zero was defined when the pre-set conditions were reached (temperature, 160 °C and oxygen pressure at 10 bar). The reaction was performed for 180 min. The samples in the effluent were taken at intervals of 10 min through lh, and the phenol content (C), intermedíate content and Total Organic Carbón (TOC) were analyzed. Total Organic Carbón (TOC) of the samples was measured with a TOC 5000 Shimadzu Analyzer. The conversión of phenol for the different materials and the TOC was calculated using:
Where TOC0 is Total organic carbón at t=0 (ppm), Co is the phenol concentration at t = 0 (ppm), C180 is the phenol concentration at t=3 h of reaction (ppm), TOC180 is Total organic carbón at t=3 h of reaction (ppm). So the selectivity was calculated according to following equation (Luck, 1999):
The initial rate (ri) was calculated from the phenol conversión as a function of time, using the following equation:
Where,
The chromatographic system to be used with this experiment consists of a Perkin Elmer Model Clarus 580 Gas Chromatograph which has a ñame ionization detector (FID). The injector temperature maintained at 200 °C and the detector temperature maintained at 275 °C. The injection volume was 1-μL. The column was a VF-lms: with dimensions: 30 m x 0.25 mm x 0.25 pm. The oven temperature maintained at 180 °C. The carrier gas used was helium with 99.999% of purity.
3. Results and discussion
3.1 Physical-chemical characterization
Figure 1 shows the results of high angle XRDs of the synthesized materials (Co20/SBA-15, Co20/SBA-15Tmb) and, it was verified by XRDs at a low angle (Figure not shown) the obtaining of the hexagonal phase in two dimensions (6pmm) characteristic of the SBA-15 support.
Catalysts impregnated with 20 % by weight of cobalt (Co20/SBA-15) and, calcined at 350 °C show 4 peaks at 26: 37°, 4-00-043-1003), which correspond to the CO3O4 phase, this coincides with that reported by other authors (Akça, 2006; .Jia et al., 2011; Ohtsuka, Arai, Takasaki, & Tsubouchi. 2003; Martínez & López, 2006; Shukla et al, 2011; Wang et al., 2001). In the Co20/SBA-15Tmb catalyst, the typical shoulder of the SBA-15 is not observed at 23°, which we can infer as a disorder in the ordered mesoporous structure due to the addition of TMB that causes phase change (mesocellular foam) as reported by other authors (Johansson, Córdoba, & Odén, 2009; Lettow et al., 2000: Schmidt-Winke et al., 1999).
The textural properties are summarized in Table 1. the specific surface área (Sbet) was determined by the Brunauer-Emmett-Teller method (BET) (Brunauer. Emmett, & Teller, 1938) and the BHJ method was used to determine the volume and distribution of pore size (Barret et al., 1951). SBA-15 was obtained under strongly acidic conditions (pH = 0.6) using a triblock copolymer (Pluronic 123). These conditions have been previously reported (Bagshaw, Prouzet, & Pinnavaia, 1995: Flodstrón, & Alfredsson, 2003; Fukuoka et al., 2003; Lee, & Cheon, 2001; Zhao et al., 1998; Ryoo, Joo, & Jun, 1999: Yamada, Zhou, Asai, & Honma, 2002).
Table 1 Textural properties (BET Surface SBET pore volumen Vp, and pore diameter Dp) of SBA-15, Co20/SBA-15, SBA-15TMB and Co20/ SBA-15TMB
| Sample | Sbet (m2/g) | Vp (cm3/g) | Dp* (nm)ads |
|---|---|---|---|
| SBA-15 | 835 | 0.70 | 6.7 |
| C020/SBA-15 | 356 | 0.50 | 7.6 |
| SBA-15t.vib | 737 | 0.97 | 19.0 |
| Co2o/SBA-15tmb | 396 | 0.65 | 18.5 |
*Maximun value in the pore size distribution
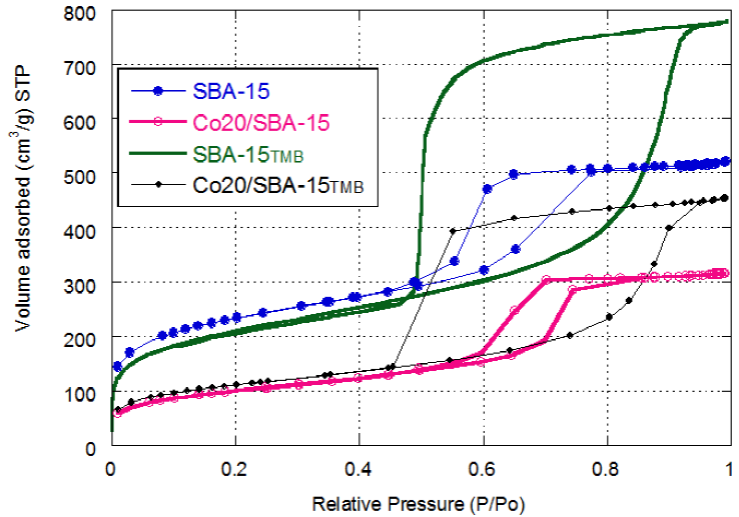
Figure 2a shows the nitrogen adsorption-desorption isotherms (N2) of SBA-15, SBA-15Tmb, Co20/SBA-15 and Co20/SBA-15tmb and, in Figure 2b we can see the diameter distribution of pore of the supports (SBA-15 and SBA-15tmb)- It is observed that the isotherms are of type IV with a hysteresis cycle type Hl (according to the IUPAC classification) typical of these materials, which reílects that they are compact packings of cylindrical pores in an orderly manner (Huo, Margolese, & Stucky, 1996: Kruk, Jaroniec, & Sayari, 1997; Sing, 1985; Thieme & Schüth, 1999). However, in the solid with TMB (SBA-15tmb) the hysteresis loop is different: it is of type H1. which indicates that there are non-cylindrical pores known as pores in the form of an inkwell of McBain. This pore is characterized by the radius of the wider (spherical) cavity and the radius of its narrowest cavity (cylindrical capillary) (Everet & Sidney, 1958; Gregg, Sing, & Salzberg, 1967), in which the body is larger than the mouth, henee we have different pore diameter in the adsorption and desorption (Figure 3) with a wider pore diameter distribution (Leofanti, Padovan, Tozzola, & Venturelli, 1998).
When the TMB was added, the pore diameter and volume were increased, the valúes were 19 nm and 0.97 cm3/g respectively. The SBA-15tmb with a larger pore diameter had a higher pore volume, while the SBA-15 with the diameter of 6.9 nm provides a higher specific área of 835 m2/g, this coincides with that reported in other studies that they use expanding agents to expand the pore size (Lettow et al, 2000; Wang et al., 2001), with TMB being the most common compound for increasing the pore size of SBA-15, (Calin, Galarneau, Cacciaguerra, Denoyel, & Fajula, 2010; Doadrio et al., 2004; Jaladi, Katiyar, Thiel. Guliants, & Pinto 2009; Shih-Yuan et al., 2011; Wang et al., 2001).Likewise, it is reported that with the addition of TMB a phase transition from hexagonal to a mesocellular foam oceurs when the TMB/P123 ratio exceeds 0.3 (Schmidt-Winkel et al., 1999), the máximum theoretical valué of the size of the pores before the phase transition is 12 nm, which has also been demonstrated experimentally (Lettow et al., 2000). This valué has been exceeded in this study as reported by other authors who have used other swelling agents such as hexane in the presence of NH4F (Sun et al., 2005), where NH4F increases the hydrophilic volume of P123, also the use of heptane seems to efficiently expand the volume of the hydrophobic micelle, which results in larger pores (Johansson et al., 2009).
The addition of 20 % cobalt in the SBA-15 produces the reduction in the textural properties (Figure 2a). The volume of N2 adsorbed at relative pressures P/Po > 0.4 suggests partial plugging of the porosity by large particles of cobalt (CO3O4). The characteristic of the isotherms of mesoporous systems is maintained as well as the distribution of the pores that show a unimodal distribution. The narrow porch size distribution is shown in SBA-15 and Co20/SBA-15 confirms the uniformity of the mesoporous system. These results coincide with what was previously reported in the literature (Chytil. Haugland, & Blekkan 2008; Zhao et al., 1998; Flodstrón, & Alfredsson, 2003; Impéror-Clerc, Davidson, & Davidson, 2000; Martínez, López, Márquez, & Díaz 2003; Newalkar & Komarneni, 2001; Ryoo, Ko, Kruk, Antochshuk, & Jaroniec, 2000; Zhang et al., 2006).
With the intention of obtaining additional information on the nature of the siliceous skeleton in the SBA-15, the infrared spectroscopy technique was used in a range of 4000-400 cm-1. Figure 4 shows a peak at 3450 cm-1, associated with adsorbed water. The absorption band that appears at 1635 cm4 corresponds to δ(HOH) physisorbed water (Busca 1996; Gomez-Cazalilla, Mérida-Robles, Gurbani, Rodríguez-Castellón, & Jiménez-López 2007; Li. Shi, Zhang, Xiong, & Yan, 2004; Posada, Giraldo, & Cardona, 2011; Xia & Mokaya, 2004). The 4 characteristic bands of the SBA-15 appear in the spectrum (.Tin et al.. 2004; Selvaraj et al, 2005; Martínez & Ruiz, 2002; Xiao 2005). Three of these bands correspond to vibrations of silicon-oxygen bonds and can be classified by the type of movement of the oxygen atom with respect to the silicon atoms in the beam, bending and stretching (Kirk, 1998: Shen et al, 2006).
In Figure 5, the absorption spectra of the FT-IR in the range of 4000-500 cm-1 of the supports and catalysts are shown: SBA-15, SBA-15TMD, CO20/SBA-15 and CO20/SBA-15TMD and, in Table 2 the frequencies λ (cm-1) of them are indicated. In Figure 5, a small shoulder around 568 cm4 is seen, this signal is attributed to Co-O bonds, suggesting the presence of a small amount of cobalt oxide crystallites as previously reported (Shukla et al., 2011; Tsoncheva, Ivanova, Rosenholm, & Linden 2009). It is observed that when supporting the cobalt in the SBA-15 there is a displacement of the δ(Si-O-Si) band to 471 cm. However, when we introduced the TMB the vibrations δ(Si-O-Si) originally present in the SBA-15 did not observe it, nor the one corresponding to the simmetrical vibrations at 803 cm-1, which indicates the presence of trimethyl in the skeleton of silica, and we observe that there is a displacement of the band 1080 cm4 to 1091 cm-1 and, when introducing the cobalt in this support the characteristic band to the flexión of the silanol groups (Si-OH) disappears, the above can be related to the transformation of Si-OH groups into Si-O-Si (CIL^, as reported by .lia et al. (2011) in the silylation of Co/SBA-15 catalysts for its application the synthesis by Fischer Tropsch.

Fig. 5 FTIR absorption spectra in the 4000-400 cm-1 región of puré SBA-15, SBA-15tmb, CO2O/SBA-15 aud CO2O/SBA-15tmb.
Table 2 Band assignmcnt in the FTIR spectra of SBA-15 and Co-SBA-15X catalysts.
| Sample | 6(Si-0-Si) | λ (cm1) u.(Si-O-Si) | 6OH(Si-OH) or u(Si-O-M) | uas(Si-0-Si) |
|---|---|---|---|---|
| SBA-15 | 479 | 803 | 967 | 1080 |
| CO20/SBA-15 | 471 | 803 | 967 | 1083 |
| SBA-15tmb | ___ | ___ | 969 | 1091 |
| Co20/SBA-15tmb | - | - | - | 1084 |
It was also observed that the effect of heat treatment led to the formation of Co-O-Si species during calcination and significantly inhibited Co leaching; these results coincide with Hu, Yang, and Dang (2011) where their results show that CO3O4 was the main cobalt species present both inside and outside the support; Therefore, a synergic effect between the textural properties and the active centers for the degradation of phenol is explained in our work.
The images obtained by electrón microscopy scanning (SEM) in the support and in the catalysts are shown in Figure 6 (a, b and c). The morphology observed was the typical forms of SBA-15 such as fibers, spheres, rods and short bars of ~ 0.5-2 pm in length that coincide with that reported by other authors (Chao et al., 2002; Bjórk, 2013: Sierra, Mesa, Ramírez, López, & Guth 2004; Katiyar, Yadav, Smirniotis, & Pinto, 2006; Zhao, Sun, Li, & Stucky 2000) the morphology does not change when introducing 20% by weight of cobalt. However, we observe mainly amorphous particles when the support is modified with TMB and impregnated with cobalt. The different forms are related to the conditions of synthesis as mentioned by Van Grieken, Hernández, and Calleja, (2003), they observed that the speed of agitation is one of the main variables that control the size and morphology of the particles of the mesoporous materials and, mention that the variation of this parameter allows preparing mesoporous silica spheres with a narrow particle size distribution in the range of 200-700 pm (Van Grieken, et al, 2003).

Fig. 6 Scanning electrón micrographs (SEM) images of a) SBA-15, b) CO20/SBA-I5 and c) CO20/SBA-15TMB.
In addition, according to Johansson (2009), the length and diameter of the particles can be influenced by variations in the concentration of hydrochloric acid (HC1). This is due to the effect of HC1 on the hydrolysis of TEOS. A higher concentration of this acid increases the hydrolysis which generates fewer micelles that can adhere to each other and the final particle shape is thinner. Simultaneously, the rapid rate of hydrolysis increases the rate of cylindrical micelle formation. Therefore, at the highest concentrations of HC1, the micelles are more elongated, narrower and more homogeneous in length compared to those synthesized with HC1 of lower concentration. In this study, we used a lower concentration of acid (0.5 M) compared to the typical synthesis using 2M HC1 (Zhao et al., 1998) and a constant agitation of 600 rpm, which seems to be related to the final morphology of the supports and obtained catalysts.
Figure 7 (a, b, c, d, e, f) shows transmission electrón microscopy (TEM) images of SBA-15 (a and b), Co20/SBA-15 (c and d), and Co20/SBA15Tmb (e and f). In the first image (a) a typical morphology of the SBA-15 is observed as "honeycomb", where the mouth of the pores can be seen and, in Figure 7 (b) the hexagonal arrangement of pores is distinguished, whose longitudinal direction is perpendicular to the plañe of the image. We can appreciate the walls of the pores, which form channels distributed in parallel with each other. With these TEM images characteristic of the ordered mesoporous material, the hexagonal structure (p6rnrn) in two dimensions previously reported in the literature is confirmed (Impéror-Clerc et al., 2000; Saika, Srinivas, & Ratnasamy 2006; Shukla et al., 2011) and is according to the results of low angle XRDs. In the micrograph corresponding to the Co20/SBA-15 (e and d) catalyst, the presence of CO3O4 aggregates on the external surface observed in the form of small particles confined to the mesopores is observed, which coincides with that reported by other authors (Hu et al., 2011; Lu et al., 2004; Martínez et al., 2003; Martínez & López, 2006).

Fig 7 Transmission electron microggraph (TEM) images of sample SBA-15 (a and b), Co20/SBA-15 (c and d), Co20/SBA-15TMB (e and f)
In the analysis of the catalysts by TEM (Figure 7c), it reveáis that the mesoporous silica presents an ordered structure with hexagonal symmetry that is not possible to appreciate when modifying the support with TMB (Figure 7e). In Figures 7e and 7f corresponding to Co20/SBA-15tmb, the Co particles disseminated heterogeneously on the silica matrix can be distinguished. The introduction of an expanding agent such as TMB in the case of mesoporous materials such as MCM-41 that use an ionic surfactant does not alter the cylindrical shape of the pores. however, when the tribloque PEO-PPO-PEO is used as a témplate (P123) in the synthesis of SBA-15, the hexagonal structure of cylindrical pores is maintained only for low concentrations of TMB, as the ratio of TMB/P123 increases, a phase change (mesocellular) and the morphology of the particles are obtained. is affected (Letow et al., 2000; Schmidt-Winkel et al., 1999), this can be seen in the micrographs corresponding to Figures 7e and 7f ; the average size of the cobalt particles (Figure 8) was estimated at ~ 18.8 nm for SBA-15 and of the order of _ 6.4 nm on average for Co20/SBA-15tmb.
Figure 9a and 9b show the EDS spectra of the solids with 20 % by weight of cobalt in the support SBA-15 and SBA-15tmb respectively (Co20/SBA-15 and Co20/SBA-15tmb). It is observed that the relative amount of the metáis varíes in the two materials because of the possible diffusion effect when changing the structure of the puré silica (SBA-15) by the addition of the swelling agent 1,3,5 TMB. The strong signáis of Co can be clearly detected. which confirms the presence of this element, however, we observed that the theoretical load deposited in the supports by impregnation at pore volume was 20 % by weight and this by EDS we see that it is lower: 16.33 % in Co20/SBA-15 and 8.55 % in Co20/SBA-15Tmb. Proving thereby that the method of preparing the materials results in cobalt dispersed heterogeneously in the supports as can be seen in the micrographs corresponding to Figure 7 (c. d, e, and f).
3.2 Catalytic evaluation
The catalytic activity of the mesoporous material SBA-15 modified with 1,3,5 TMB and with 20 % by weight of cobalt (Co20/SBA15tmb), in the degradation of phenol was compared with puré silica (SBA-15). In Figure 10 we observe the degradation of phenol as a function of time as an indicator of the activity of the materials that was carried out at 180 minutes. It is observed that the phenol conversión of the support (SBA-15) differs significantly from the materials SBA-15tmb and Co20/SBA-15. The order of the conversión curves coincides with the degradation capacity; the most active material was Co20/SBA-15tmb- This seems to be related to the effect of the heat treatment that generated the formation of Co-O-Si species during the calcination and significantly inhibited the leaching of the Co, these results coincide with Hu et al. (2011) where their results show that the CO3O4 is the main cobalt species present both inside and outside the support; Therefore, a synergic effect between the textural properties and the active centers for the degradation of phenol is explained in our work.

Fig 10 Phenol conversions as a function of the time for SBA-15, SBA-15TMB and supported catalysts (T=160°C,PO2=10 bar)
Figure 11 shows the results of the total organic carbón (TOC) normalized as a function of time at 180 minutes of the reaction. It also shows the conversión percentages of total conversión of organic compounds to CO2 and H2O. In the reference material (SBA-15), we can observe that at 120 minutes only reached 20 % oxidation and the degradation with Co20/SBA15tmb shows the highest valúes in total organic carbón that is 86%, this indicates that some intermediary by products were formed during the oxidation reaction. Studies on the degradation of phenol with Co/SBA-15 report that the catalyst by itself does not have a strong adsorption of phenol, however with Co/SBA-15-oxone (Saputra et al., 2012; Shukla et al.. 2011), the concentration of phenol gradually decreased and the rate of degradation appeared to be constant and the elimination of 100 % phenol was achieved in 180 min. This high rate of degradation was due to the activation of peroxymonosulfate (PMS) to produce sulfate radicáis for the oxidation of phenol in heterogeneous solutions by CO3O4. We only obtained 86% in the degradation of phenol with Co20/SBA15tmb catalyst; PMS was not used in oxidation experiments. Shukla et al. (2011) show that cobalt precursora significantly influence the activity of Co/SBA-15 catalysts. The catalysts derived from cobalt chloride and acétate have shown similar activities and would achieve 100 % elimination of phenol in 200 min. However, the reaction rate from cobalt nitrate (as in this work) was much slower, reaching 100 % elimination in 390 min (Shukla et al., 2011).
In this study, the fact of reaching 86 % of phenol oxidation catalytic reaction may be related to the metallic particle size since the size decreased when the TMB was added; in the Co20/SBA-15 material, a particle size of 18.8 nm was estimated which is much larger than in the Co20/SBA15tmb which was 6.4 nm on average. The addition of TMB as a swelling molecule promotes better catalytic properties in materials synthesized with 20 % Cobalt, which can be attributed to the fact that this molecule distorts the mesoporous structure to the extent of disappearing the hexagonal array as observed by XRD. This disorder, in addition to allowing the formation of larger diameter pores (Figure 2b), modifies the structure of the surface favoring the distribution of cobalt particles to a size of ~ 6.4 nm, this being the most active, and which generates an adequate metal-support interaction, which manifests itself in the greater activity of the catalysts synthesized with TMB and with 20 % cobalt weight.
As mentioned, the total oxidation of phenol was not achieved in the catalysts at 120 minutes of reaction and the number of intermedíate by products (catechol, hydroquinone, benzoquinone, oxalic acid, maleic acid and formic acid), were identified by gas chromatography in the solution after the reaction. Only traces of hydroquinone (less than 2 ppm), catechol (less than 1 ppm), traces oí maleic acid and oxalic were found in these intermedíate products. Oxidation of hydroquinone decreases more slowly than in the case of catechol oxidation because di-hydroxybenzene groups are oxidized through different pathways.
The proposed mechanism by which the oxidation reaction of phenol is carried out is by free radicáis, which can be either a phenolic or a hydroxyl group. Oxidation of an aromatic group can begin with the activation of the oxygen molecule or the hydrocarbon molecule and oxygen can particípate in the reaction as an adsorbed species on the surface of the catalyst or from the structure of the metal oxide. Phenol degradation reaction can be described as follows: during the first steps of the reaction, phenol is degraded to aromatic compounds (such as catechol, hydroquinone, and benzoquinone), what was observed in the color change of the solution. The latter intermediates such as maleic acid, oxalic acid, and formic acid were formed after the opening of the aromatic ring, causing the solution to become colorless. These intermedíate products were mineralized to CO2 and H2O. The color of the solution at the end of the reaction was colorless; which indicates that the aromatic intermediates were no longer present in the solution, these results according to what was reported by other authors (Bhargava et al., 2006; Hu et al., 2011; Ohta, Goto, & Teshima, 1980).
In Table 3, the results of reaction rate (mmol Ir-1). TOC, the percentage of conversión of phenol and the selectivity to C02 of the support and catalysts are shown. We can observe that the material Co20/SBA-15tmb presents the highest selectivity (93 %) as we can see in Figure 12. The conversión levéis of phenol were higher when the Co was supported in the SBA-15 modified with TMB which is related to the smaller cobalt particle size and, the selectivity of the reaction towards complete mineralization was not observed in the synthesized materials.
Table 3 Activity and selectivity for the catalyst Wet-Air Oxidation of phenol after 160 min o reaction
| Catalysts | r1 a (mmolh-1) | Abatement TOC (%)a | Phenol Conversion (%)a | Selectivity to CO2 a |
|---|---|---|---|---|
| SBA-15 | 0.9095 | 26 | 28 | 19 |
| SBA-15tmb | 0.9980 | 36 | 46 | 35 |
| Co20/SBA-15 | 1.663 | 60 | 58 | 2 |
| Co20/SBA-15tmb | 2.520 | 86 | 85 | 93 |
4. Conclusions
The Co supported on the ordered mesoporous material SBA-15 and modified with 1,3,5 trimethylbenzene was found to be effective in phenol degradation. The modification of SBA-15 with the organic agent affected the textural, structural and catalytic properties of the synthesized materials, enlarged pore size and shape, changing the hysteresis loop from type I to type II indicating non-cylindrical pores and the average size of 19 nm in the SBA-15 modified with TMB.
The physical-chemical characterization of the support shows ordered structures of a mesoporous material two dimensions (6pmm) characteristic of the SBA-15 with the high specific área, narrow pore distribution and, when 20 % by weight of cobalt is supported, the textural properties and structural elements are maintained.
The morphology of the SBA-15 support is the typical one known as "honeycomb", and hexagonal structure of cylindrical pores and, when modifying the SBA-15 with TMB, there is a phase change (mesocellular) and the morphology of the particles. It is affected and the cobalt disperses heterogeneously in the support.
The addition of TMB expanded the pore size of the support, reducing the metallic particle size, this characteristic seems to give the material greater dispersión and be more selective in the conversión to CO2, so that there is a greater activity for the degradation of phenol.
In Catalytic Wet Oxide Oxidation of phenol, the material of SBA-15 showed a degradation of 26 %, the catalyst with Co/SBA-15 reached 60 %. The Co20/SBA-15tmb catalyst obtained an oxidation of 86 % at 120 min of reaction. The effect of TMB on these catalysts was the increase in activity and selectivity to CO2.











 nueva página del texto (beta)
nueva página del texto (beta)