1. INTRODUCTION
The global economy is increasingly dependent on go green concepts in industrial and technological sectors. Of late, the world community is highly apprehensive of the depletion of fossil fuels and is concerned about the carbón footprints. Our developmental life style is left behind and is hard pressed for going for alternative sustainable materials. In every nook and cranny of the world, Research and Development is in full swing to chance upon bio-based substances and environmental friendly sustainable materials for industry producers and consumers (Kengkhekit & Amornsakchai, 2014). In this context, a variety of natural fibers such as sisal, flax, jute, coconut and banana are being effectively utilized as reinforcement phase in composite materials since it has a host of advantages over its synthetic counterparts such as low-priced, easily separated, and are abundantly available (Khoshravan & Paykani, 2012; Nikmatin, Syafiuddin. Kueh & Maddu, 2017).
Lagenaria siceraria (Bottle Guard) originates from southern Africa and commonly cultivated in tropical and subtropical áreas of the world. It is a biennial/annual crop which can be harvested young and used as a vegetable. After harvesting the vegetables of the plant, the stem of the plants is remained as agro-waste. Extensive research is being carried out at the LS plant to explore the various pharmacological activities such as anti-diabetic, cardio-protective, hepato-protective so on, and so forth besides been used in the green synthesis of nano particles for various industrial applications (Laouchedi, Bezzazi & Aribi, 2017; Prithiviraj, Muralikannan, Senthama-raikannan & Saravanakumar, 2016).
The present study is aimed at a better understanding of physical-chemical, thermal, and mechanical properties of Lagenaria siceraria fibers (LSFs) extracted from their agro-waste stem by the comprehensive characterization for the first time.
2. MATERIALS AND METHODS
2.1 LAGENARIA SICERARIA FIBER AND ITS EXTRACTION
The Lagenaria siceraria (LS) plants were collected from the fields of the plantation after harvesting the vegetable in Therkkuppallam village, Erode District, Tamil Nadu, India. The agro waste stems of LS plant were separated by removing their leaves and tendrils using knife and washing it with water to remove the impurities and dirt. The fibers can be extracted from the skin of the collected stems of water-retting process (Saravanakumar, Kumaravel, Nagarajan & Moorthy, 2014). The long and continuous stems were cut into pieces at the joints having an approximate length of 10-20 cm each and kept immersed in water for fourteen days to facilítate microbial degradation. Then, the fibers were separated from the stem piths and washed thoroughly with distilled water to remove their impurities. The obtained fibers were sun-dried for 72 h to remove the moisture. The LSFs plant and extracted fiber from LSFs is shown in Figure 1 and 2.
2.2 CHARACTERIZATION OF THE LSFs
2.2.1 Determination of chemical composition of LSFs
By opting standard analytical procedures, the chemical compositions of LSFs such as cellulose, hemi-cellulose, lignin, and ash content were determined (Suryanto, Marsyahyo, Irawan & Soenoko, 2014). The Mettler Toledo XS 205 balance method was adopted to estimate the density of the LSFs (Vignesh, Balaji & Karthikeyan, 2016). Conrad procedure was followed in the determination of the wax content of LSFs. According to the ASTM E1755-01standard, the ash content of LSFs was determined (Justiz-Smith, Virgo & Buchanan, 2008) and the moisture content was determined by standard method described elsewhere (Boopathi, Sampath & Mylsamy. 2012).
2.2.2 Fourier Transform-Infrared Spectroscopy
The existences of various major functional groups present in the LSFs were ascertained by using an FTIR spectrometer (Perkin Elmer RXI). The LSFs powder was made into a pellet by mixing with Potassium bromide (KBr) followed by scanning in the wavelength range of 500 to 4000 cm-1 with 30 scans per minute. The resultant peaks were recorded.
2.2.3 X-ray Diffraction assay
The X-Ray Diffraction (XRD) assay was performed to assess the Crystalline Index (CI) and crystal size of the extracted LSFs. The assay was carried out by using XPERT-3 diffractometer fitted with CuKa radiation (wavelength 1.5406 Ǻ nm) source at a current of 30 mA, with an application of 45kV tensión. The sample was examined at a temperature of 25° C in the 20 ranges between 10° and 80°. The valué of the peak intensities of the LSFs was used in the calculation of crystalline index, CI (%) with the help of Segal"s equation (1) (Natarajan, Kumaravel & Palanivelu, 2016)
Where I002 is the máximum intensity of crystalline peak and IAMthe intensity of amorphous peak. By applying Scherrer"s formula as shown below (Equation 2), the average crystal size (CS) of the LSFs was determined (French & Cintrón, 2013).
Where D is the crystal size, K the Scherrer"s constant (K = 0.94), λ the wavelength of the Kα radiation (1.5406 Ǻ), β the full peak width at half of the máximum, and θ the Bragg"s diffraction angle.
2.2.4 Thermo gravimetric Analysis (TGA)
A thermo gravimetric analyzer (Model STA 449 F3, NETZSCH, Germany) was used to examine the thermal stability of the LSFs. The lOmg of the powdered LSFs sample was taken in an alumina crucible and heated continuously at a rate of 10°C min1 from 30 to 550°C in a nitrogen atmosphere at a flow rate of 20 ml/min.
2.2.5 Single fiber tensile testing
INSTRON 5500 universal testing machine was used in the examination of tensile properties of the LSFs at a crosshead speed rate of 10 mm min-1. A gauge length of l00mm was maintained with a load cell capacity of 1 kN. As per the ASTMD3822/D3822 M-14 standards, 20 samples were analyzed for the study with 65% of relative humidity at 21°C (NagarajaGanesh &Muralikannan, 2006a).
2.2.6 Morphological analysis
The surface morphology of the LSFs was studied with the help of scanning electrón microscope (JEOL JSM-6390) by applying high energy beam of electrons with an accelerating voltage of 10 kV and an attainable vacuum level of 1.5 x l0-3 Pa. The images were recorded at different magnifications.
3. RESULTS AND DISCUSSIONS
3.1 CHEMICAL COMPOSITION OF THE LSFS
The major components of lignocellulosic fibers are cellulose, lignin, hemicellulose, pectin, wax, and ash. The percentage of each component varíes for different type of fiber. The amount of these components in a fiber depends on the geographical área, climate, age, source of the fiber and methods of extraction. The quantity of the fiber constituents directly influences the physicochemical properties of the fiber (Arthanarieswaran, Kumaravel & Saravanakumar, 2015). The chemical composition and density of LSFs were compared with a few natural fibers and are shown in Table l.
Table 1 Chemical compositions of the raw LSFs compared with the other common fibers (Saravanakumar et al., 2014).
| Fiber | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Wax (%) | Moisture (%) | Ash (%) | Density (g/cm3) |
| LSFs | 79.91 | 12.69 | 7.62 | 0.31 | 9.96 | 0.92 | 1.216 |
| Jute | 64.4 | 12 | 11.8 | 0.7 | 1.1 | - | 1.3 |
| Flax | 64.1 | 16.7 | 2 | 1.5-3.3 | 3.9 | - | 1.5 |
| Hemp | 68 | 15 | 10 | 0.8 | - | - | 1.47 |
| Kenaf | 31-72 | 20.3-21.5 | 8.0 - 19 | - | - | - | 1.45 |
| Ramie | 68.6-85 | 13-16.7 | 0.5-0.7 | 0.3 | 7.5 -17 | - | 1.5 |
| P.Foetida | 77.96 | 12.63 | 10.47 | 0.35 | 9.54 | 0.94 | 1.328 |
| A. leucophloea | 68.09 | 13.6 | 17.73 | 0.55 | 8.83 | - | 1.395 |
| I. staphylina | 72.76 | 13.6 | 19.56 | 1.51 | 8.28 | 1.4 | 1.4 |
The LSFs have significantly high cellulose content 79.91 wt %, to withstand the hydrostatic pressure gradient of the fiber. The LSFs have a considerable amount of lignin 7.62 wt %, which wind α - cellulose micro fibrils, into a bundle of cellulosic fiber and as well serves as a chemical adhesive between cellulose and hemicelluloses and ensure fiber rigidity and thermal stability. The high percentage of hemicellulose 12.69 wt % of content in LSFs strongly influences the moisture absorption, bio and thermal degradation of the fiber. The wax content 0.31 wt % LSFs may render poor interfacial binding between fibers and polymer matrices while preparing the composites (Gopinath, Ganesan, Saravanakumar & Poopathi, 2016). The LSFs consists of moisture 9.96 wt % and ash 0.92 wt %. Furthermore, the density of the LSFs was measured and found to be 1.216 g /cm3, which render it suitable for lightweight composite materials.
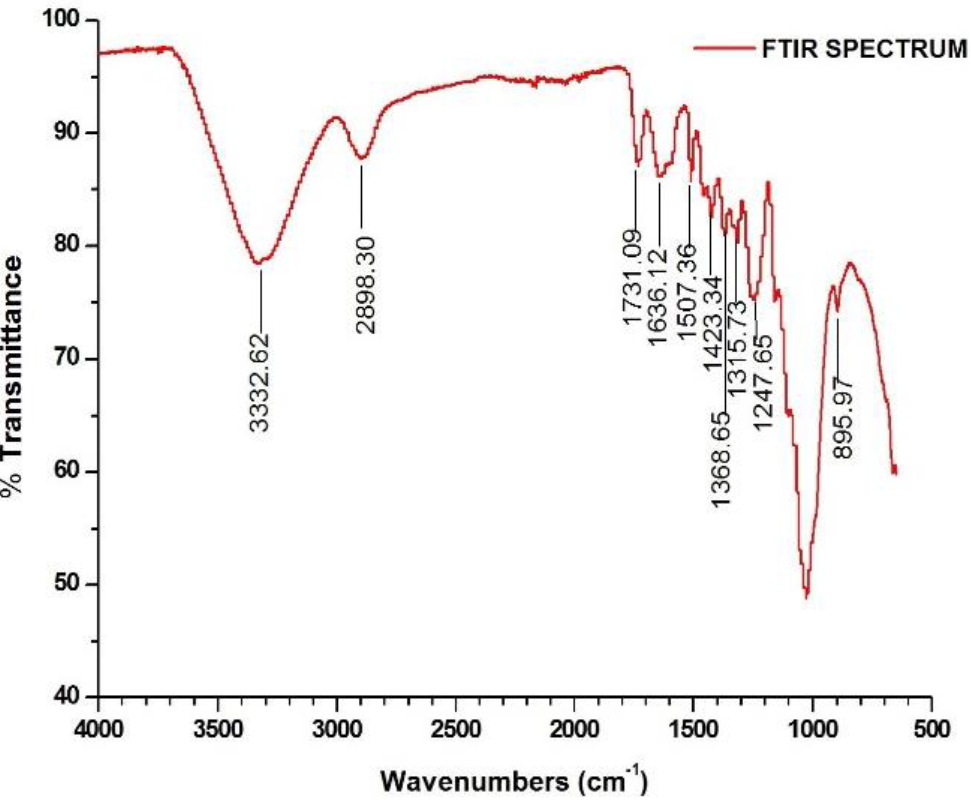
3.2 FT-IR ANALYSIS OF LSFS
The FT- IR spectrum of LSFs is shown in Figure 3. & Muralikannan, The absorption peaks at 3332, 2898, 1731, 1636, 1507, 1423, 1368, 1315, 1247, 1155, 1058 and 896cm-1 revealed the presence of three major components of LSFs such as cellulose, hemi-cellulose, and lignin. The absorption peaks at 3332, 2898, 1423, 1315, 1155, 1058 and 896 cm1 were due to free and hydrogen bonded O - H stretching, asymmetric stretching of the methylene group (> CH2), -CH2 scissoring, - OH bending, C - O antisymmetric bridge stretching, C - O - C stretching and C - O stretching vibrations of of α - cellulose component of LSFs (NagarajaGanesh & Muralikannan, 2016b). The absorption peaks observed at 1731and 1247cm_1 were due to C=0 stretching and C - O - C stretching vibrations of acetyl group of hemicellulose component of LSFs. The absorption peaks that appeared in 1636,1507,1423,1368, and 1058 cm4 were due to C=C stretching, C - O stretching, - CH3 asymmetric stretching, - CH symmetric stretching, and aromatic plañe deformation of lignin components of LSFs. The peak observed at 896 cm-1 corresponds to the presence of β (1-4) - glycosidic linkages between the monosaccharide in cellulose and hemi cellulose (Maepa et al., 2015).
3.3 XRD ANALYSIS OF LSFS
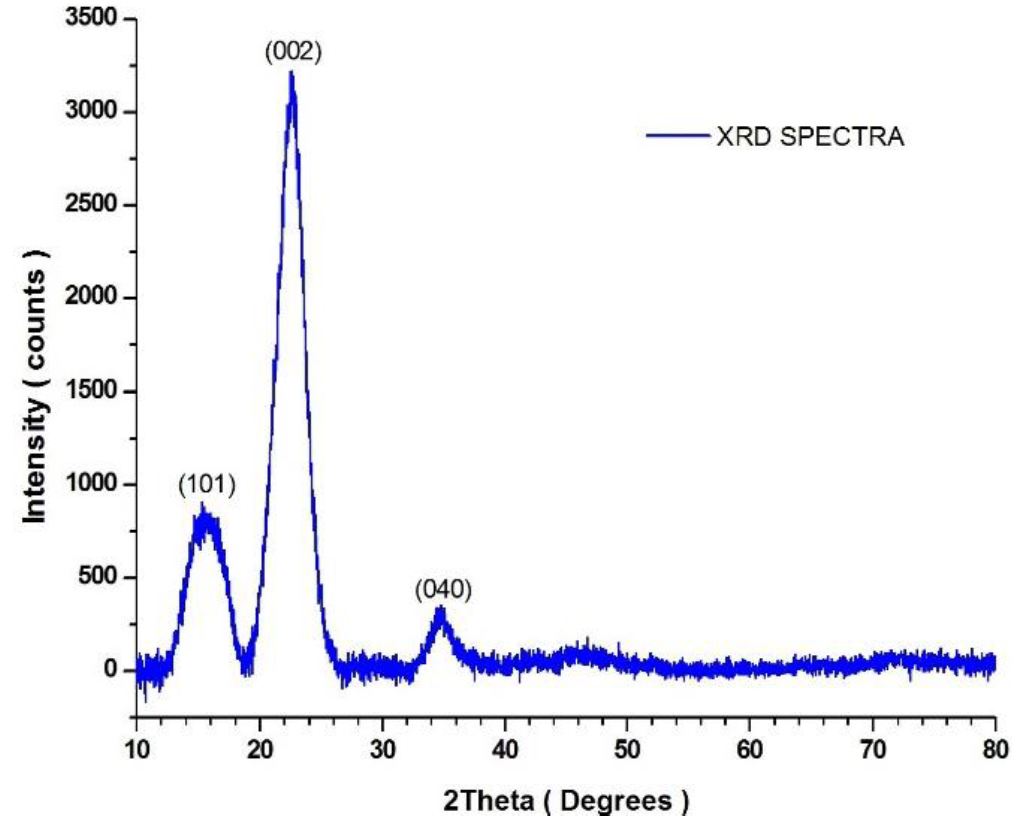
The amount of cellulose and non-cellulosic content of fibers determines its crystallinity. The X-ray diffractograms of LSFs is shown in Figure 4.
The XRD clearly indicates three well-defined peaks at 2θ= 22.68° (002 plañe), 2θ= 17.28° (101 plañe), 2θ= 34.66° (040 plañe), which correspond to crystalline and amorphous peaks of the cellulose of the natural fiber. The crystalline index (CI) of LSFs 92.4% is calculated from equation (1), which is abnormally higher than that of the other reported natural fibers such as hemp (80%), jute (65.8%) and flax (70%), cotton (68%), mending grass (58.6%), sisal (55%), and Wheat straw (45.57%). The average crystal size of LSFs calculated by using Scherrer"s formula (equation 2) is found to be 7.2 nm lower than that of jute (29.25 nm), sisal (16.99 nm), mending grass (14.3 nm) but higher than that of ramie (6.6 nm), wheat straw (6.4 nm) and cotton (5-7 nm) (Reddy et al., 2014).
3.4 THERMO GR A VIMETRIC ANALYSIS OF LSFS
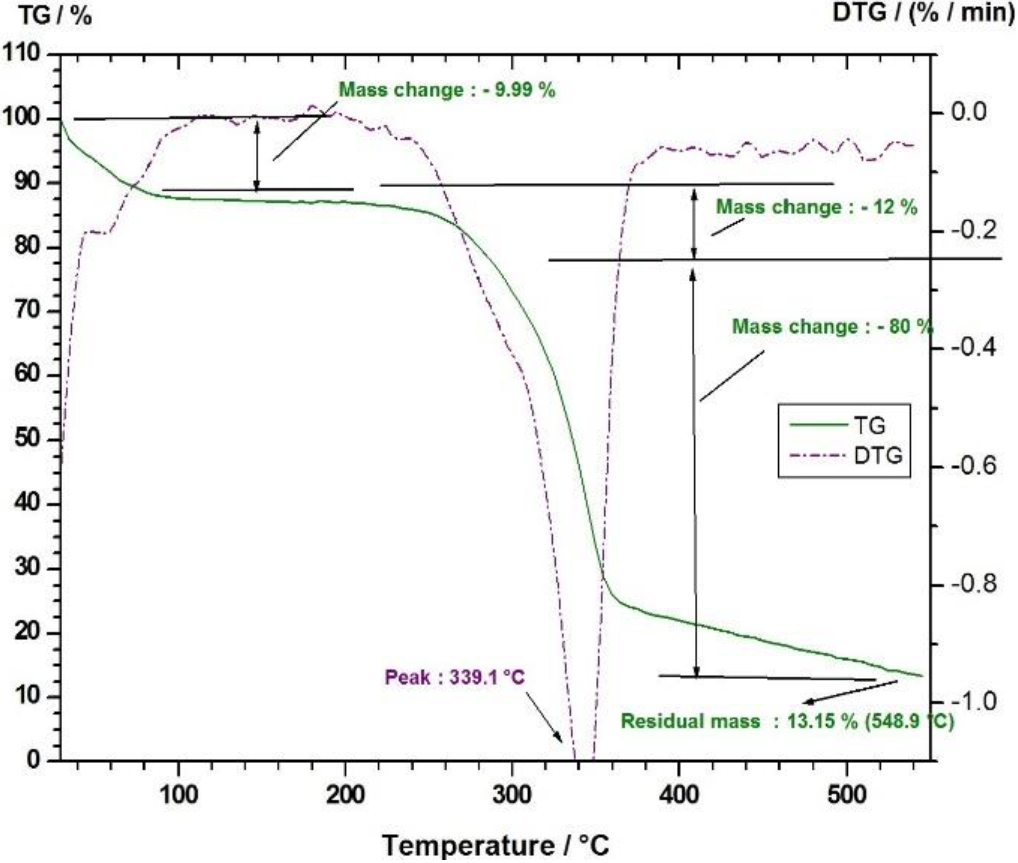
The thermal stability of LSFs examined by TG and DTG curves is shown in Figure 5. The initial minor degradation of the fiber noticed at 88.4°C with a weight loss of 9.9% due to the evaporation of the moisture content present in the fiber (Mayandi, Rajini, Pitchipoo, Jappes & Rajulu, 2016). The second major degradation observed in the temperature range between 240°and 400°C with a weight loss of 12 % is due to the thermal depolymerisation of hemicellulose. A very prominent peak at 339.1°C with a major weight loss of 80% is due to the degradation of cellulose. The results were significantly comparable with that of the other natural fibers such as bamboo, hemp, jute, and kenaf fibers were observed at 321°, 308.2°, 298.2°, and 309.2°C respectively (Manimaran, Saravanakumar, Mithun & Senthamarai-kannan, 2016). Later, there is a minor degradation after 400°C with a weight loss of 12 %, which is due to the decomposition of lignin and wax content of the fiber (Kathiresan, Pandiarajan, Senthamaraikannan & Sara-vanakumar, 2016).
3.5 TENSILE PROPERTIES OF LSFS
During the single-fiber tensile testing, the tensile properties such as strength, modulus, and elongation at the break of the LSFs were found to be 257-717 MPa, 7 42 GPa, and 1.38-4.67% significantly comparable with that of the other natural fibers such as flax (345-1500 MPa, 27.6 GPa, and 2.7-3.2%), hemp (690 MPa,70 GPa, and 1.6%), jute (393-773 MPa, 26.5 GPa, and 1.5-1.8%), and kenaf (930 MPa, 53 GPa, and 1.6%). The higher cellulose content of the lignocellulosic LSFs with a lower micro fibril angle significantly influences the tensile strength of the LSFs. The micro fibril angle (α) of the LSFs was calculated using Equation (3), which is as follows:
Where ɛ is the global deformation (or) strain (mm/mm), α the micro fibril angle (deg.), LO the gauge length (mm), and L the elongation at break (mm). The calculated micro fibril angle (α) of the LSFs was in the range of 8.52 - 25°.
3.6 SURFACE MORPHOLOGICAL ANALYSIS OF LSFS
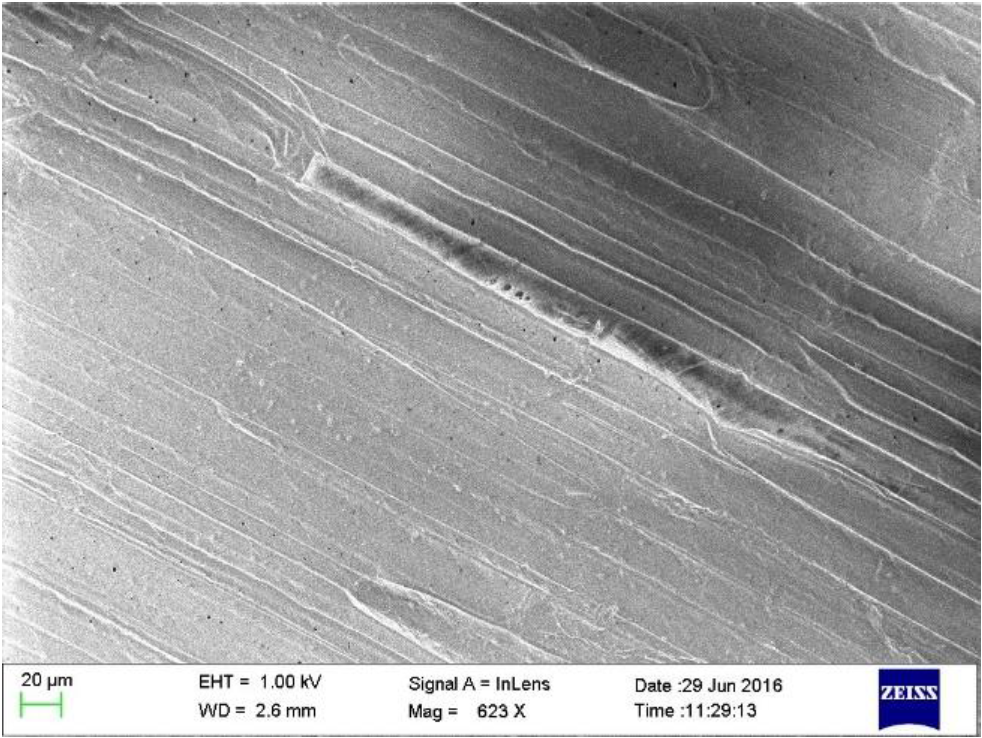
The scanning electrón microscopio image of LSFs is shown in the Figure 6. The surface morphology of the LSFs consists of several fiber cells covered with non-cellulosic components, wax and other impurities over the entire length of the fiber. It has a clean and smooth surface and appears as a thick layer that facilitates both mechanical properties and binding efficiency with matrix materials (De Rosa, Kenny, Puglia, Santulli & Sarasini, 2010).
4. CONCLUSIONS
Owing to the increasing significance of the use of natural fibers in the field of biodegradable composite materials, the agro waste of Lagenaria siceraria stem was chosen for the study of extraction, characterization, mechanical and thermal behavior of the fiber. The fiber from the waste stem has high cellulose content (79.91%) with an abnormal high crystalline index (92.4%) and crystalline size (7.2 nm) playing an imperative role in the tensile strength (257-717 MPa) of the fiber, which improves the mechanical properties of the composite materials. The high thermal stability (withstand up to 339.1°C) renders the fiber to be processed with various thermo polymer resins. The light density (1.216 g/cm3) of the lignocellulosic LSFs from the agro waste renders the possibility of using it as an alternative reinforcement for lightweight eco-friendly composite materials for various industrial applications.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.











 text new page (beta)
text new page (beta)