Introduction
The awareness of pollution, scarce of fossil fuels and the environmental footprint, are rising and they are subject of important research around the world, regarding fuels there is an important concern about the development of ecological friendly combustibles and two main research lines have been developed, the first one is the introduction of the so-called biodiesel, and the second one is the improvement of the existing fuels [1-5].
Regarding fuels, vegetable oils had appeared as the option of choice, not only as biodiesel source but also as additives in the fossil fuels. Among different varieties of seeds, available as biodiesel source and additive [1,3,4,6-10], Castor oil (ricinus comunis) is well known as renewable source of chemical industry [11-13] as well as biodiesel starting material [14-16]. Additionally, results from rheological behaviour of castor oil biodiesel suggest that pure castor oil increase viscosity when is used as oil additive and its chemical structure had been already studied [17], even though there is scarce information about its thermal behaviour of this oil as lubricant when it is used as oil additive.
As a way to give more insight about thermal properties of castor oil -synthetic oil blends SAE40W, which is a highly refined mineral oil, the present work deals with the thermal characterization of those mixes, using two well-known photopyroelectric methods: BPPE and FPPE techniques [18-20].
Experimental details
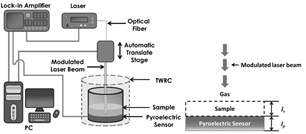
Photopyroelectric techniques are non-destructive methods used in thermal and optical characterization of many materials including liquid samples these techniques are characterized by using information carried on thermal waves generated over the pyroelectric detector as well as sample under study. BPPE is used to analyze thermal diffusivity of liquids and, it is based on the variation of the sample thickness by means of a setup showed in Figure 1.
In this experimental setup, the sample is enclosed in a chamber of variable length which is formed by a metal foil (Cu 100 μm thick) and PZT (500 μm thick) pyroelectric temperature sensor. A laser diode beam, impinges on the surface of the metallic foil, which acts as light absorber as well as thermal wave generator, laser beam is modulated by the internal oscillator of a lock-in amplifier, this arrange is known as thermal wave resonator cavity (TWRC).
Absorbed light at the metal foil induces thermal waves at sample metal interface
those thermal waves travel through the sample and, have the same modulation
frequency that the incident beam (f). The temperature oscillations at x =
l can be measured using the pyroelectric (PE) sensor as a function of
the sample thickness l
s
. For a thermally thin copper foil (aculcu ≪ 1),
thermally thick sample (asls ≫ 1) and, thermally thick PE
detector (aplp ≫ 1) (where
where A is an instrumental factor, η s is the nonradiative conversion efficiency for the absorbing sample, κ p is the thermal conductivity of the pyroelectric sensor, b sp = e s /e p , with ej the thermal effusivity of the j-th element in the PE cell and ω 0 the angular frequency of the laser beam (ω 0 = 2πf). When the light modulation frequency is fixed, the constant A and all the terms before the first exponential in Equation 1 remains constant and can be joined in a second constant (B), therefore the output voltage can be reduced as:
From Equation 2 is possible to see that the measured voltage over the PE depends only on the sample thermal diffusivity (αs) and thickness. By performing a sample thickness scan, it is possible to get the sample thermal diffusivity from the slope of the logarithm of the photopyroelectric (PPE) signal amplitude as function of l s or from the slope of the linear PPE signal phase as a function of l s .
The light modulation frequency used in the present study was chosen at 0.5 Hz, in order to guarantee that sample and pyroelectric sensor were thermally thick; the light source was a diode laser beam, 40 mW power, at 785 nm wavelength.
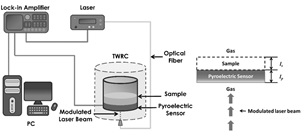
Thermal effusivity of oil blends were obtained by using the so-called Front
Photopyroelectric Technique (FPPE),the experimental setup of this technique is shown
in Figure 2, in this configuration the sample
is placed on an intimate thermal contact with the PE detector and on the opposite
site a modulated laser beam is applied, under the assumption that the sample is
thermally thick, i.e. sample thermal diffusion length,
where C is a fitting parameter depending on every pyroelectric sensor, σ p is the PE complex thermal diffusion coefficient σ p = (1+i)/µ p , where: i=(-1) 1/2 and µ p = (α p /(πf)) 1/2 , with α p the PE sensor thermal diffusivity, l p is the PE thickness, b = e s /e p , g = e g /e p , with es, eg and ep, thermal effusivities for sample, air and pyroelectric sensor respectively, using Equation 3 and suitable frequency scan, sample thermal effusivity can be extracted by fitting the theoretical expression to the experimental data. All measurements were performed at room temperature.
Results and discussion
Thermal effusivity as function of castor oil concentration was obtained using the
IPPE setup, PE thermal properties were determined a priori by using reference
samples with well-known thermal properties. For castor oil- SAE40W oil blends in the
0 to 20 % range castor oil content, thermal effusivity behavior is showed in Figure 3, black squares represent the obtained
experimental values, with the respectively confidence interval (± 15
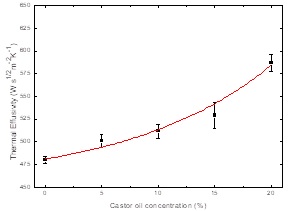
Figure 3 SAE 40W-castor oil blends thermal effusivity behavior as function of castor oil, solid line is a fit of thermal effusivity with an exponential function.
Figure 4 shows the sample thermal diffusivities
obtained by BPPE setup, using the sample thermal diffusivity as fitting parameter in
Equation 2, confidence interval
for each value was calculated by means of least squares method with a value of ± 3
x10-9
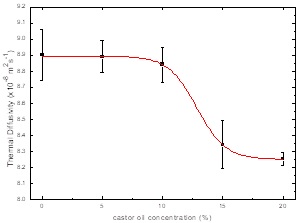
Figure 4 SAE 40W-castor oil blends thermal diffusivity behavior as function of castor oil, solid line is a fit of the thermal diffusivity with a sigmoidal function.
Since the thermal diffusivity ( α
s
=
By using the relation
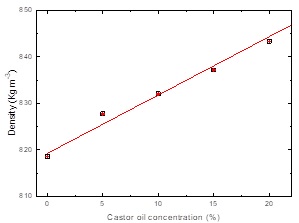
It was also measured the densities of the castor oil blends as a function of their concentration which are showed in Figure 5. Densities were measure using a pycnometer method at room temperature results show that castor oil blends present linear increment when castor oil content increase.
Conclusions
Depending on castor oil percentage, thermal effusivity increases as Figure 3 shows, this can be due to pure castor oil thermal effusivity is higher than pure oil.
Blend densities increases as castor oil content increase; SAE 40W density is similar that Sperm whale and castor oil presents higher value [28-30], therefore it is expected and increase in density as function of castor oil content.
Castor oil content provokes that thermal diffusivity diminishes as sigmoidal
function, this could be due to the increment in the oil blend densities, by using
the relationship











 text new page (beta)
text new page (beta)