Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Superficies y vacío
Print version ISSN 1665-3521
Superf. vacío vol.25 n.2 Ciudad de México Jun. 2012
Effect of energy in the size of pyrrole-derived particles synthesized by plasma
Gómez L. M.&, Olayo M. G.*,°, Cruz G. J., López-Gracia O. G.*, González-Torres M.,&, de Jesús C.#, González-Salgado F.I
Departamento de Física, Instituto Nacional de Investigaciones Nucleares Apdo. Postal 18-1027, D.F., CP 11801, México.
& Programa de Maestría en Ciencia de Materiales, Facultad de Química, Universidad Autónoma del Estado de México.
* Programa de Maestría en Ciencias Químicas, Facultad de Química, Universidad Autónoma del Estado de México, Paseo Tollocan esq. Paseo Colón, Toluca, Estado de México. CP 52000, México °guadalupe.olayo@inin.gob.mx
# Instituto Tecnológico de Toluca, Ex-Rancho La Virgen S/N. Metepec, Edo. Mex., CP 52140, México.
Recibido: 24 de noviembre de 2011
Aceptado: 2 de mayo de 2012
Resumen
Se presenta un estudio sobre la síntesis por plasma de partículas derivadas de pirrol a diferentes potencias con la finalidad de estudiar el efecto que produce la energía aplicada en el tamaño de partícula y en las propiedades estructurales y morfológicas. Las partículas se sintetizaron por plasma con descargas resistivas de resplandor a 13.56 MHz, 10-1 mbar y 40-120 W. Las partículas son de forma esférica con diámetro promedio entre 109 y 205 nm reduciendo linealmente en función de la energía de la descarga. La estructura de las partículas conserva parte de la funcionalidad aromática de los anillos de pirrol combinada con grupos C=O y C=N que pueden ayudar a la biocompatibilidad del material.
Palabras clave: Partículas; Pirrol; Energía; Plasma.
Abstract
This work presents a study about the synthesis by plasma of particles obtained from pyrrole with the purpose of evaluating the influence of the energy applied to the synthesis on the size and morphology of particles. The syntheses were performed with resistive glow discharges at 13.56 MHz, 10-1 mbar and 40-120 W. The particles have spherical morphology with mean diameter between 109 and 205 nm decreasing linearly with the energy of discharges. The structure of particles preserved great part of the aromatic functionality of pyrrole combined with C=O and C=N chemical groups that may help in the biocompatibility of the material.
Keywords: Particles; Pyrrole; Energy; Plasma.
1. Introduction
Polymeric materials have increased their applications in the field of biomaterials due to the advances in the molecular manipulation of structures that enhance their physical and chemical properties. With this strategy, many nanostructured biomaterials have been developed with advantages in the size and superficial morphology compared with those of macrostructured materials, because the size of particles, protrusions and pores on the surfaces have almost as much influence in the biocompatibility as the functional groups on the main structure [1-3].
Polymers derived from pyrrole are potential biomaterials through their electrical properties and amine groups that promote cell interaction [4-6]. In this area, polypyrroles synthesized by plasma have special advantage because they do not use other chemicals except the initial monomers and dopants in the syntheses and maybe partially because of that, they have shown special biocompatibility with spinal cord tissues. Plasma polymerizations use high energy particles in gas-phase and electric fields instead of initiators, accelerators or solvents of chemical liquid-phase syntheses that may generate toxic wastes to cells.
In the area of chemical liquid-phase syntheses, microemulsion polymerization was used for obtaining spherical nanoparticles of polypyrrole and ammonium peroxydisulfate [7]. Nanostructures of polypyrrole with lactic acid have been synthesized by emulsion by Shi [8]. Polypyrrole powders were synthetized chemically using ammonium persulfate [9] and anhydrous iron chloride [10] as oxidants.
In the field of gas-phase plasma synthesis, Yang studied the synthesis of polypyrrole nanoparticles synthetized by plasma with Ar as carrier gas [11], while Cao studied the ratio of aromatics rings as precursors of hollow-core spherical nanoparticles by plasma in Ar atmosphere [12].
Nano and meso particles of polypyrrole (PPy) with spherical morphology using glow discharges of pyrrole, similar to those of this work, were synthesized previously to be used in biological applications [13]. Now, this paper presents a study about the evolution of particle size as a function of the energy applied to the discharges in the synthesis by plasma.
2. Experimental Section
Particles derived from pyrrole were synthesized by glow discharges of pyrrole in a tubular glass reactor of approximately 1500 cm3. Only pyrrole and the residual atmospheric gases participated in the synthesis, there were no other reagents as carrier gases or dopants. The reactor had stainless steel electrodes and flanges at the ends with three access ports each. In the central ports, two electrodes of 6.5 cm in diameter with a spacing of 6 cm between them were introduced. The electrodes were connected in resistive mode to a RFX-600 Advanced Energy radio frequency power supply. The other accesses are inlet ports of a Pirani pressure gauge and an Alcatel Pascal 2015C1 vacuum pump with an Alcatel LNT 25S gas condenser.
Pyrrole (Aldrich, 98%) was polymerized by plasma with glow discharges at 13.56 MHz, 10-1 mbar and power between 40 and 120 W, during 180 min. The polymers were obtained in powder and thin films on the reactor walls. Particles of nano, meso and micro dimension composed the powder which was removed from the reactor with a small brush. After that, the films were swollen with distilled water and removed with a spatula.
The structure of particles derived from pyrrole was analyzed with a FT-IR Spectrum 1600 Perkin-Elmer Infrared Spectrophotometer using 32 scans. The morphology was studied with a Jeol JSM-5900LV scanning electron microscope and the micrographs were processed with the Olympus Measure IT program. X-ray photoelectron spectroscopy (XPS) analysis was performed using a Thermo K-Alpha photoelectron spectrometer equipped with a monochromatic Al X-ray excitation source.
3. Results and Discussion
3.1 Structure of particles derived from pyrrole
Figure 1 shows the IR spectra of pyrrole particles synthesized at 40, 80 and 120 W. The dominant absorption in the particles, wide and centered around 3437 cm-1, belongs to N-H bonds of pyrrole amines [14]. The absorption in 1455 cm-1 can be associated with the substitution of hydrogens in heteroaromatic rings. There is a slight absorption centered at 2932 cm-1 indicating the presence of aliphatic C-H bonds most likely caused by pyrrole fragments originated by the collisions with the highest energy particles in the plasma.
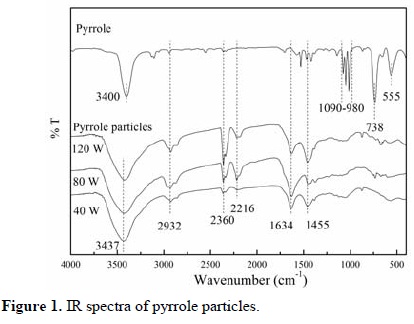
Triple bonds, C≡N and C≡C, caused by the intense oxidation of molecules are manifested in the peak centered at 2216 cm-1. Consecutive double bonds in different combinations between N, C and O (N=C=C, O=C=C, C=C=C, ...) can be associated with the absorption at 2360 cm-1. Individual double bonds as C=O, C=C and C=N can be associated with the absorption centered at 1634 cm-1 interval. All of these multiple bonds are indication of dehydrogenation and/or oxidation of the polymers.
The pyrrole spectrum has absorptions centered at 555, 738 and between 1090-980 cm-1 indicating the vibration of C-H bonds in different modes of the conjugated structure of the pyrrole skeleton. These absorptions are not shown in the spectra of pyrrole particles probably due to the disappearance of C-H bonds in the heteroaromatic rings as a consequence of networking in the pyrrole particles.
Figure 2 shows an XPS survey spectra of particles derived from pyrrole with its associated atomic distribution. The main signals detected are C1s at 285.1 eV, N1s at 399.8 eV and O1s at 532.1 eV. Traces of Si2p usually appear in almost all surfaces due to atmospheric dust contamination. As in the case of the IR analysis, C and N atoms are attributed to the pyrrole structure and O atoms can be attributed to the oxidation in the material.
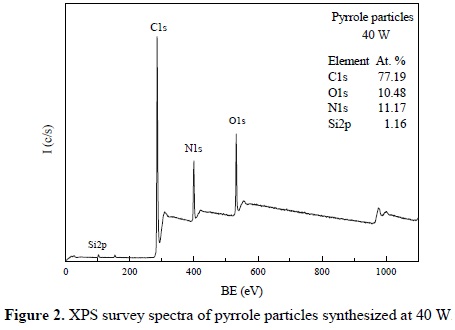
The stoichiometric C/N atomic ratio of pyrrole and polypyrroles is 4, however this ratio in pyrrole particles is approximately 7 in the case of 40 W synthesis. One reason for such increase is that the XPS analysis considers only the first nanometer depth of the beam incidence, which is always affected by the superficial C, O and Si contamination, increasing their content in the elemental analyses.
Independently of this characteristic, the elemental data shows an increase in the C participation in the particles, which can also be associated to partial damage of pyrrole rings to form small alkenes with the fragments, as discussed in the IR sections. This effect can also be named carbonization of the material due to the high energy of the plasma particles. However, it is difficult to differentiate the C content of contamination, carbonization or that part of the intrinsic structure of the material.
3.2 Morphology of particles
Figures 3-5 (4) show powder synthesized from pyrrole at different power arranged in random clusters of spherical and smooth particles. Each power has associated a micrograph and a normal diameter distribution calculated with Equation 1 using the most representative particle size. Approximately 100 particles were measured per synthesis, see Figure 6. The harmonic mean corresponds to the maximum value of f(ɸ) in the particles.

Where: f(ɸ): normal distribution function, ɸ: diameter of particles, µ: harmonic mean, and σ: standard deviation.
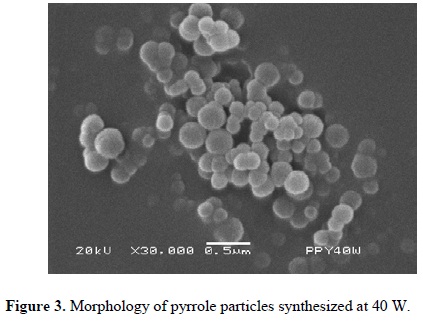
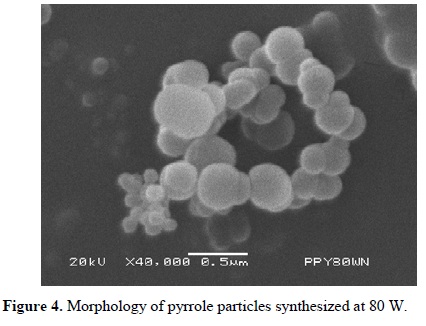
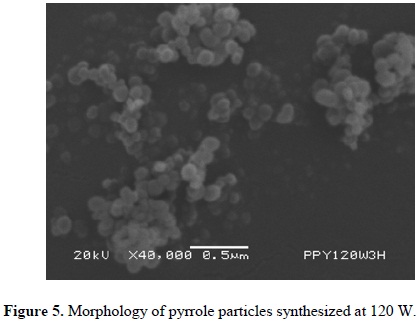
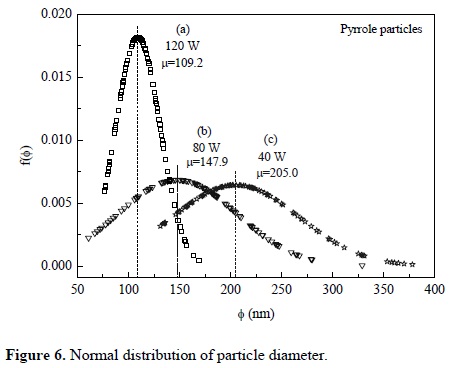
Particles synthesized at 40 W have a diameter between 131 and 378 nm with center at 205 nm, see Figure 6(a). Figure 6(b) shows the diameter distribution of particles synthesized at 80 W with center at approximately 148 nm and sizes between 61 and 329 nm. Particles synthesized at 120 W have a diameter between 76 and 169 nm with center in 109 nm, see Figure 6(c). In the three cases, the diameter distribution is enlarged in the zone of the larger diameter, which indicates a tendency in the synthesis to create particles towards this zone.
Figure 7 presents a comparison of average particle diameter of PPy at different power of synthesis. A first observation is that the particle diameter and the dispersion of values decrease linearly with power. The decrease rate of particle size is 1.19 nm/W.
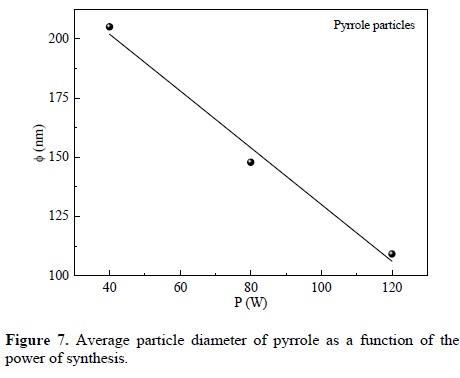
Extrapolating the diameter tendency to very low power, the maximum mean diameter would be reached approximately at 250 nm. However, this is a limit value, hypothetical, under similar reactivity conditions that at very low power could be different. A similar analysis can be done to calculate the minimum power required to achieve average diameters below 100 nm, where the particles come into nano-dimensions. The result in this case would be at a power greater than 125 W.
4. Conclusions
Pyrrole-derived particles were synthesized by plasma with spherical profile and smooth surface. Only pyrrole and the residual atmospheric gases participated in the synthesis as chemical agents. There were no other reagents as carrier gases or dopants. The diameter of the particles synthesized in the 40-120 W interval was between 61 and 378 nm. The energy used during the synthesis influences the particle size, decreasing linearly with the applied power. Extrapolating this trend under similar experimental conditions, the minimum power required to achieve average diameters below 100 nm would be 125 W. N-H groups, usually found in some biomaterials, were found in the IR analysis of the particles of this work. The particles have indication of multiple bonds in their structure, which are not in the structure of pyrrole, and can be a consequence of dehydrogenation during the synthesis.
Acknowledgements
The authors thank C. Jorge Pérez for his help in the scanning electron microscopy and to Conacyt for the financial support to the projects 80735 and 130190.
References
[1]. P.A. Gunatillake, R.Adhikari. European Cells and Materials. 5, 1 (2003). [ Links ]
[2]. J.L. Ifkovits, B.S., J. A. Burdick. Tissue Engineering. 13, 2369 (2007). [ Links ]
[3]. J. Jozefonvicz, M. Jozefowicz. Pure and Applied Chemistry. 64, 1783 (1992). [ Links ]
[4]. S.P. Massia, P. Stark, D.S. Letbette. Biomaterials. 21, 2253 (2000). [ Links ]
[5]. E. Colín, M.G. Olayo, G.J. Cruz, L. Carapia, J. Morales, R. Olayo. Progress in Organic Coatings. 64, 322 (2009). [ Links ]
[6]. N. Guimard, N. Gómez, C.E. Schmidt. Progress in Polymer Science. 32, 893 (2007). [ Links ]
[7]. A. Reung-U-Rai, A. Prom-Jun, W. Prissanaroon-Ouajai, S. Ouajai. Journal of Metals, Materials and Minerals. 18, 27 (2008). [ Links ]
[8]. G. Shi, M. Rouabhia, Z. Wang, L.H. Dao, Z. Zhang. Biomaterials. 25, 2477 (2004). [ Links ]
[9]. C.F. Hsu, L. Zhang, H. Peng, J. Travas-Sejdic, P.A. Kilmartin. Synthetic Metals. 158, 946 (2008). [ Links ]
[10]. S.A. Waghuley, S.M., Yenorkar, S.S. Yawale, S.P. Yawale. Sensors and Actuators B. 128, 366 (2008). [ Links ]
[11]. P. Yang, J. Zhang, Y. Guo. Applied Surface Science. 225, 6924 (2009). [ Links ]
[12]. J. Cao, T. Matsoukas. Journal of Nanoparticles Research. 6, 447 (2004). [ Links ]
[13]. G.J. Cruz, M.G. Olayo, O.G. López, L.M. Gómez, J. Morales, R. Olayo. Polymer. 51, 4314 (2010). [ Links ]
[14]. J. Morales, M.G. Olayo, G.J. Cruz, R. Olayo. Journal of Polymer Science Part B: Polymer physics. 40, 1850 (2002). [ Links ]














