Introduction
Vanadium oxides (VOx) and VOx doped with different metal oxides are interesting semiconductor materials because of their abundance in the Earth’s crust, as well as its multi-oxidation states and various crystalline structures (Silversmit et al., 2004, 2006). As a semiconductor material, VOx shows a band gap ~2.6-3.5 eV that makes it a good semiconductor material for diverse electronic application. In the literature, VOx thin films have been deposited with different methods for diverse potential applications employing techniques such as: magnetron sputtering and spin-coating for thermochromic applications (Ho et al., 2019; Zhan et al., 2020; Yuan et al., 2021); pulsed laser deposition for cathodes in Lithium and sodium-ion batteries (Petnikota et al., 2018); sprayed solution for NO2 gas sensing (Khatibani, Abbasi and Rozati, 2016; Mane and Moholkar, 2017); solution-processing method for smart windows applications (Peng et al., 2018; Wang et al., 2020; Shen et al., 2021).
On the other hand, modifications of optical or structural properties of semiconductor thin films are necessary to improve application of these materials. Which by adding a different type of metal ions into a VOx host matrix, known as a doping process, is one of the most effective ways to modify vanadium oxide structure, leading to different electrochemical, catalytic, and magnetic properties (Lu et al., 2019; Sharma et al., 2021; Geng et al., 2022). Metal doped vanadium oxide thin films have been obtained with different deposition techniques: Y-doped VOx thin films by DC reactive magnetron sputtering for electrical modulation applications (Zhou et al., 2020); Cs-doped VOx thin films deposited by spun-cast as a hole extraction layer in perovskite solar cells (Yao et al., 2018); Fe-doped VOx by sol-gel spin-coated for electrochromic device applications (Bae, Koo and Ahn, 2019); In-doped VOx thin films obtained by spray pyrolysis coating system for optical transparency modulation (Tabatabai Yazdi, Pilevar Shahri and Shafei, 2021); Co-doped VOx thin films by microwave irradiation process for battery-type supercapacitor applications (Liu et al., 2020), among others (Ji et al., 2018; Li et al., 2019; Xu et al., 2019).
Solution-processing spin-coating is an economical method to obtain homogeneous pure or doped VOx thin films. Compared to other chemical solution deposition methods, this method has the advantage that it enables the deposition of metal oxide thin films without seed layer on substrates such as glass, fluorine doped tin oxide (FTO), indium doped tin oxide (ITO) or coated polyethylene (PET) for transparent or flexible electronics applications (Hajzeri et al., 2012). In particular, metal ion doping in VOx thin films could be an in-situ process in a spin-coating method by adding the metal source into the vanadium precursor solution. However, expensive organometallic compounds are usually employed to prepare metal precursor solutions. In this work, we propose a simple method to prepare vanadium oxide and cobalt doped vanadium oxide solutions, without the use of organometallic compound or any complex agent that would result in a slow metal cation release, and an increased time and temperature deposition. Chemical photoelectron analysis of vanadium oxide samples, with or without cobalt doping, confirms the formation of a vanadium oxide VO2 compound in the film samples. Cobalt oxide CoO also prevails in VO2:Co2+ samples, with some neighboring atoms arranged in a short-range order. Porous surface of VO2:Co2+ thin films can be obtained, giving an optical absorption onset at 2.3 eV.
Experimental procedure
Synthesis of VOx powders and solution
Precursor solutions for VOx powders were prepared by using 0.1 M solution of vanadium (IV) oxide sulfate hydrate (VOSO4·xH2O) and 1 M solution of sodium hydroxide (NaOH), as previously suggested [25]. These two precursor solutions were mixed, resulting in a blue color solution with a pH ~2 tested with a MColorHastTM. After that, the blue solution was heated at 80 °C for 3 h to obtain VOx precipitates. These precipitates were dispersed in methanol to dissolve possible impurities and then centrifuged. After centrifugation, VOx precipitates were dried at 80 °C for 2 h to obtain VOx powders.
To prepare a VOx sol-gel solution, VOx powders were dispersed in isopropyl alcohol under ultrasonic vibration at room temperature for 10 min. Then, the mixture solution was filtered with a cotton filter to remove the undispersed precipitates and obtain a transparent solution for thin film deposition by spin-coating. Pure VOx films were called samples S1.
Preparation of VOx:Co solutions
To obtain cobalt doped VOx precursor solutions, dried VOx powders and CoCl2·6H2O salt were dispersed/dissolved in isopropyl alcohol. Then, the solutions were subjected to ultrasonic vibration at room temperature for 10 min. Finally, the solutions were filtered with a cotton filter to remove the undispersed precipitates and obtain transparent VOx:Co solutions for thin film deposition by spin-coating. Four VOx:Co solutions were prepared with VOx/CoCl2⋅6H2O weight ratios of 0.7/0.3, 0.5/0.5, 0.3/0.7 and 0.1/0.9, called samples S 2 , S 3 , S 4 and S 5 , respectively.
VOx and VOx:Co thin film deposition
The five precursor solutions, VOx and VOx/Co, were used to form thin films by spin-coating. Microscope glass slides from VE-P10 Velab™, to be used as substrates for vanadium oxide solutions, were washed with Alconox® detergent, rinsed with distilled water and air-dried. The clean substrates were placed in the Chemat Thecnology Spin-Coated KW-4A, 50 µL of the VOx or VOx:Co solutions were deposited at 2500 rpm for 2 min on the glass substrates. The obtained coatings were thermally annealed in air at 200 °C for 1 h and became solid thin films.
Thin films characterizations
X-ray photoelectron spectroscopy (XPS) was used to assess the chemical composition of the films. The tool employed was PHI 5000 Versa Probe II, with a monochromatic Al Kα (1486.6 eV) X-ray source with a 0.1 eV step size and a constant pass energy of 23.50 eV. The scale of binding energies was corrected by calibrating the main C 1s peak at a binding energy of 284.8 eV. Atomic compositions of VOx/Co films were estimated from the XPS results. Crystalline structures of VOx/Co films were probed with X-ray diffraction (XRD) employing a Rigaku DMAX-2200 diffractometer equipped with a Cu Kα (λ = 1.54 Å) X-ray source. A Jeol JSM-7800F field emission scanning electron microscope (FE-SEM) was used to evaluate the surface morphology of the films. The surface morphology of each film sample was analyzed by atomic force microscopy (AFM) with a Veeco Dimension Icon, Bruker instrument. Optical transmittance spectra of VOx/Co films were measured with a Perkin Elmer Lambda 20 UV-visible spectrometer. Thicknesses of VOx and VOx:Co thin films were measured with an Ambios Technology XP 200 profilometer.
Results and discussion
Profilometry and main characteristics
The first assessment of the possible formation of continuous VOx and VOx:Co thin films from the above mentioned precursor solutions is film thickness measurement by profilometry. Table 1 lists the five types of VOx:Co thin films with their respective thickness. In the Alpha-Step profiles of all film samples, large grains of 100 to 400 nm of height were observed and were embedded in continuous solid coatings with thickness from 133 to 246 nm.
Optical properties
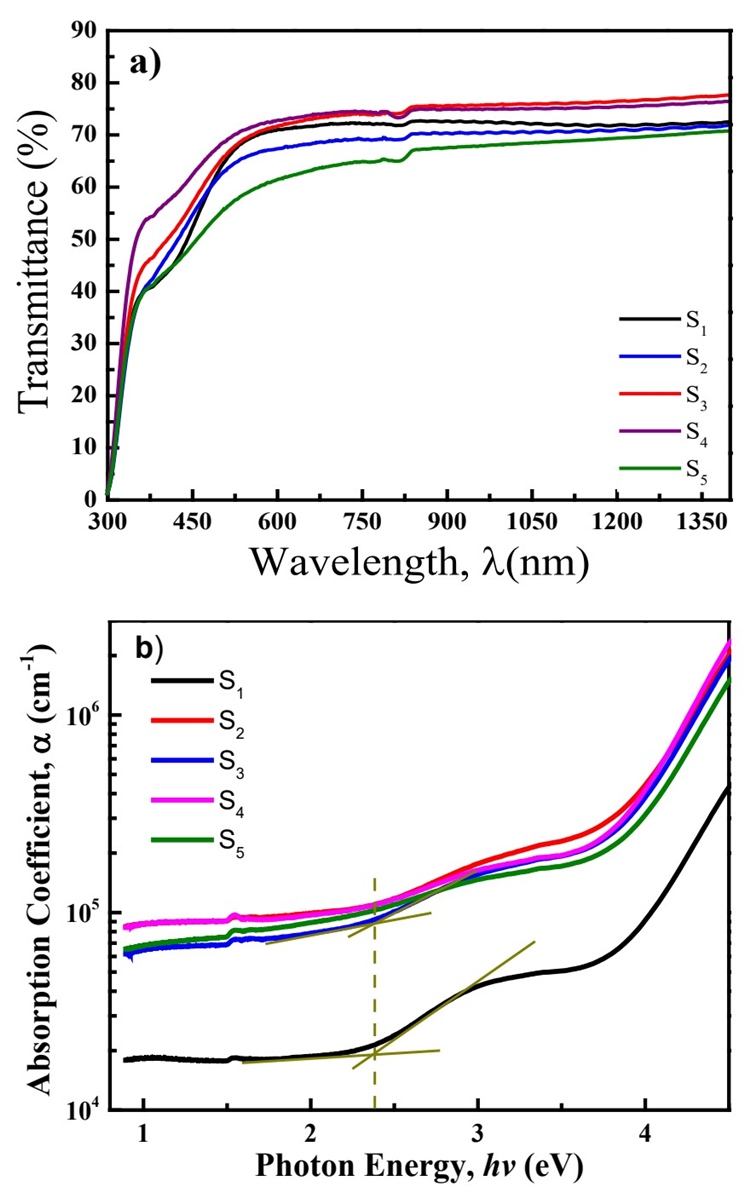
Optical transmittance spectra of VOx:Co thin films can be appreciated
in Figure 1a with transmittance percentage
of 65 - 75 % in the 800 -1350 nm wavelength (λ) range. The main influence of
Co2+ inclusion is observed in UV-A to blue region, at 325 - 500
nm, as reported elsewhere (Hajzeri et al.,
2012; Martínez-Gil et al.,
2020). Furthermore, samples with larger Co2+
concentration, such as S
4
and S
5
, show lower transmittance at the visible region (400 - 700 nm). Optical
absorption coefficient spectrum of each film sample, α(λ) (Figure 1b), is calculated from the simplified Beer-Lambert
equation:
X-ray diffraction
X-ray diffraction pattern of VOx film sample (S 1 ) is presented in Figure 2, which appears to correspond to an amorphous material due to the broad signal around 22°, plus a monoclinic phase of HNaV6O16•4H2O with the plane orientation (100) at around 2θ ∼ 8° (JCPDS# 49-0996 (Channu et al., 2011)). This crystalline compound could be a residual from VOSO4·xH2O + NaOH, reaction and was not be eliminated during the 200 °C annealing or the thin film processing. Interestingly, after Co2+ addition, the reflection peak around 2θ ∼ 8° gradually reduces. The higher the concentration of Co2+, the smaller the intensity of that peak, until its complete elimination in the sample with the highest concentration of Co2+ (sample, S 5 ). S 5 shows the diffraction pattern typical of an amorphous film, hence it is a predominantly amorphous material. Since no diffraction peaks of CoOx or VCoOx compounds appear in the five VOx:Co2+ samples, it seems that low temperature annealing leads to amorphous materials, as indicated in (Hu et al., 2017; Martínez-Gil et al., 2020).
Thin films surface morphology
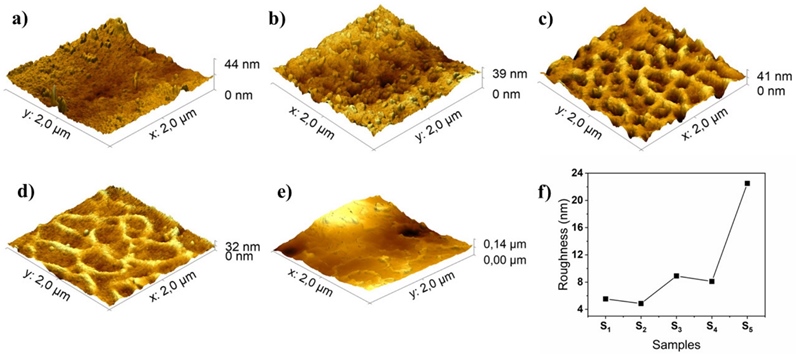
Figure 3 shows AFM three dimensional surface topography images in scales of 2 m x 2 µm of (a) S1, (b) S2, (c) S3, (d) S4 and (e) S5 VOx:Co2+ film samples. The root-mean-square roughness of the same samples is plotted in Figure 3f. The surface topography of pure VOx film (S 1 ) presents lumps and the lowest roughness. As the cobalt ions are incorporated into VOx host, variations in shape and roughness are observed in AFM images. Notable changes are observed in samples S 3 and S 4 that exhibit a ring like morphology and increased roughness (8 - 9 nm) in comparison with S1 and S2 (4 - 5 nm) samples. sample S 5 that contains the highest cobalt concentration loses the ring pattern in its surface morphology and shows the highest roughness (∼23 nm) among the five samples. Similar results are reported elsewhere, suggesting that the inclusion of Co2+ in VOx host by solution methods modifies the superficial morphology of cobalt doped vanadium oxide thin films (Fuentes-Ríos et al., 2021). Such porous morphology encourages potential use of VOx:Co2+ thin films as a cathode material for lithium battery applications (Wang et al., 2011).
To complement the AFM results, SEM micrographs of S 1 , S 3 and S 5 samples were analyzed and are shown in Figure 4. Without cobalt addition, vanadium oxide sample S 1 shows a granular ground with shallow leaves. The S3 sample (VOx:Cobalt salt = 50:50), presents an interconnected rings or porous arrangement, very similar to the AFM image of the same sample (Fig. 3c). The pore size distribution histogram of this film sample is in Figure 4d. It shows that the pore size varies from 100 to 300 nm, and those with ~150 nm predominates. By chemical element mapping in the SEM image of sample S 3 , vanadium and cobalt elements have been detected to have a homogeneous distribution inside the pores. Finally, the sample with the highest concentration of cobalt, S 5 , shows an irregular porous granular pattern at the surface, and under such pattern a continuous film is observed.
Chemical composition
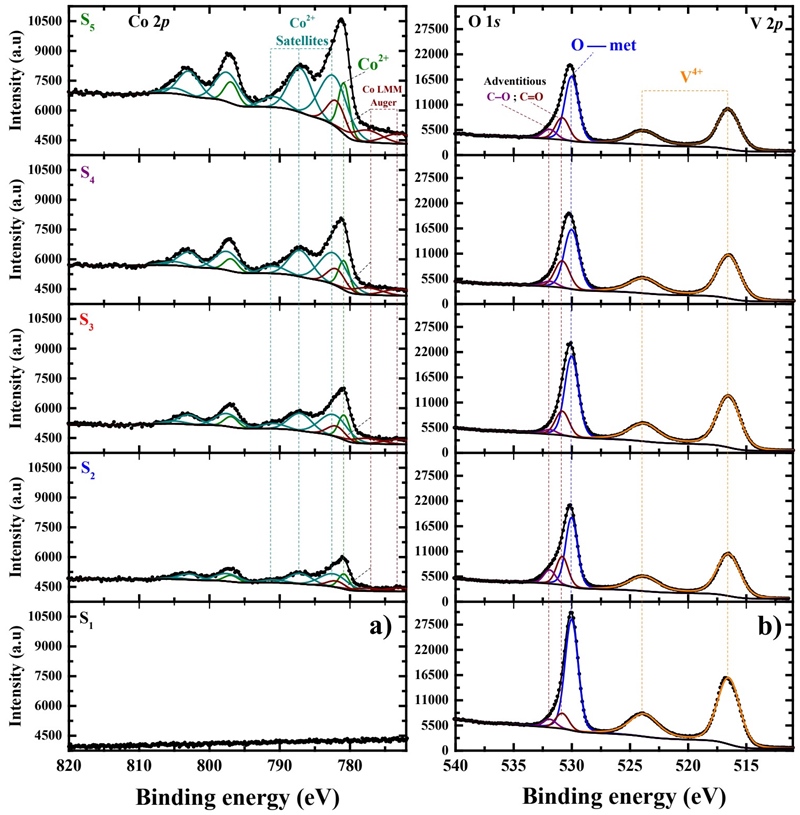
To assess the chemical state of the VOx:Co2+ film samples, an accurate peak-fitting analysis of their X-ray photoelectron spectra (XPS) (A. Herera-Gomez, no date) was carried on. Figure 5 shows the photoelectron spectra of the five samples corresponding to the (a) Co 2p and (b) O 1s and V 2p core-levels. From the Co 2p spectra (Figure 5a) we observe that the experimental data are congruent to that of the Co2+ chemical state, showing the main peak centered at 780.80 eV, but at the same time, three satellite peaks at higher binding energies that are characteristics of the Co2+ state (Yang et al., 2010; Biesinger et al., 2011; Martínez-Gil et al., 2020). It is important to mention that the relative intensities of those satellite peaks suggest that there are neighboring atoms arranged in a short-range order, possibly having distortions that deviate from the octahedral Co2+ symmetry. That is, the intensities of the satellite peaks are proportional to the molecular defects or amorphous state of the material, which correlates to the amorphous XRD results for all samples.
The V 2p and the O 1s spectra share the same binding energy range (Figure 5b), where we can observe the sole presence of a vanadium oxide. Due to the large spread of the reported binding energy values of this compound, to assess the present films we have employed the method proposed by Hryha et al. (Hryha, Rutqvist and Nyborg, 2012), where instead of using only the energy position of the V 2p 3/2 for chemical state determination, we have taken into account the difference in the binding energy between the O 1s and the V 2p 3/2 core levels (Δ= BE(O1s) - BE(V2p 3/2 )). The results show that for all samples the energy separation is around 13.50 eV, which suggests that the oxide obtained with our chemical process is VO2 (Hryha, Rutqvist and Nyborg, 2012). Here it is interesting to note that the energy position of the V 2p 3/2 in our samples is at 516.50 eV, which is almost the average value of those reported for VO2 (515.95 eV) and V2O5 (517.20 eV), however, due to also the large spread in binding energy calibrations in the literature, we discard the possibility of the V2O5 compound.
From peak intensities directly derived from the photoelectron spectra, chemical compositions of our VO2:Co2+ film samples have been determined. We have corrected the peak intensities with the appropriate physical parameters accounting for the photoemission signal attenuation due to scattering (Cabrera-German, Gomez-Sosa and Herrera-Gomez, 2016). The results expressed in terms of atomic percentage for each of the observed chemical species are presented in Figure 6a. Here, we observe that as the Co2+concentration in the precursor solution increases, the amount of Co2+ in the deposited films also increases, from 0 to 16.55 at %. On the other hand, the V4+ atomic concentration in all five samples vary slightly, from 31 at % (sample S 1 ) to 27 at % (sample S 5 ), which suggests that the VO x yield is high in all the probed samples. We can also observe that the percentage of oxygen atoms bonded to metals is coherent to the chemical species: VO2 and CoO, reaching 69 at % for sample S1 and decreasing up to 56.4 at % for sample S5 as the Co2+ in the films is the largest. The atomic ratio of vanadium versus cobalt (V:Co) in each sample is listed in Table 1, changing from 6.6:1 (S 2 ), 4.9:1 (S 3 ), 2.9:1 (S 4 ) to 1.6:1 (S 5 ).
For a better assessment of metal oxide compositions in the film samples, the ratio between oxygen and metal concentrations is plotted in Figure 6b. Here, the O/V atomic ratio lies around the expected stoichiometry of VO2 for all the samples, which further confirms our previous analysis. Yet, if we determine the atomic ratio of oxygen to the sum of the two metallic species, O/(V+Co), we observe that such ratio decreases with increasing concentration of Co2+ in the reaction solution, a trend that follows the expected stoichiometry of the CoO compound.
Conclusions
We demonstrate that amorphous thin films of cobalt doped vanadium dioxide can be prepared from spin-coating with a simple solution method without using any ligand or surfactant. Optical band gaps of those films are of ∼2.3 eV, with higher absorption coefficients for cobalt doped samples. From detailed quantitative photoelectron analysis, we conclude that there is only one oxidation state of vanadium in all the film samples, V4+, giving predominantly VO2 products. The formation of cobalt oxide is also confirmed by XPS, showing a Co2+ oxidation state corresponding to possibly disordered oxygen atoms in an octahedral, correlates to the amorphous nature of the films. Furthermore, the surface morphology of the VO2:Co2+ thin films is largely influenced by the cobalt concentration, giving interconnected circle pore network when the V:Co atomic ratio is around 4.9:1. The wide band gap properties with porous surface structure make these semiconductor thin films promising for cathode material in lithium battery applications.











 text new page (beta)
text new page (beta)