Introduction
Human milk (HM) is a biologically active food that contains high-biological value compounds and immunological constituents, that play a vital role in the modulation of the immune system in newborns and particularly in premature infants. For this, human milk banks (HMB) promote, protect, and support breastfeeding. Besides, these institutions are responsible for collection, conservation, and distribution of milk to feed premature infants and newborns with nutritional disorders (Lowry et al., 1951; Slutzah et al., 2010; Solís-Pacheco et al., 2019). The high availability of nutrients in fresh HM provides an ideal culture media for several microbial groups, such as Staphylococcus aureus, one of the most important bacteria that can contaminate HM from origin, due to mastitis by infection (Koenig et al., 2005; Mediano et al., 2017). Besides, secondary contamination can occur due to unsatisfactory hygienic and sanitary handling conditions during extraction and storage (Mediano et al., 2017). To control HM microbial populations, HMB implements several food conservation techniques, with refrigeration and pasteurization as the most widely studied methods (Koenig et al., 2005; Novak and Cordeiro, 2007; Slutzah et al., 2010; Wesolowska et al., 2019). In line with this, HM can be stored at refrigeration temperature (4 °C) for up to 96 h, without compromising its nutritional value and microbiological safety (Slutzah et al., 2010). Instead, pasteurization is the most common technique for food conservation. Microbial inactivation by this process is based on the maintenance of food temperature at 62.5 °C for 30 min and rapid cooling at 5 °C. Pasteurization inactivates vegetative bacteria and most viruses, including human immunodeficiency virus, herpes, and cytomegalovirus (Cavazos-Garduño et al., 2016; Wesolowska et al., 2019). After pasteurization, HM should be stored in airtight containers and frozen at -18 ºC for preservation for four months. However, during this period, the nutrients of the milk are not guaranteed (Wesolowska et al., 2019).
Some innovative technologies have been proposed to extend HM shelf-life preserving its nutritional value, including high-temperature short-time pasteurization, high hydrostatic pressure (HHP) processing, and microwave irradiation (Wesolowska et al., 2019). However, once the HM microbial load has been inactivated, HM handling is still complicated due to the high volume required for storage. Recently, the spray-drying process demonstrated to be an alternative for reducing HM volume while maintaining more than 95 % of the macronutrients (Solís-Pacheco et al., 2019). During this process, polysaccharides, proteins, and fiber can be added as wall materials, preventing volatilization and protecting encapsulated material against environmental conditions (Afoakwah et al., 2012). In addition, the use of prebiotic fibers in the HM drying process could act as a source of oligosaccharides for newborns, who cannot obtain them directly from the mother’s diet. The value of breastmilk oligosaccharides and dietary fibers, in complementary nutrition for the development of the infant’s microbiome with both short- and long-term health complications, has been lately highlighted (Çavdar et al., 2019). NUTRAFLORA® is a synthetic short-chain fructooligosaccharide, made by enzymatic reaction with sucrose. This prebiotic fiber is low in calories (1.5 kcal/g) and viscosity, and has stability at temperatures used during the High-Temperature Short-Time process (> 169 ºC). Furthermore, is easy to dry and extrude, and does not participate in Maillard reactions. Its high solubility makes NUTRAFLORA® ideal for dry or liquid formulations (Ingredion, 2016). Hence, this study aimed to evaluate i) the effect of HHP pretreatment and the spray-drying process on the preservation of the macronutrients of HM added with prebiotic fiber, ii) the inhibition of microorganisms naturally present in HM, and iii) the reduction of S. aureus artificially inoculated in HM.
Materials and methods
Biological material and ethical considerations
Hospital Civil of Guadalajara “Fray Antonio Alcalde” provide frozen samples from its HMB. The samples were transported in a cooler to the Laboratorio de Microbiología at Instituto Tecnológico de Tepic for analyses. The Ethical Research Committee approved this study on October 2019 No. HCG/CEI-1225/17.
Inoculation of S. aureus in HM
S. aureus ATCC 25923 was provided by the Laboratorio de Microbiología Industrial, Universidad de Guadalajara. The stock cultures were kept at -80 °C in tryptic soy broth (TSB; Becton Dickinson Bioxon, Le Pont de Claix, France) with 15 % (v/v) glycerol. Before experiments, 25 μL of stock cultures were transferred to 3 mL TSB and incubated at 35 °C for 18 h, then 25 μL were added to new TSB and incubated at 35 °C under static conditions for 18 h, to yield a final concentration of 106 CFU/mL of microorganisms at stationary phase. Bacterial cells were harvested by centrifugation (9390 × g for 5 min), washed twice in sterile saline solution [SS, 0.85 % (w/v), sodium chloride at pH 7.0 ± 0.2], and centrifuged under the same conditions. Then, the bacterial cells were resuspended in the raw HM to a final concentration of 106 CFU/mL (Bulut and Karatzas, 2021).
HM conservation process
For the HHP process, HM was thawed and homogenized. Then 250 mL of non- and inoculated-samples with S. aureus were vacuum-sealed in sterile FoodSaver® plastic bags (Newell Brands, Hoboken, NJ, USA) and pressurized at 300 MPa (Avure Autoclave Systems, Model LCIP402260NCEP1MLN, Eri, PA, USA) under different conditions (Table 1). HM was dried using a Mini Spray Dryer B-290 (Büchi, Flawil, Switzerland) with an inlet temperature of 165 °C and an air outlet temperature of 110 °C. The milk was fed with a 7 mm-diameter nozzle at a constant flow rate (2 mL/min); during the whole process, the sample was stirred at 100 rpm at 25 °C. Before drying, the spray dryer was stabilized with sterile distilled water (SDW) under the required operating conditions for 10 min. Soluble fiber (NUTRAFLORA® P-95 / L95-S, Ingredion, Ciudad de México, Mexico) was used as wall material at 5 % (w/v). The process yield was estimated (Eq. 1). Three replicates were used for the test and the experiment was repeated twice.
Where: W DS is the weight of dried milk and W SF is the weight of solids in raw milk (Solís-Pacheco et al., 2019). The powder was stored in PET/BOPP/PE zipper bags at 25 ± 2 °C.
Proximal analyses of HM and HM powder
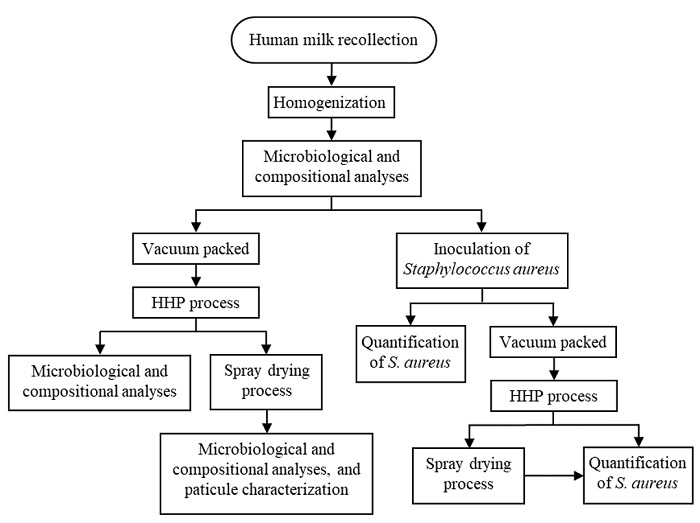
Total protein was estimated by the method of Lowry et al. (1951), lipid content was determined by the gravimetric method described by Folch et al. (1957), and lactose concentration was obtained with Anthrone reagent following the method of Dubois et al. (1956). Protein and lactose quantification were carried out using calibration curves with casein at 595 nm and lactose at 490 nm; respectively. On the other hand, ashes were estimated by drying the samples in an oven at 240 °C for 24 h and the content was expressed as a percentage (Sögüt et al., 2013). Each determination was carried out in triplicate and repeated once. The values were compared with the data obtained from the tests carried out using fresh HM. The methodological strategy realized in this study is represented in Figure 1.
Microbiological analyses in HM and milk poder
Quantification of mesophilic aerobic bacteria, total coliforms, lactic acid bacteria, S. aureus, molds, and yeasts was carried out in raw milk according to Mexican standards (NOM-111-SSA1-1994, NOM-113-SSA1-1994, NOM-115-SSA1-1994, NOM-184-SSA1-2002). Microbial counts were carried out on Petri dishes with the corresponding culture media for each microbial group and incubated under different conditions. For yeast and molds, potato dextrose agar (PDA, Bioxon, Becton Dickinson and Company, Queretaro, Mexico) was used and the plates were incubated for 3 and 5 days at 25 °C. The culture media used for aerobic bacteria (tryptic soy agar, TSA; Becton Dickinson, Le Pont de Claix, France), total coliforms (Red violet bile agar, Merck, KGaA, Darmstadt, Germany), lactic acid bacteria (MRS agar, Oxoid, Basingstoke, UK), and S. aureus [Baird-Parker agar base (Difco, MI, USA) supplemented with egg yolk and 100 pg/mL potassium tellurite] were incubated at 37 °C for 24-48 h. Lactic acid bacteria were incubated under anaerobic conditions. Besides, after each conservation process yeast, molds, lactic acid bacteria, and coliforms were estimated as previously described in non-inoculated samples. Whereas, S. aureus was quantified in un- and treated inoculated samples. For dried milk, rehydration was carried out considering the moisture content of each sample. The logarithmic microbial reduction was calculated (Eq. 2).
where N 0 is the initial count and N is the survival count after treatment (Coroller et al., 2006). Each test was carried out in triplicate and repeated once.
Moisture content (MC) of spray-dried milk
The MC was gravimetrically determined (da Silva Carvalho et al., 2016). For this, 2 g of sample were used and the thermobalance (Sartorius MA 35, Göttingen, Germany) was operated at 105 °C, the test was finished until weights remained constant. The test was carried out in triplicate and repeated twice.
Water activity (aw)
The measurement was made by using 5 g of the sample in the hygroscopic Aqualab 4TEV (METER Group, Inc., Pullman, WA, USA). Calibration was carried out with a sodium chloride solution at 0.75 aw. The test was carried out in triplicate and repeated twice.
Powder milk solubility
The test was carried out according to the protocol proposed by Cano-Chauca et al. (2005). Briefly, 1 g of the powder was added to 100 mL of distilled water and solubilized using a Vortex mixer (Genie 2, Daigger, Wheaton, IL, USA) at 3200 rpm for 5 min. The sample was centrifuged (Hermle Z326K, Wehingen, Germany) for 5 min at 3000 × g, then 25 mL of the supernatant were transferred to pre-weighed Petri dishes and immediately oven-dried at 105 °C for 5 h. The solubility percentage was calculated by weight difference. The test was carried out in triplicate and repeated twice.
Statistical analyses
Factorial designs were carried out. The data were subjected to analysis of variance, then; the post-hoc least significant difference (LSD) Fisher test (p ≤ 0.05) was used for means comparison. Analyses were performed by using Statgraphics Centurion XVI.I software (Statpoint Technologies, Inc., Warrenton, USA).
Results and discussion
Microbiological quality of HM
HM is an ideal medium for the growth of microorganisms due to the high availability of nutrients. Some microorganisms, such as S. aureus, E. coli, and Salmonella may contaminate HM due to inadequate procedures during its extraction and/or storage (Mediano et al., 2017; Novak et al., 2008). In this study, the HM from the Bank of Hospital Civil de Guadalajara showed low levels of aerobic mesophilic bacteria (2.48 ± 0.25 Log10 CFU/mL), S. aureus (2.21 ± 0.17 Log10 CFU/mL), and lactic acid bacteria (1.31 ± 0.44 Log10 CFU/mL). Whereas coliforms, molds, and yeast were not detected by the traditional counting plate. The initial counts of all tested microorganisms are below the established limits for pasteurized milk or infant formula (‘NOM-131-SSA1-2012, Products and services. Formulas for infants, continuation, and special nutritional needs. Food and non-alcoholic beverages for infants and young children. Provisions and specifications’, 2012; ‘NOM-184-SSA1-2002, Products, and Services. Milk, Milk Formula, and Milk Product Combined. Sanitary specifications’, 2012; ‘NOM-243-SSA1-2010, Milk, milk formula, combined milk product, and milk derivatives. Provisions and sanitary specifications. test methods’, 2010) considering that the samples were still untreated. These results are in concordance with previous reports of fresh milk obtained from the same HMB (Cavazos-Garduño et al., 2016; Solís-Pacheco et al., 2019; Aguilar-Uscanga et al., 2021). This fact allows us to infer the establishment and compliance of good practices for the obtention and handling of HM by the mentioned HMB. This is of vital importance to maintain microbiological quality and nutritional value, and to avoid infections in newborns via the presence of microorganisms in the milk (Hartmann et al., 2007). However, the microbial load in HM can be higher than that obtained in this research due to some infections, such as mastitis. In HM samples from women with mastitis, the mean microbial load was 4.11 Log10 CFU/mL. The staphylococcal group is the most frequently isolated (97.57 %), with Staphylococcus epidermidis (91.56 %) and S. aureus (29.74 %) as the most common species isolated from mastitis samples (Mediano et al., 2017). Inadequate sanitation and handling during milk extraction result in its contamination with E. coli and Salmonella, while S. aureus contamination of HM occurs in breastfeeding women as a result of clinical mastitis (Kaavya et al., 2021; Mediano et al., 2017). In addition, S. aureus is present in the oropharynx and skin of humans, and its presence in HM may be due to secondary contamination or to unsatisfactory hygienic and sanitary conditions of the extraction apparatus used (Mediano et al., 2017).
Proximal analysis of HM
The initial estimated values of lactose, lipids, proteins, and ashes in raw HM were 7.36 ± 0.94, 2.46 ± 0.26, 1.65 ± 0.59 g/dL, and 0.26 ± 0.02 %; respectively. These results are in concordance with previous reports for Mexican fresh HM (Cavazos-Garduño et al., 2016; Solís-Pacheco et al., 2019) and with the generally assumed HM composition (Boquien, 2018). After the HHP processing, the assessed components were maintained at more than 95 % (Table 2). This behavior agrees with the results of Solís-Pacheco et al. (2019) for spray-dried HM under the same conditions. However, after the spray-drying process lactose showed a significant reduction in comparison to raw milk (p ≤ 0.05), but without change in comparison to unpressurized dry HM (p > 0.05). During the spray-drying process, chemical and physical changes such as crystallization and nonenzymatic browning as the Maillard reactions can occur, reducing the lactose concentration (Kinsella and Morr, 1984; Uscanga et al., 2021). Besides, the concentration of macronutrients in HM can be influenced by various processes, such as storage, freezing, and thawing. It has been reported that freezing, thawing, and spray-drying processes can decrease the lipid content in HM by up to 9.0 %, attributable to its adherence to the wall of container and equipment, incomplete homogenization, lipolysis, or lipid peroxidation (Cavazos-Garduño et al., 2016; Chang et al., 2012). In this research, the variation in lipid content after HHP and spray-drying were not significant (p ≤ 0.05; Table 3). The increment in ashes content in the HM powder was attributed to the use of soluble fiber as wall material, which can contain between 1.60 and 2.30 % of ashes depending on the source and variety (Ragaee et al., 2012).
Tabla 2: Efecto de alta presión hidrostática en la composición de la leche humana.
| Treatment | Lactose (g/dL) | Lipids (g/dL) | Proteins (g/dL) | Ashes (%) | Humidity (%) |
| 1 | 6.71 ± 0.69a | 2.89 ± 0.17a | 1.49 ± 0.10a | 0.27 ± 0.03a | 86.19 ± 0.50a |
| 2 | 7.42 ± 0.06a | 2.44 ± 0.84a | 1.37 ± 0.64a | 0.20 ± 0.05a | 86.13 ± 0.07a |
| 3 | 6.98 ± 0.63a | 1.90 ± 0.70a | 1.44 ± 0.22a | 0.31 ± 0.06a | 86.81 ± 0.68a |
| 4 | 6.85 ± 0.60a | 2.34 ± 0.14a | 1.39 ± 0.43a | 0.30 ± 0.09a | 86.37 ± 0.34a |
Values are expressed as mean ± standard deviation (n = 6). Parameters of raw human milk: lactose 7.36 ± 0.94 g/dL; lipids 2.46 ± 0.46 g/dL; proteins 1.65 ± 0.59 g/dL; and ashes 0.26 ± 0.02 %. Values in the same column followed by different letters are significantly different according to Fisher’s LSD test at p ≤ 0.05.
Tabla 3: Efecto del secado por aspersión en la composición de la leche humana.
| Treatment | Lactose ( g/dL ) | Lipids ( g/dL ) | Proteins ( g/dL ) | Ashes (%) |
| Control | 6.25 ± 0.75ab | 2.32 ± 0.52a | 2.01 ± 0.63a | 1.50 ± 0.29a* |
| 1 | 6.99 ± 0.18a | 2.23 ± 0.11a | 1.83 ± 1.00a | 1.32 ± 0.07a* |
| 2 | 5.88 ± 0.39b* | 2.18 ± 0.41a | 1.32 ± 0.82a | 1.49 ± 0.11a* |
| 3 | 6.83 ± 0.39a | 2.06 ± 0.24a | 1.73 ± 0.37a | 1.57 ± 0.32a* |
| 4 | 5.74 ± 0.13b* | 1.95 ± 0.17a | 1.30 ± 0.30a | 1.34 ± 0.05a* |
Values are expressed as mean ± standard deviation (n = 6). Parameters of raw human milk: lactose 7.36 ± 0.94 g/dL; lipids 2.46 ± 0.46 g/dL; proteins 1.65 ± 0.59 g/dL; and ashes 0.26 ± 0.02 %. Control: raw or unpressurized dried milk. *: Indicates a significant difference in comparison with the initial value in raw milk. Values in the same column followed by different letters are significantly different according to Fisher’s LSD test at p ≤ 0.05.
Microbial reduction and inactivation of S. aureus by conservation process
Microbial inactivation by HHP depends on several factors including treatment conditions (surrounding media, temperature, pressure, time, etc.) and microbial characteristics (vegetive or spore cells, composition of the cell wall, physiological state, and so on) (Kaavya et al., 2021; Salleh-Mack and Roberts, 2007). The lethal effect of treatments on bacterial cells increased as a temperature function (p ≤ 0.05; Table 4). This is in agreement with a previous report on the inactivation of S. aureus and Bacillus cereus spores in HM after 4 cycles of 5 min at 38 °C and 350 MPa (Demazeau et al., 2018). In line with this, it has been demonstrated that S. aureus inactivation in cow’s whole milk at 20 °C occurs at >250 MPa and 8 min under these conditions, a decimal reduction after 3.7 min at 300 MPa was reported (Erkmen and Karataş, 1997). The composition of the cell wall and shape of the bacteria are related to their resistance. Spherical organisms ought to be more resistant to crushing than those rod-shaped ones, being consistent with the idea that the lethal action of pressure changes that accompany their passage through liquids (Jacobs and Thornley, 1954). The HHP disrupts the bacterial membrane, being Gram-positive more resistant than Gram-negative bacteria (Costello et al., 2021; Huu et al., 2021; Repine et al., 1981). This is related to the different peptidoglycan content, which is higher in Gram-positive (30-70 %) than in Gram-negative (< 10 %) bacteria, making them more resistant to pressure (Schumann, 2011). Microorganisms can resist high pressures, due to membrane fluidity. However, the synergistic effect of the temperature contributes to the destabilization of the outer and inner membrane, releasing intracellular components (Huu et al., 2021; Kaavya et al., 2021; Salleh-Mack and Roberts, 2007).
Table 4 Reduction of Staphylococcus aureus after the conservation process.Tala 4. Reducción de Staphylococcus aureus después de los tratamientos de conservación.
Tabla 4: Color of squash fruit peel (Cucurbita pepo L.) var. ‘Grey Zucchini’s.
HHP: High hydrostatic pressure. Values are expressed as mean ± standard deviation (n = 6). Initial load 6.98 ± 0.23 CFU/mL. Values in the same column followed by different letters are significantly different according to Fisher’s LSD test at p ≤ 0.05. ND: the microorganism was not detected after the treatment.
Spray drying is a technique widely used for milk powder production. This process is not aimed to cause microbial inactivation (Alvarenga et al., 2018). However, it showed a synergistic effect in pressurized HM samples contributing to the complete inactivation of S. aureus during the drying process, because cells suffered previous damage by the HHP (Bulut and Karatzas, 2021) (Table 4). Temperature can have a cumulative or synergistic effect (depending on the environmental conditions, particularly the media) on the HHP treatments, favoring microbial reduction (Zenker et al., 2003; Zhang et al., 2020). According to Calvoa et al. (2018), human milk should be discarded after a pasteurization process, when it has a total microbiological content equal to or greater than 10 CFU/mL, in our case a higher number of bacteria was not found in the human milk samples, after high-pressure processes and spray drying.
Properties of HM powder
The MC of the powders ranged from 2.08 to 2.88 % (Table 5), whereas the microparticles aw showed values between 0.15 and 0.33. These results agree with the previous report of spray-dried HM without wall materials for which the MC and aw estimated were 1.7± 0.74 % and 0.21± 0.15, respectively (Solís-Pacheco et al., 2019). MC and aw values lower than 5 % and 0.6, respectively, are optimal to reduce microbiological spoilage and prevent lipid oxidation (Sun et al., 2020).
Tabla 5: Color of squash fruit peel (Cucurbita pepo L.) var. ‘Grey Zucchini’s.
| Treatment | Moisture content (%) | Water activity | Solubility (%) | Yield (%) |
| Control | 2.71 ± 0.79a | 0.33 ± 0.14a | 99.1 ± 0.04a | 97.02 ± 0.13a |
| 1 | 2.39 ± 0.35a | 0.21 ± 0.04a | 99.0 ± 0.02a | 91.73 ± 1.74b |
| 2 | 2.88 ± 0.49a | 0.20 ± 0.10ª | 99.0 ± 0.05a | 97.02 ± 0.09a |
| 3 | 2.08 ± 0.38a | 0.15 ± 0.05a | 99.4 ± 0.02a | 88.66 ± 1.98b |
| 4 | 2.69 ± 0.89a | 0.18 ± 0.01a | 99.2 ± 0.03a | 92.24 ± 2.21b |
Control: raw or unpressurized dried milk. Values are expressed as mean ± standard deviation (n = 6). Values in the same column followed by different letters are significantly different according to Fisher’s LSD test at p ≤ 0.05.
On the other hand, the HM powder solubility was up to 99 %, demonstrating that the macronutrients in HM did not developed insoluble complexes with the fiber, without affecting their availability. This parameter rarely has been assessed for HM, however, is widely studied in dried milk from other species. For dromedary and cow’s milk, the estimated solubility ranged from 91-95 % and 78-88 %; respectively, at an inlet temperature of 230 °C during the drying process (Felfoul et al., 2022). Otherwise, for dried camel milk the solubility was in function of the inlet temperature, being higher at 140 °C than at 200 °C (Habtegebriel et al., 2018). Therefore, the difference between this study and other reports is related to the specie, and the drying processing parameters, due to the milk powder obtained at a less severe processing temperature, the powder retains higher solubility of milk proteins (Habtegebriel et al., 2018).
The yield of the spray-drying process for HM added with soluble fiber was up to 99 %. This result is higher than the obtained for groundnut (82.72 %, inlet temperature 186 °C) (Saha et al., 2019), dromedary and cow milk (94.99 %, inlet temperature 230 °C, and 95.54 %, inlet temperature 230 °C, respectively) (Felfoul et al., 2022). The yield of the spray-drying process is strongly dependent on inlet temperature. Generally is assumed that the use of high inlet temperatures (180-230 °C) provides a higher yield (Felfoul et al., 2022; Saha et al., 2019). However, this is not the only important variable in the yield of dried milk. For camel milk, the total solids recovery in the cyclone increased by an increment of the inlet temperature and airflow rate, whereas decreased with an increase in the total solids content of milk (Habtegebriel et al., 2018). An increment in the total solids of the milk is related to a viscosity increment, difficulty feeding, and aspersion rate resulting in droplets sticking with the drying chamber (Habtegebriel et al., 2018; Langrish et al., 2006). Otherwise, the high yield obtained in this research could be related to the use of soluble fiber, which, additionally, can contribute to macronutrient maintenance. In milk, the concentration of macronutrients in HM can be influenced by various processes, such as storage, freezing, thawing, and spray-drying processes. These techniques can decrease the lipid content in HM by up to 9.0 %, attributable to its adherence to the wall of the dryer chamber and equipment, faults homogenization, lipolysis, or lipid peroxidation (Cavazos-Garduño et al., 2016; Chang et al., 2012). Otherwise, aggregation of casein micelles produces variation in the protein concentration (Chang et al., 2012).
Conclusions
The combination of HHP and spray-drying technique is an alternative process that allows the maintenance of the value of lactose, lipids, proteins, and ashes in HM. These values were not affected by the use of each treatment alone or in combination. HHP (300 MPa) by itself reduced the microbial loads to undetectable levels, even for S. aureus artificially inoculated, if the process was carried out at 45 °C. However, the use of spray-drying after the HHP at 35 °C reduced the remanent microorganisms. Hence, to assure the HM safety the treatment recommendable would be HHP at 45 °C for 10 min followed by the spray-drying process. The transformation of an HM into a powder facilitates its storage and handling in an HMB. The low aw and MC values are of great importance as are related to food spoilage reduction in dried foods. Besides, the use of soluble fiber as wall material allowed us to obtain a high yield from the spray-drying process.











 text new page (beta)
text new page (beta)