Introduction
Coronavirus disease 2019 (COVID-19), caused by the SARS-CoV-2 virus, affects children and adults. Respiratory involvement is heterogeneous in children, with varying degrees of severity. The most severe respiratory manifestations are pneumonia and severe acute respiratory distress syndrome1. Thrombotic complications involving various organs have also been described in children and adults: venous thrombosis, pulmonary embolism (PE), or hemorrhagic events2,3.
Thromboembolism or PE in children is rare. The incidence is estimated at 2.1 cases per 100,000 pediatric emergency room visits. The most common risk factors are body mass index ≥ 25, women using contraceptives or previous episodes of thrombosis without PE4.
Adult patients with severe SARS-CoV-2 infection are at increased risk of thrombosis, with PE being the main complication5. For example, a multicenter study in France involving 150 adult intensive care unit (ICU) patients with acute respiratory distress syndrome due to COVID-19 reported that 25 (16.7%) patients developed PE5. Another study conducted with medical records of adults with COVID-19 from four hospitals in New York (United States) reported 29% incidence of PE in ICU patients and 24% outside the ICU6. In children, this complication has been poorly described. A study in the United States analyzed data from 693 pediatric patients hospitalized for COVID-19 and found that only eight (1.2%) presented PE, three were admitted to the ICU, one patient required mechanical ventilation, and none died7. The associated risk factors were obesity, oncologic pathology, recent surgery, and contraceptive use7. Another study in a hospital in Texas (United States) during the wave of the Delta variant of SARS-CoV-2 reported a frequency of 1.7% (nine out of 543 patients hospitalized for COVID-19) of PE, with obese adolescents being the most affected. In addition, none of the patients had a complete vaccination schedule8.
Based on the above, this study aimed to report an unusual case of PE in an adolescent with severe COVID-19 pneumonia, describe the clinical picture and management, and compare it with reports on children published in the literature.
Clinical case
We describe the case of a 15-year-old male adolescent with Down syndrome who went to the emergency department for odynophagia, demanding wet cough, fever, and general malaise of 9 days' evolution. He received ambulatory treatment with amoxicillin/clavulanic acid 50 mg/kg/day orally for 6 days without improvement. One day before hospital admission, the patient had respiratory distress. The patient had a history of hospitalizations for pneumonia at 2, 4, and 5 years of age, with no other pathological history. Seven months prior to his current condition, the patient's father had COVID-19. During physical examination on admission, the patient presented saturation of 88% with ambient oxygen, which improved to 98% with a nasal cannula at 4 L/min; heart rate: 110/min; respiratory rate: 38/min; temperature: 39°C; blood pressure: 100/70 mmHg; and body mass index: 32 kg/m2. On examination of the chest and lungs, the patient presented subcostal retractions and decreased vesicular murmur in the lower third of the right hemithorax with sub-crepitus in both lung bases. The antigen detection study for SARS-CoV-2 was positive, so the patient was transferred to the COVID contingency area.
Laboratory results showed elevated acute phase reactants: elevated C-reactive protein, ferritin, and lactate dehydrogenase (LDH). In addition, the coagulation profile showed hyperfibrinogenemia and elevated D-dimer (2.5-fold its normal value). Conversely, prothrombin time and activated partial thromboplastin time within normal ranges, and hemogram and differential count with no alterations (Table 1). Chest X-ray showed multifocal opacities with peribronchial and peripheral distribution with right predominance and right perihilar and paracardiac consolidation associated with small inferior consolidations (Figure 1). Due to the clinical picture and hypoxemia associated with laboratory and radiological findings, the patient was diagnosed with severe COVID-19 pneumonia plus bacterial coinfection. Treatment was initiated with intravenous ceftriaxone 80 mg/kg/day and dexamethasone 0.15 mg/kg/day.
Table 1 Laboratory findings on admission and during hospitalization
| Laboratory (reference values) | Hospital stay | ||||
|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 7 (PE diagnosis) | Day 15 (change from enoxaparin to rivaroxaban) | Day 37 (discharge) | |
| Hemoglobin (≥ 11.5 g/dL) | 17.5 | 15.6 | 15.8 | 15.0 | 14.0 |
| Hematocrit (≥ 34%) | 50.7 | 45.6 | 45.1 | 43.2 | 43.8 |
| Leukocytes (4.5-13.5×103/mm3) | 4.92 | 6.56 | 15.86 | 10.74 | 6.85 |
| Neutrophils (1.5-8.0×103/mm3) | 2.50 (51%) | 2.75 (42%) | 12.94 (80%) | 6.87 (64%) | 2.23 (32.6%) |
| Lymphocytes (1.5-6.0×103/mm3) | 2.06 (42%) | 3.15 (48%) | 1.36 (8.6%) | 2.42 (22.5%) | 3.53 (51.5%) |
| Platelets (150-350×103/mm3) | 155 | 162 | 149 | 289 | 305 |
| CRP (≤ 0.5 mg/dL) | 9.04 | 1.52 | 15.7 | 17.8 | 0.48 |
| Ferritin (7-140 ng/mL) | 1024 | 497 | 857 | 708 | - |
| ALT (0-39 U/L) | 46 | 57 | 56 | 110 | 51 |
| AST (0-47 U/L) | 50 | 35 | 51 | 34 | 26 |
| Urea (10-38 mg/dL) | 34 | 40 | 32 | 25 | 23 |
| Creatinine (0.7-1.1 mg/dL) | 0.98 | 0.89 | 0.83 | 0.82 | 0.97 |
| Serum Na+ (135-148 mmol/L) | 141 | 137 | 134 | 138 | 142 |
| LDH (230-460 U/L) | 976 | 734 | 834 | 884 | - |
| CPK-CK (24-195 U/L) | 236 | 44 | 449 | - | - |
| CK-MB (0-24 U/L) | 15 | 13 | 17 | 17 | - |
| PT (11.68-14.21 s) | 11.64 | 10.74 | 13.49 | 11.7 | 12.48 |
| aPTT (27.12-44.21 s) | 31.15 | 25.24 | 31.88 | 40.2 | 36.97 |
| Fibrinogen (160.16-369.42 mg/dL) | 760 | 537 | 662 | 842.4 | 359.58 |
| D-dimer (< 0.5 mg/L) | 1.25 | 1.48 | 24.97 | 3.5 | 2.58 |
ALT: alanine aminotransferase, aPTT: activated partial thromboplastin time, AST: aspartate aminotransferase, CPK-CK: creatine kinase, CPK-MB: creatine kinase MB, CRP: C-reactive protein, LDH: lactate dehydrogenase, PE: pulmonary embolism, PT: prothrombin time.
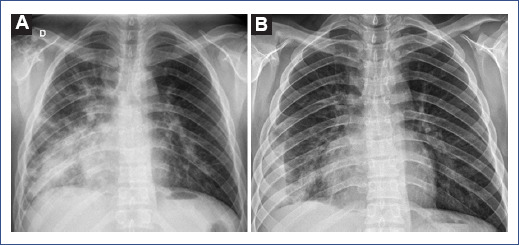
Figure 1 The chest radiograph shows multifocal opacities with peribronchial and peripheral distribution and right predominance. A: right perihilar and pericardiac consolidation associated with small inferior consolidations in the lateral pleural base. B: after treatment, decrease in opacities and right pleural effusion.
The patient initially had a favorable evolution. The fever resolved 36 h after admission, and there was a progressive decrease in oxygen requirement (1.5 L/min). Laboratory tests on day 3 of hospitalization showed a marked decrease in acute-phase reactants (Table 1). On day 7 of hospitalization, the patient referred chest pain at the right lower costal level, fever, and hemoptysis (± 100 cc) on two occasions associated with respiratory distress and oxygen requirement (2 L/min). Blood pressure was normal. Control tests showed leukocytosis with neutrophilia, mild lymphopenia, and increased C-reactive protein (CRP), ferritin, LDH, creatine kinase, and fibrinogen, and a marked increase in D-dimer (50-fold its baseline value) (Table 1). Based on respiratory deterioration, hemoptysis, elevated D-dimer, and acute phase reactants, PE was suspected. Pulmonary angiography showed a ground glass infiltrate in the right lung field and left lower lobe, extensive consolidation in the right lower lobe, and extensive thrombus in the right inferior lobar artery of 2.5 × 1.2 cm extending into the posterior branch. These results were consistent with the diagnosis of PE plus pneumonia reported in COVID-19 CO-RADS 5 (COVID-19 data and reporting system) (Figure 2). In addition, an electrocardiogram was performed to assess cardiac involvement, which showed a right bundle branch block and left anterior hemiblock; however, the echocardiogram did not show any alterations, and there was no clinical evidence of cardiac compromise. Anticoagulant treatment was initiated using low-molecular-weight heparin (enoxaparin 40 mg) subcutaneously every 12 h, followed by antibiotic coverage and systemic corticosteroid. In addition, immunological studies were also performed: anticardiolipin antibodies, anti-B2 glycoprotein, antinuclear antibodies, anti-neutrophil cytoplasm antibodies, and anti-DNA antibodies with negative results, however, lupus anticoagulant was positive.
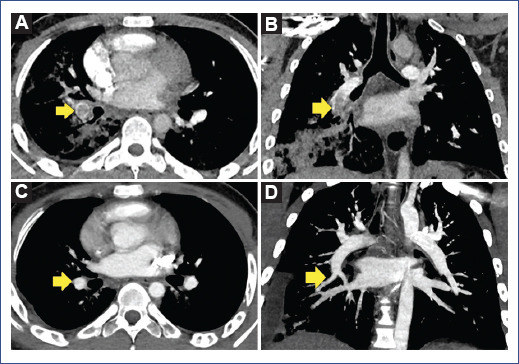
Figure 2 A and B: angiotomography showing an extensive acute thrombus in the right inferior lobar artery with extension to the apical and posterior branches associated with extensive consolidation in the right lower lobe. C and D: after treatment, the resolution of the thrombus and right pleural effusion associated with ipsilateral pulmonary basal consolidation was observed.
During his evolution, the patient did not present hemodynamic compromise and did not require ICU admission or mechanical ventilation. Therefore, supplemental oxygen was withdrawn on day 10 of hospitalization. On day 15, we switched from enoxaparin to oral anticoagulation with rivaroxaban 15 mg orally every 12 h for the first 3 weeks and then 20 mg every 24 h continuously for 3 months. Control CT pulmonary angiogram 1 month after diagnosis showed complete thrombus resolution. The patient's clinical, laboratory, and radiological evolution was favorable. At present, he continues to be monitored by the hematology, cardiology, and pneumology departments.
Discussion
Adult patients with acute respiratory distress syndrome secondary to COVID-19 significantly develop thrombotic complications and PE5. This condition has been rarely described in children. In these cases, it has occurred more frequently in those hospitalized for COVID-19 than other causes7. Recently, it has been reported in the pediatric population, mainly in those with severe respiratory complications7,9.
Our patient presented with severe COVID-19 pneumonia, and then abruptly developed increased respiratory distress with hemoptysis associated with elevated D-dimer. This clinical picture was similar to recent publications of children with this condition (Table 2). Most reports describe patients with a similar age as our case; however, a recent study during the SARS-CoV-2 delta wave in Texas (United States) reported ages < 15 years in five of nine children with PE. A 6-year-old girl was the youngest patient documented who presented PE with COVID-19 pneumonia8.
Table 2 Published cases of pulmonary embolism and COVID-19 in children and adolescents < 18 years of age (PubMed and Scopus updated to July 2022)
| Author (year) | Sex | Age (years) | Duration of illness (days) | Associated diseases/comorbidities | Main symptoms | SARS-CoV-2 infection | Anticoagulant treatment |
|---|---|---|---|---|---|---|---|
| Martinelli et al. (2020)24 | Female | 17 | 3 | COVID-19 pneumonia; 29 weeks' gestation; and obesity | Fever and dyspnea | Antigen Test (+) | Enoxaparin |
| Visveswaran et al. (2020)14 | Female | 12 | 5 | Antiphospholipid syndrome | Increased volume and pain in left leg | IgM (+) | Mechanical thrombectomy, tissue plasminogen activator, heparin, and enoxaparin |
| Odièvre et al. (2020)25 | Female | 16 | 7 | COVID-19 pneumonia and sickle cell disease | Fever, shortness of breath, and chest pain. | RT-PCR (+) | Not specified |
| Anastas et al. (2021)10 | Female | 15 | 3 | MIS-C; asthma; and obesity | Dyspnea and syncope. | IgM (+) | Tissue plasminogen activator and enoxaparin |
| Cristoforo et al. (2021)23 | Male | 11 | 3 | Nephrotic syndrome; obesity; and COVID-19 pneumonia (8 weeks before event) | Fatigue, shortness of breath, vomiting, and edema in extremities. | - | Tissue plasminogen activator, heparin, and warfarin |
| Ouarradi et al. (2021)26 | Female | 14 | 3 weeks | Obesity | Wet cough, dyspnea, and chest pain. | IgG (+) | Non-fractionated heparin, vitamin K, and aspirin |
| Panjabi et al. (2021)27 | Female | 15 | 1 h | SARS-CoV-2 infection; obesity; and use of OCPs (3 months, but discontinued 1 month before admission) | Chest pain and respiratory distress. | Antigen Test (+) | Heparin infusion and enoxaparin |
| Female | 16 | 2 weeks | COVID-19 pneumonia; obesity; and diabetes | Cough, respiratory distress, and syncope. | IgG (+) | Pulmonary embolectomy, heparin, and apixaban | |
| Kotula et al. (2021)12 | Female | 15 | 3 | Suspicion of MIS-C; obesity; asthma; and appendectomy 3 days before admission. | Respiratory distress and syncope | IgM (+) | Tissue plasminogen activator and heparin |
| Bin Ali et al. (2021)13 | Female | 12 | 1 week | COVID-19 pneumonia | Wet cough and chest pain | RT - PCR (+) | Enoxaparin |
| Mitchell et al. (2021)9 | Female | 8 | - | COVID-19 pneumonia | - | - | Heparin |
| Male | 11 | - | COVID-19 pneumonia; genetic syndrome | - | - | Enoxaparin | |
| Ioannidou et al. (2022)15 | Male | 15 | 4 | Low height (received anastrozole) | Hip pain, right knee edema and respiratory distress. | RT-PCR (+) | Low-molecular-weight heparin (tinzaparin) and warfarin |
| Kavthekar et al. (2022)11 | Male | 16 | 3 | MIS-C; Guillain–Barré syndrome | Fever and weakness in extremities | IgG (+) | Heparin |
COVID-19: coronavirus disease 2019, IgG: immunoglobulin G, MIS-C: multisystem inflammatory syndrome in children, RT-PCR: reverse transcription-polymerase chain reaction.
Body mass index ≥ 25 has been described as a risk factor4 and is a frequent feature observed in the published cases (Table 2). COVID-19-related PE in children is an uncommon but potentially life-threatening finding, so these patients require ICU monitoring and mechanical ventilation, especially those with multisystem inflammatory syndrome (MIS-C)10-12 or bilateral pulmonary involvement12,13. Our patient had no evidence of hemodynamic compromise; however, he was under continuous monitoring and constant medical evaluation. In contrast to other publications8,14,15, no evidence of venous thrombosis was found, so an ultrasound of the extremities was not performed.
Among the pulmonary histopathological changes found in patients with SARS-CoV-2, organized fibrin in most of the intra-alveolar foci and epithelial lesions in alveoli and blood vessels have been described16. These changes could explain the prothrombotic state of the patients with COVID-19 pulmonary involvement. The development of PE is the result of a prothrombotic state caused by the storm of cytokines derived from sepsis, which in turn produces inflammation of the pulmonary vessels leading to thrombosis when combined with the viral factor that interferes with the coagulation cascade17.
In patients with Down syndrome, prothrombotic states are rare; however, factor V Leiden mutations, methylenetetrahydrofolate reductase deficiency, factor VII deficiency, and some physiological conditions that promote venous stasis such as decreased muscle strength in the extremities may predispose to this condition18,19.
Prophylactic anticoagulation was not considered in our patient since D-dimer was < 5-fold the upper limit and because the evolution had been favorable with antibiotic and corticosteroid treatment, although some consensus guidelines recommend this measure, especially in patients with obesity20.
PE management should be performed according to high, intermediate, or low-risk stratification21. Although our patient was hemodynamically stable; and his echocardiogram was normal and we were not able to assess cardiac involvement better due to the lack of troponin or B-type natriuretic peptide levels to perform these tests at our institution. Consequently, the case was considered an intermediate-risk case due to the need for supplemental oxygen and COVID-19 pneumonia. Therefore, we initiated anticoagulant therapy with low-molecular-weight heparin, followed by oral anticoagulant therapy. Oral anticoagulants are the first line of treatment in most patients21 as they reduce the risk of recurrent venous thrombosis, similar to vitamin K antagonists, and the risk of bleeding is low, thus continuous monitoring is unnecessary.22. Anticoagulant therapy should last at least 3 months, with monthly check-ups depending on clinical status and right ventricular involvement21. In other cases reported7-10,12-14,23-27 (Table 2), the most frequent treatments were unfractionated heparin, enoxaparin, and some oral anticoagulants such as apixaban7,27. In this case, rivaroxaban was chosen due to its oral administration, accessibility, and adequate adherence to treatment.
During follow-up, our patient presented tomographic evidence of PE resolution at 1 month of treatment. However, due to the risk of recurrence of thrombosis, treatment was continued for up to 3 months. He is currently free of dyspnea and functional limitation and continues to undergo follow-up examinations.
In patients with a first episode of PE and no predisposing risk factors such as cancer, previous surgery, or any associated coagulation disorder, as in this case, the risk of recurrence of venous thrombosis and fatal PE at one-year follow-up is 10% and 0.4%, respectively, and, and 1.5% and 36% at 10 years, being higher in males28.
For economic reasons and due to the clinical improvement of the patient after therapy, it was not possible to perform the study of thrombophilias such as screening for protein C and S, antithrombin III, Leyden factor V mutation, or genetic disorders of hypercoagulability as in other reports10,27. Only the lupus anticoagulant was positive, possibly induced by the viral infectious process since it is considered a factor that contributes to thrombosis in patients with COVID-195.
In conclusion, PE associated with severe COVID-19 has been scarcely reported in children. However, it can have a favorable course with timely diagnosis and management. It is important to perform more studies to have recommendations for effective and safe anticoagulation in children with COVID-19 coagulopathy since most of them are extrapolated from the adult population or are based on expert recommendations20.
Furthermore, scales to stratify the risk of coagulopathies associated with COVID-19 in the pediatric population should be established, especially while the pandemic continues.
The present study was approved by the ethics committee of the Instituto Nacional de Salud del Niño-Breña (Lima, Peru) (N° 066-2022-CIEI-INSN).











 text new page (beta)
text new page (beta)


