Introduction
Few data exist on the incidence of spontaneous ventricular tachycardia (VT) in a random pediatric population. The prevalence of VT detected in asymptomatic children is low according to school-based heart screening (2 to 8 per 100 000 children)1,2. VT after palliative repair of congenital heart disease is relatively infrequent but is a known cause of early and late morbidity and mortality. Pediatric VT is primarily idiopathic in patients without underlying heart disease. Idiopathic VT in children with structurally normal hearts although not related with risk of sudden arrhythmic death, it may be associated with significant deterioration in their quality of life. Idiopathic VT includes right ventricular infundibulum tachycardia, left VT involving the fascicular conduction system, and fascicular VT (also called verapamil-sensitive VT). Fascicular VT has been classified into four subtypes: left posterior, left anterior, upper septal, and papillary muscle fascicular VT3,4. Fascicular VT that involves the left posterior fascicle is the most common type of ifiophatic VT. In cases with high diagnostic suspicion, specific 12-lead electrocardiogram (ECG) signs and typical responses to intravenous drug therapy are essential features to consider.
We conducted a retrospective study on the experience regarding clinical presentation, definitive treatment with catheter ablation, and long-term outcomes of pediatric patients with idiopathic left fascicular VT.
Methods
Study sample
This single tertiary heart center study received 62 children with VT treated between September 1995 and February 2018. Of these, 43 patients had right or left idiopathic type VT. Here, we studied 18 children with idiopathic left fascicular VT variant who were referred to the arrhythmia clinic for electrophysiological study and definitive treatment with catheter ablation. Structural heart disease was excluded after a detailed medical history and examination, with 12-lead ECG, chest X-ray, echocardiography, and, if necessary, right or left ventriculography. Echocardiography excluded the presence of false tendon or fibromuscular band extensions from the posteroinferior left ventricle (LV) to the basal septum in all patients. Follow-up was conducted jointly with the interventional electrophysiology and congenital heart disease services. All parents signed informed consent for the interventional electrophysiological study and catheter ablation.
Electrophysiological study and catheter ablation protocols
Antiarrhythmic drugs (AAD) were withdrawn for at least five half-lives before the procedure. Electrophysiological study and catheter ablation protocols were performed under local anesthesia. In younger children or patients with anxiety, procedures were performed under conscious sedation, and in infants, under general anesthesia. 6-Fr Josephson-curve quadripolar catheters were inserted through the femoral vein and placed in the high right atrium, the His-bundle region, and the right ventricular apex. In selected cases, a steerable decapolar catheter was put into the coronary sinus or along the LV septum under fluoroscopic guidance. In infants, three 2-Fr fixed-curve quadripolar catheters were introduced through a single 6-Fr long sheath trio multi-lumen introducer (Trio/Ensamble Arrow Medical, Pasadena CA, USA) via the femoral vein. Programmed pacing was performed from the right ventricular apex and right atrium and, when necessary, intravenous infusion of orciprenaline or aminophylline for VT induction. Intracardiac bipolar electrograms were filtered between 30 to 500 Hz and displayed at a sweep speed of 100 to 200 mm/s. Left fascicular VT was confirmed by the relative activation times of Purkinje and His-bundle electrograms; the origin of VT was determined by identifying the site of earliest ventricular activation during VT, and mapping was performed searching for Purkinje electrograms extending from the left basal septum to apical areas. In four cases, a three-dimensional (3D) electroanatomical mapping system, EnSite Velocity NavX (St Jude Medical, St Paul, MN, USA), and the Electroview 3D (Bard Electrophysiology, Lowell, MA, USA) were used.
LV endocardial geometry was performed in these cases, followed by local activation time mapping. Catheter ablation was advanced retrogradely across the aortic valve. Radiofrequency current (RFCA) was delivered between a quadripolar 7-Fr, 4-mm distal-tip electrode steerable catheter (B-curve Mansfield/Webster, Watertown, or RF MARINR MC Medtronic, Minneapolis, MA, USA) or the Therapy Cool Path ablation catheter irrigated (St Jude Medical) and a dispersion pad applied to the left subscapular region in selected cases. Heparin was administered intravenously at 100 U/kg after femoral artery access, followed by additional doses every hour as needed. RFCA was delivered setting power of 30 to 35 W and a maximum temperature of 65°C; for the cooled catheter, 35 W and 45°C. After each RFCA pulse, VT reinduction was attempted by programmed stimulation. For successful pulses, RFCA was applied for 120 seconds; otherwise, it was interrupted at 10 seconds. For infants, a 5-Fr ablation catheter (RF MARINR SC, Medtronic, curve reach 35 mm) was used with RFCA power of 30 W at 55°C for 60 seconds. All patients were observed overnight in the pediatric postoperative intermediate intensive care ward and then transferred to the pediatric cardiology area for clinical observation, ECG, and echocardiographic monitoring for possible complications before discharge after 24 hours. Patients were discharged on aspirin 100 mg/day for the next 2 months. Follow-up was performed in the outpatient clinic every 3 months until discharge, at least up to one-year post-ablation. Recurrence was defined as the return of clinical symptoms or ECG documented left fascicular VT.
Results
Over 22 years and 5 months, 18 patients (0.8 patients/year) were followed up to evaluate idiopathic left fascicular VT: 14 (77.8%) males and four females. The mean age at the first episode of VT was 8.5 ± 5 years (7 months-15 years, median 9 years) with a mean evolution time of 1.3 ± 0.8 years (1 month-3 years, median 1.2 years). The mean age at the time of catheter ablation treatment was 11.1 ± 3.8 years (8 months-16 years, median 12 years) with a mean weight of 36.8 ± 16.4 kg (8.7-58 kg, median 35 kg). The 8-month-old infant weighed 8.7 kg and measured 0.76 cm (BSA 0.41 m2).
Clinical characteristics
All patients presented symptoms with a predominance of palpitations and dyspnea (60%), palpitations only (40%), and coexisting dizziness or fainting sensation (10%); no patient presented syncope or was resuscitated (Table 1). An 8-month-old infant male showed fatigue, food refusal and irritability, and incessant tachycardia as auscultatory findings. A 14-year-old female with two years of evolution, initially with episodes of paroxysmal VT, progressed to the persistent form of left fascicular VT with symptoms of congestive heart failure. The echocardiogram showed a pattern of dilated cardiomyopathy (categorized and confirmed as tachycardia-induced cardiomyopathy) published as a case report5.
Table 1 Clinical characteristics
| Variables | Patients (n = 18) |
|---|---|
| Mean weight, kg (range) | 36.8 ± 16.4 (8.7-58) |
| Age of onset, mean ± SD (range) | 8.5 ± 5 (7 months-15 years) |
| Age at ablation, mean ± SD (range) | 11.1 ± 3.8 (8 months-16 years) |
| Male, n (%) | 14 (78) |
| Female, n (%) | 4 (22) |
| Symptoms | 100 % |
| Palpitations/dyspnea | 60 % |
| Only palpitations | 40 % |
| Coexisting dizziness or fainting sensation | 10 % |
| Congestive heart failure, n (%) | 1 (5.5) |
| Syncope | 0 |
| Cardiac arrest | 0 |
| Previous antiarrhythmic drugs, mean ± SD | 2 ± 1 |
SD: standard deviation.
Antiarrhythmic treatment and outcome
In paroxysmal fascicular VT, acute treatment in the emergency room was always successful with intravenous (IV) verapamil but not autonomic maneuvers, adenosine, esmolol, or amiodarone (which only partially slowed the VT rate). During clinical follow-up, an outpatient regimen of oral AAD was prescribed, with a mean of 2 ± 1 without success, including AADs such as verapamil, β-blockers, propafenone, and only in one case, amiodarone. Initially, AADs were used as monotherapy, followed by a combination of AADs in 86.5%.
ECG characteristics
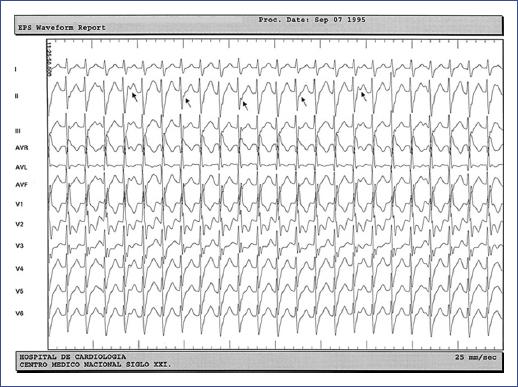
In 17 of the 18 cases (94.4%), VT was detected on the resting 12-lead ECG during an episode of paroxysmal VT, and in one patient, VT was identified by a 24-hour-Holter ECG (Table 2). The sustained monomorphic form of VT was identified in 16 patients, the non-sustained or premature ventricular complexes (PVCs) ran in one patient, and the third patient showed both types. The 12-lead ECG pattern indicative of a left posterior fascicular type of VT [right bundle branch block (RBBB) with left axis deviation] was found in 17 (94.4%) children. Of this group, one case involved both left posterior and left anterior (RBBB with right axis deviation) fascicles (Figure 1), another case involved left posterior and upper septal fascicular VT type (narrow QRS and normal axis) (Figure 2), and one more case involved the Purkinje network of the posterior papillary muscle (RBBB with extreme right axis deviation). The mean VT rate was 176 ± 17 beats/min (150-205 bpm, median 170 bmp), and the mean QRS width, relatively narrow, of 106 ± 10 ms (90-120 ms, median 100 ms), except for the upper septal VT with narrow QRS. Indications for interventional treatment were refractoriness to AAD and hemodynamic intolerance during VT in 16 patients and incessant VT in two, one of whom had tachycardia-induced cardiomyopathy.
Table 2 Ventricular tachycardia characteristics.
| Variable | Cases (n = 17) |
|---|---|
| Median VT rate (bpm), mean ± SD (range) | 176 ± 17 (150-205) |
| Paroxysmal VT, n (%) | 16 (89) |
| Incessant VT, n (%) | 2 (11) |
| Non-Sustained, n (%) | 1 (5.5) |
| Verapamil-sensitive | 100% |
| Adenosine-sensitive | 0 |
| QRS morphology | |
| QRS width (ms), mean ± SD (range) | 106 ± 10 (90-120) |
| RBBB—left axis, n (%) | 17 (94) |
| RBBB—right axis, n (%) | 1 (5.5) |
| RBBB—extreme right axis, n (%) | 1 (5.5) |
| Induced in basal conditions, n (%) | 15 (100) |
| Right ventricular apex, n (%) | 13 (87) |
| Atrial and ventricle, n (%) | 4 (27) |
| Burst ventricular pacing, n (%) | 2 (13) |
| Constant VA conduction, n (%) | 3 (17) |
| Ablation procedure | |
| Conventional mapping, n (%) | 14 (78) |
| Total procedure time (min), mean ± SD (range) | 148.7 ± 95 (60-250) |
| Acute success rate, n (%) | 15 (83.3) |
| Recurrences, n (%) | 3 (16.6) |
| Re-ablation, n (%) | 3 (16.6) |
| Total success, n (%) | 18 (100) |
| RF (pulses), mean ± SD (range) | 6.0 ± 5.0 (1-17) |
| Fluoroscopy time (min), mean ± SD (range) | 15.7 ± 6.9 (5-19) |
| Complications | 0 |
| Follow-up (years), mean ± SD (range) | 2.0 ± 1.2 (1-4) |
RBBB, right bundle branch block; RF, radiofrequency; SD, standard deviation; VA, ventricular-atrial; VT, ventricular tachycardia.
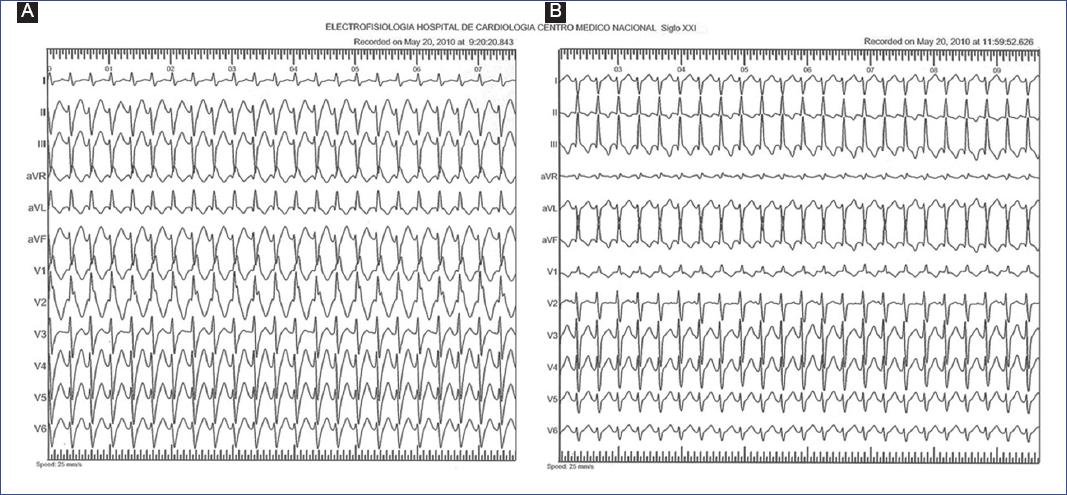
Figure 1 Fascicular ventricular tachycardia in a 12-year-old male. A: left posterior fascicular VT (right bundle branch block with left axis deviation). B: left anterior fascicular VT (right bundle branch block with right axis deviation).
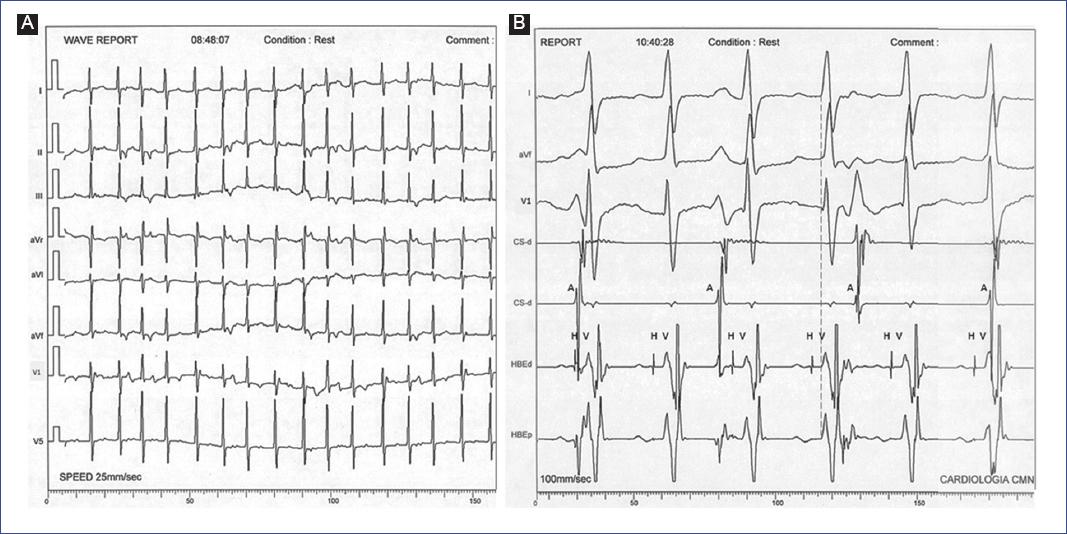
Figure 2 Fascicular ventricular tachycardia in an 8-month-old male infant. A: upper septal fascicular VT (narrow QRS and normal axis). B: intracardiac electrograms of the same case, where the ventricular-atrial dissociation can be observed. A, left atrial electrograms; HBE, His-bundle electrograms (H); V, ventricular electrograms; CS, coronary sinus electrograms.
Mapping and catheter ablation
Clinical left fascicular VT was reproducibly induced under baseline conditions in the 15 patients with a history of sustained paroxysmal VT: 13 patients by right ventricular apex extra stimuli (86.7%), four by atrial pacing and ventricular extra stimuli (27%), and two patients (13.3%) by ventricular burst pacing. We also found one case with non-sustained recurrent spontaneous VT or non-sustained PVC runs despite orciprenaline or aminophylline infusion. A reentrant mechanism was considered in all cases except for non-sustained recurrent VT (with a possible triggered activity mechanism). The rationale was the inverse relationship between the coupling interval and the first beat of the tachycardia echo interval. Also, the finding that the VT was reproducibly induced and terminated by pacing was considered. Entrainment pacing was not routinely used. The mean baseline AH interval was 69.2 ± 18.7 ms, and the HV interval of 45.7 ± 9 ms, both within normal limits before and post-RFCA. During VT, 15 patients (83.3%) had ventricular-atrial (VA) dissociation, and three patients (16.7%) had constant VA conduction. In these cases, the use of adenosine caused temporary VA dissociation and allowed the differential diagnosis of VT.
In most cases (14), only conventional endocardial mapping was performed during sustained VT, and in one patient, it was performed during both VT and sinus rhythm (SR). In the left posterior fascicular VT, the earliest ventricular activation was recorded in the apical posteroinferior zone; in the left anterior fascicular VT, in the anterosuperior zone; and in upper septal fascicular VT, in the superior medial septum. In all cases, the His-bundle was retrogradely activated during VT with a mean VH interval of 11.7 ± 5 ms, except in the case of upper septal fascicular VT with a retrograde His-bundle recorded before the QRS complex. Mapping was conducted to search for the earliest presystolic Purkinje (PP) potential and mid-diastolic Purkinje potential (DP) electrograms. In 16 patients, the earliest local activation of the left ventricle during sustained VT was preceded by a sharp, high-frequency PP potential electrogram that was the main target of ablation. Two patients obtained PP and mid-DP potentials simultaneously (Figures 3, 4, and 5).
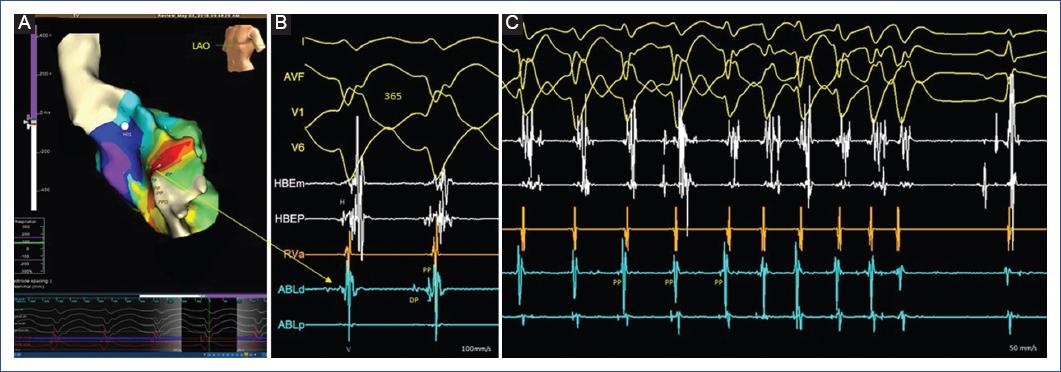
Figure 3 Electroanatomical mapping (EnSite NavX) in a 16-year-old female. A: LV endocardial geometry with isochronal local activation time (LAT) map during sustained VT. Purkinje electrogram (PP) recordings extend from the left basal septal to the apical sites marked on the LV geometry. Early and late transition areas (from the small white to the purple area) can be identified. B: intracardiac electrograms from the ablation catheter (ABLd) corresponding to the LAT map with simultaneous recording of a mid-diastolic Purkinje potential (DP) and an early presystolic Purkinje (PP) potential (yellow arrow). C: at this site, the radiofrequency pulse interrupts ventricular tachycardia. HBE: His-bundle electrograms (H); V: ventricular electrogram.
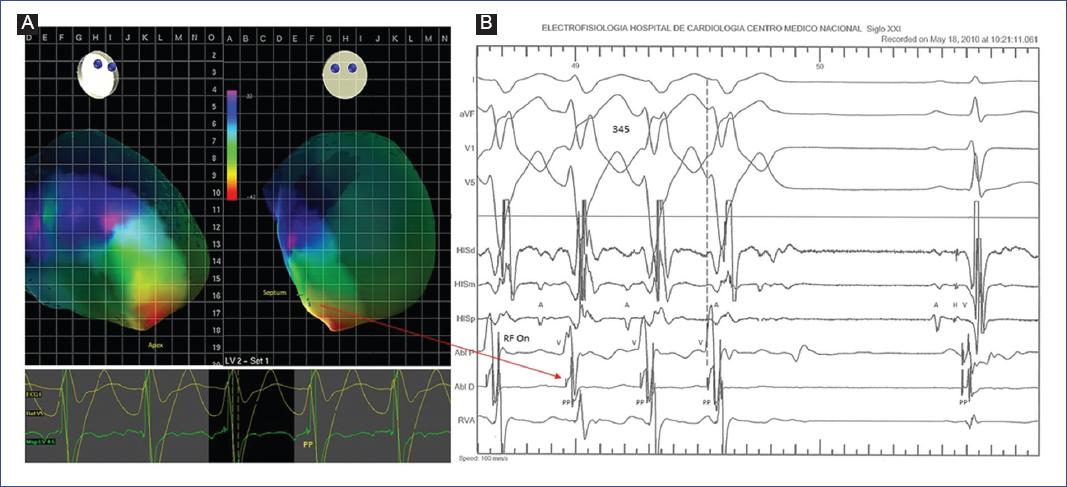
Figure 4 Mapping and catheter ablation in a 10-year-old male. A: electroview 3D mapping system with local activation time (LAT) map. During sustained VT, the early activation site (PP potential) is recorded within the posterior-apical third of the left ventricular septum (red arrow). B: intracardiac electrograms from the ablation catheter (ABL D) recording PP potentials at -8 ms of QRS onset (dotted line) corresponding to the LAT map; successful radiofrequency site (RF On, second pulse).
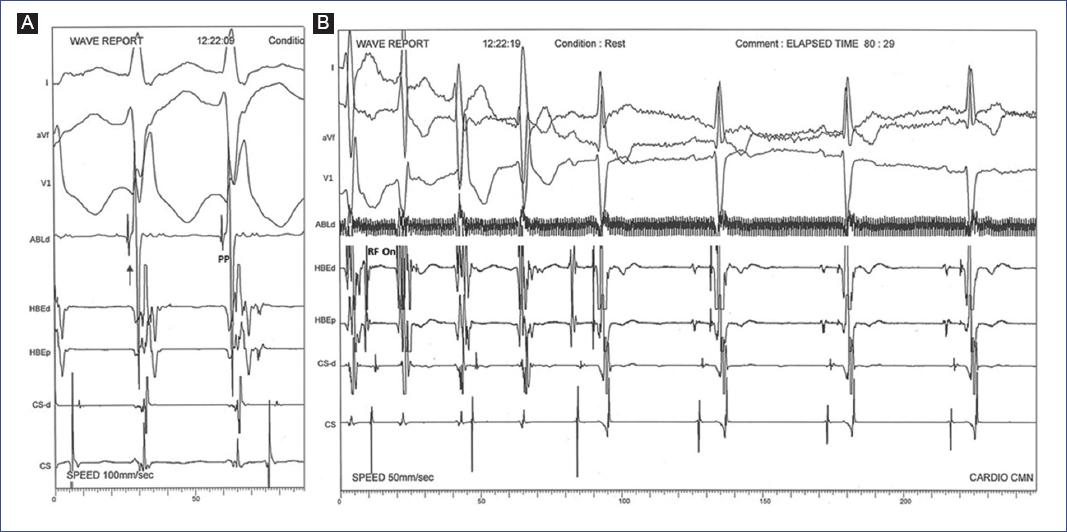
Figure 5 Mapping and catheter ablation in an 11-year-old female. A: conventional mapping during sustained VT in the left posterior fascicle; intracardiac electrograms from the ablation catheter (ABL d) recording PP potentials (arrow) simultaneously with QRS onset. B: target site of the successful radiofrequency pulse (RF On).
During VT, the mean earliest PP potential preceded QRS onset by -19 ± 7 ms and was recorded within the posteroapical third of the LV septum. Total procedure time had a mean of 148.7 ± 95 min (60-250 min, median 142.5 min) and a mean fluoroscopy time of 15.7 ± 6.9 min (5-19 min, median 12.9 min). Fluoroscopy time in 3D electroanatomical mapping cases was between 4 and 7 minutes of intermittent fluoroscopy. The procedure was used to assist in the placement of diagnostic catheters, ablation catheters for retrograde passage of the aortic valve, and construction of the LV endocardial map. The interventional procedure with RFCA was acutely successful in 15 of 18 (83.3%) children; two children underwent a second and one child a third intervention to achieve 100% success. The mean number of RFCA pulses was 6.0 ± 5.0 (1-17, median 5).
Recurrence of left fascicular VT with the same 12-lead ECG morphology was documented in three patients (16.6%: 14, 43, and 86 days, respectively, after the initial ablation procedure). These patients underwent a second session with satisfactory results. In the case of the 8-month-old infant, RFCA was successful for the presentation of incessant left posterior fascicular VT but not for the induced paroxysmal upper septal fascicular VT. In the latter, each application caused transient left bundle branch block; therefore, it was decided to abandon the ablation procedure because of the high risk of atrioventricular block. The outcome was the spontaneous disappearance of this type of tachycardia.
The mean follow-up time of all children after RFCA was 2.0 ± 1.2 years (1-4 years, median 1.5 years), with permanent success and no need for AAD in all groups. No major or minor complications were related to the vascular approach or passage through the aortic valve (confirmed by echocardiography). One case had a periprocedural transient left bundle branch block during RFCA pulses applied in the region above the LV mid-septal region derived from the ablation procedure. After the RFCA session and follow-up, no 12-lead ECG showed the criteria of a new fascicular block.
Discussion
VT is a rare cardiac arrhythmia in the pediatric population. Clinically, most cases are idiopathic with no structural heart disease; within these, left fascicular VT stands out for its clinical characteristics and electrophysiological properties.
Clinical differential diagnosis
A clinically significant fact is that 100% of our cases were initially misdiagnosed as supraventricular tachycardia (SVT) with aberrancy, even in this or other specialized centers. This misdiagnosis might be because this pathology usually occurs in young patients with a structurally normal heart, a relatively narrow QRS during tachycardia, and the characteristic response to IV verapamil. Moreover, the diagnostic challenge is even more significant since we need to differentiate between idiopathic VT and SVT with aberrancy in infants. Thus, initial misdiagnosis of idiopathic VT as SVT can occur between 30% (in infants)6 and 100% (in adolescents) without high clinical and electrocardiographic suspicion. A practical suggestion is that if children with no structural heart disease present with a pattern of RBBB and left axis deviation during tachycardia with a lack of response to adenosine (virtually in 100% of patients), a distinct electrocardiographic entity should be assumed until proven otherwise, and the presence of left fascicular VT should be rapidly suspected7.
From the electrocardiographic perspective, and unlike VT associated with structural heart disease, there is a high incidence of AV dissociation (from 50% in infants7 to 73% in young people8) in this pathology that may be due to a relatively narrow QRS complex that facilitates the identification of P waves in the 12-lead surface ECG (Figure 6). Indirect data of AV dissociation (fusion and capture beats) are present in ≥ 25% of cases, and another relevant feature is an RS interval ≤ 80 ms in precordial leads8. As in our experience, IV verapamil is practically 100% effective in treating paroxysmal fascicular VT (hence so-called verapamil-sensitive VT). This efficacy is presumed to result from the blockade of a slow conduction area (calcium channel-dependent) within the antegrade arm of the tachycardia circuit adjacent to the left fascicle9. According to recent experience, IV verapamil is effective and safe in infants and children, following a particular dosing strategy and method of administration10. However, oral treatment and other conventional AADs have limited success, as in our case series11. During follow-up, it was present in ≥ 75% of patients; thus, all children continued with recurrent episodes of fascicular VT, a situation well recognized in other series; this determined the indication for interventional treatment in several cases.
Electrophysiological characteristics
The exact mechanism of the tachycardia circuit has not been fully elucidated. From an electrophysiological perspective, a small macro-reentrant pathway involving the posterior Purkinje network is considered. Left ventricular mapping shows a focal origin during VT; antegrade conduction descends from the basal septum through a zone of slow conduction in the ventricular septum resulting in a mid-DP potential and continues to the apical septum and the posterior septal myocardium. Retrograde conduction proceeds passively over the left posterior fascicle (≥ 94%) or the Purkinje fibers close to the left posterior fascicle originating a PP potential that precedes the onset of QRS during tachycardia12-14, whereas in the SR, the mid-DP follows the local ventricular complex and surface QRS (retro-DP), which also allows mapping and ablation in the SR.
Electrophysiological differential diagnosis
The most important feature for diagnosing left fascicular VT is recording the PP electrogram preceding the earliest local ventricular activation of the LV posteroapical septum, followed by early retrograde conduction of the His-bundle and anterograde activation of the proximal branch of the right bundle branch. At the same site, but during SR, the PP potential is recorded after the His-bundleelectrogram and before the onset of the QRS complex. During the electrophysiology study, the specialist should perform the differential diagnosis between SVT with aberrancy and actual fascicular VT with continuous conduction of the VA and other VTs. During sustained VT, if there is continuous 1:1 conduction of the VA, rapid atrial pacing may show AV dissociation favoring the diagnosis of fascicular VT. Another more straightforward maneuver is to use adenosine to block the AV node evidencing transient dissociation of the VA without interrupting or changing QRS morphology and tachycardia cycle length. In SVT with aberrancy, the His-bundle is anterograde, and the HV interval is the same as in SR; the opposite occurs in fascicular VT. Other verapamil-sensitive tachycardias, such as interfascicular VT or idiopathic mitral annulus VT, have a typical RBBB morphology with left or right axial deviation and can simulate fascicular VT. Another interesting VT to consider is verapamil-sensitive fascicular VT, which affects the Purkinje network around the posterior papillary muscle and is characterized by RBBB and extreme right axis deviation15.
Interventional treatment
Once the mechanism is determined to be a reentrant involving branches of the Purkinje network emanating from the posterior or anterior left fascicles, consideration is needed to search for the appropriate endocardial site of the reentrant circuit guided by specific electrograms of the Purkinje to perform catheter ablation. With improvements in catheter and mapping technology, catheter ablation has become the standard treatment for children and adolescents with idiopathic VT, although reports are primarily of single cases and small series16,17. Therefore, experience related to catheter ablation of VT in pediatric patients has been limited. One of the first and largest retrospective series on fascicular VT ablation procedures in the pediatric population (102 cases, mean age 12.5 ± 3.6 years) derived from an international multicenter study (22 hospitals) reported 82% of acute success rate and 72% at a median follow-up of two years18. However, some limitations of the study were the different treatment approaches, success rates, and follow-up among institutions. More recent reports of single-center experiences (44 children, 73% ≥ 10 years old, and 32 children, 8.6 ± 3.7 years, mean BW 34.3 ± 14.9 kg)6,19 showed a similar or higher acute success rate of 90% and 97%, respectively, with a recurrence rate after ablation of 10% to 14% and success rate ≥ 90% at 2 to 4 years of follow-up. Another small series (6 cases, aged 3 to 17 years) proposed that the approach technique of creating a partial fascicular block was effective and safe20. Data from a systematic review of the literature confirmed that catheter ablation is an effective and safe treatment for left fascicular VT. However, the success rate was lower in observational pediatric case series (90% after repeated procedures, 95%CI 82.1-94.6%) compared to adult cases (94.3%, 95%CI 92.2-95.9%, p < 0.001)21. Catheter ablation in children with the rare left anterior fascicular VT presents some difficulties, essentially due to the challenge of identifying tachycardia circuits, a high recurrence rate, and some complications22. Electroanatomic 3D mapping systems can facilitate the procedure and minimize the child’s exposure to fluoroscopy. In a small series of four patients with a mean age of 14.5 years (4-20 years), success was achieved in all cases using noncontact mapping (EnSite ArrayTM)23. Two recent studies with EnSite NavXTM totaling 53 children reported an acute success rate of 95% and 100%, with a median fluoroscopy time between 1.25 and 5.1 minutes24,25, although fluoroscopy was not used in 91% of cases23.
A recurrence rate of 20% and 3% was recorded during short- and long-term follow-ups. Repeat ablations achieved permanent success in all cases24,25. Only the last four cases were performed in our series with 3-D mapping after introducing this technology in our center. Overall, 3D mapping in adults improved the success rate compared to fluoroscopy, especially after the index procedure. However, there are no comparative studies in the pediatric population, and for now, the main advantage of 3D mapping is the limitation of fluoroscopy. Idiopathic infant-onset VT may be more likely to disappear spontaneously, with a mean age of 1.9 ± 1.1 years (0.1-3.2 years)26, as was the isolated case in our series with the upper septal fascicular VT, the rarest type.
Electrophysiological studies and catheter ablation procedures in infants present difficulties, essentially due to the lower weight and height of the patients. Left fascicular VT is rare in infants but can be persistent and lead to tachycardia-induced cardiomyopathy refractory to AAD. In our series, the patient with incessant VT (left posterior fascicular VT type) underwent a successful RFCA. Two isolated cases of infants aged 8 and 12 months (BW 7.7 and 10 kg, respectively) with persistent fascicular VT and heart failure refractory to AAD and electrical cardioversion have been described in the literature. Recovery of SR by RFCA, used as a life-saving procedure, was associated with improved systolic function without needing AAD. Thus, an attempt at ablation may be justified when tachycardia-induced cardiomyopathy is present or suspected, regardless of body weight27,28. In most patients, catheter ablation has no severe acute or chronic complications. Still, minor complications have been described, such as persistent left bundle branch block (0.9%)18 without major complications19,20,23-25 or permanent atrioventricular block. The latter was present in the uncommon left anterior fascial VT because, in some cases, it is located in the mid-anterior LV septum, close to the His-bundle22. Finally, in exceptional cases, life-threatening periprocedural ventricular arrhythmias have been reported18.
The prevalence and incidence of VT detected in children are relatively low, and the prognosis of idiopathic VT differs according to its origin. In the case of fascicular VT, our experience revealed an adverse clinical evolution in the pediatric population at medium or long-term follow-up, showing refractoriness to AAD in most patients. Fascicular VT represents a particular condition in which pediatricians and emergency physicians should always consider the 12-lead ECG pattern clues and its typical response to IV verapamil. The mechanism of fascicular VT is a macro-reentry involving the Purkinje system (mainly the left posterior fascicle) and the Purkinje-ventricular myocardium junction. Therefore, ablation targeting with characteristic Purkinje potential electrograms can eliminate this type of idiopathic VT. Catheter ablation is a safe treatment for fascicular VT in children with a high success rate and should represent the first-line approach in symptomatic patients. If the 3D electroanatomic mapping system is available, it should be preferred to minimize exposure to fluoroscopy.
Limitations
This study represents the experience of a single center specialized in a rare clinical condition. We report a relatively small group of patients, so the limitations are those inherent to retrospective studies; however, not enough reports were found in our setting. Although we presented relatively few cases, we had the opportunity to treat different types of idiopathic left fascicular VT. The results are consistent with some Asian reports, where the incidence and prevalence of left fascicular VT are higher than in other geographic areas.











 text new page (beta)
text new page (beta)