Introduction
Thrombotic microangiopathy (TMA) is characterized by microthrombus formation associated with subsequent thrombocytopenia, microangiopathic hemolytic anemia (MAHA), and target organ injury1. Thrombotic microangiopathies are a group of disorders primarily related to endothelial dysfunction. This category of endothelial dysfunction results from various imbalances between platelets, the endothelial and immune systems, and cytokine production2. Thrombotic thrombocytopenic purpura (TTP) is a fatal condition, rare among hematological diseases, characterized by microvascular thrombosis with platelet aggregation in patients with severe functional deficiency of ADAMTS13 (activity < 10%)3.
Furthermore, COVID-19 is a new disease with different clinical manifestations observed in children4,5. During SARS-CoV-2 infection, hematological diseases such as immune thrombocytopenia and TTP were reported in adults2,6. However, information in children is still limited. Hidalgo et al. reported a 14-year-old patient with COVID-19-associated TTP, with a favorable outcome after treatment with plasma exchange therapy (PEX)7. Verma et al. described another case of COVID-19 complicated with hemophagocytic lymphohistiocytosis and TTP in a 21-year-old male patient, who died despite treatment8. This study aimed to describe the unusual presentation of TTP associated with COVID-19 in a pediatric patient with a fatal outcome.
Clinical case
We describe the case of a 9-year-old male Hispanic patient who presented with abdominal pain and fever of 14 days of evolution to the pediatric emergency department. Five weeks earlier, the mother was positive for SARS-CoV-2 by RT-PCR (reverse transcription-polymerase chain reaction) test; on admission to the hospital, the patient’s serological test (IgG) for SARS-CoV-2 antibodies was positive. He had no previous hospitalizations or any report of illness or surgical interventions and had complete immunizations. Vital functions on admission were the following: heart rate 120 beats/min, respiratory rate 22/min, temperature 38°C, weight 30 kg, height 128 cm. On clinical examination, we detected pallor of the skin and mucous membranes, chapped lips, erythematous macular lesions symmetrically distributed on the neck, forehead, and inguinal area. Abdominal pain was present on palpation of the mesogastrium. The results of clinical tests showed hemoglobin, 13.3 g/dL; leukocytes, 18.9 x 103/μL; platelets, 528 x 103/μL; C-reactive protein, 4.6 mg/dL; normal coagulation profile; fibrinogen, 581.3 mg/dL; lactate dehydrogenase, 1317 U/L; D-dimer, 1.6 mg/L; normal complement C3 and C4; urea, 18 mg/dL; creatinine, 0.4 mg/dL; albumin, 3.7 g/dL; triglycerides, 130 mg/dL; creatine kinase, 25 mg/dL; ferritin, 1016 ng/L; serum electrolytes with normal values and urinalysis showed hematuria. Abdominal ultrasound showed hepatomegaly, and echocardiography showed no alterations. He received exogenous human immunoglobulin 2 g/kg/day, acetylsalicylic acid 3 mg/kg/day for 7 days, prednisone 2 mg/kg/day for 5 days to treat the probable multisystem inflammatory syndrome. On the second day of treatment, he was afebrile, and on the fourth day, the erythematous lesions decreased; also, there was no hematuria, and he presented a decrease in acute phase reactants, so he was discharged. Five days after discharge, abdominal pain and fever persisted. Clinical laboratory findings were as follows: hemoglobin, 11.3 g/dL; leukocytes, 10 x 103/μL; eosinophils, 1305/μL; absolute neutrophil count, 5321/μL; lymphocytes, 2710/μL; platelets, 606 x 103/μL; C-reactive protein, 8.2 mg/dL; fibrinogen, 664 mg/dL; D-dimer, 2 μg/mL; ferritin, 1030 ng/mL; lactate dehydrogenase, 890 U/L; coagulation profile, urea, creatinine, and serum electrolytes were normal. Immunological tests were performed: negative antinuclear antibodies (ANA), negative anti-dsDNA, negative antineutrophil cytoplasmic antibodies (ANCA), negative anti-cyclic citrullinated peptide antibodies (anti-CCP), negative rheumatoid factor, negative lupus antibodies, negative antiphospholipid profile, and negative IgG antibodies against Toxocara. The patient was treated with methylprednisolone at a dose of 2 mg/kg/day for 7 days, then at a dose of 30 mg/kg for 3 days, and then with prednisone at a dose of 2 mg/kg/day for 15 days. During hospitalization, he presented decreased abdominal pain and absence of fever, so he was discharged with an indication of follow-up control by rheumatology.
Four months later, the patient was readmitted to the pediatric emergency room with 12 days of evolution characterized by fever, abdominal pain, and generalized edema. On admission, he presented a heart rate of 140 beats/min, respiratory rate of 29/min, blood pressure (p90-95), and edema of the face, genitals, hands, and feet. The skin was cold, turgid, with erythematous macular lesions of urticarial appearance (positive dermographism) associated with diffuse hematic crusts and fine desquamation on the face, neck, thorax, arms, and legs. Examinations found the following values: hemoglobin, 11.6 g/dL; platelet count, 170 x 103/μL; prothrombin time (PT), 13.1 s; INR (international normalized ratio), 1.1; activated partial thromboplastin time (aPTT), 34 s; fibrinogen, 432 mg/dL; urea, 16 mg/dL; creatinine, 0.34 mg/dL; D-dimer, 4.32 mg/L; lactate dehydrogenase, 1862 U/L; serum calcium, 7.2 mg/dL; complement C3, 120 mg/dL, and C4, 28 mg/dL, and hemoglobinuria. The patient was transferred to the dermatology department due to the dermatological lesions described. Three days later, the dermal lesions increased (Figure 1), so a skin biopsy of the lesions was performed. The biopsy results described an acute spongiotic dermatitis with intraepidermal vesicles and numerous necrotic keratinocytes in the epidermis, associated with a superficial and deep perivascular and periadnexal inflammatory infiltrate, with numerous eosinophils and extravasation of red blood cells. Histochemical study with periodic acid-Schiff and Alcian blue was negative. He presented with oliguria and generalized tonic-clonic seizures associated with 86% desaturation (fraction of inspired oxygen (FiO2) 21%) on three occasions. Due to unstable hemodynamic compromise, it was decided to perform endotracheal intubation and transfer him to the Pediatric Intensive Care Unit (PICU) for ventilation and hemodynamic management. The following results were obtained in the PICU: hemoglobin, 10.6 g/dL; schistocytes, 6+/field (blood); positive direct Coombs test; leukocytes, 13.4 x 103/μL; platelets, 19 x103/μL; haptoglobin not detectable; triglycerides, 280 mg/dL; ferritin, 3442 ng/μL; lactate dehydrogenase, 1862 U/L; D-dimer, 4 mg/L; complement C3, 120 mg/dL, and C4, 28 mg/dL; creatinine, 1.23 mg/dL; creatine kinase, 248 mg/dL; erythrocyte sedimentation rate, 19 s, and ADAMTS13 activity (von Willebrand factor-cleaving protease) in 54% (RV: 40-130%) after transfusion support of blood products. Flow cytometry showed a decrease in T lymphocytes, CD4+ lymphocytes, CD8+ lymphocytes, an increase in CD4/CD8 ratio for age, and a decrease in natural killer (NK) cell count. Viral load for Epstein-Barr virus, cytomegalovirus, and parvovirus B19 was negative.
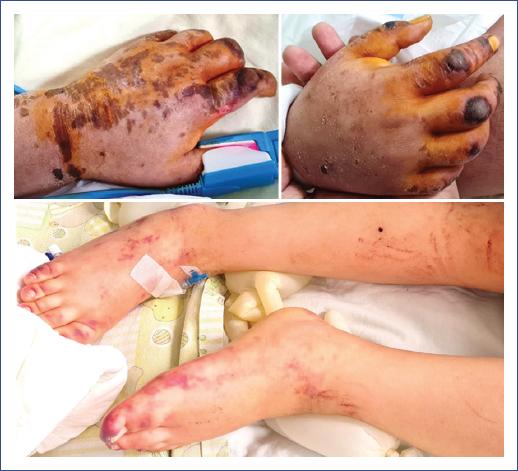
Figure 1 Erythematous macular lesions with excoriated hematic scabs in the hands associated with purpuric dermatosis in the patient’s lower limbs on the Pediatric Intensive Care Unit admission day.
Renal ultrasound showed the left kidney with asymmetry compared to the contralateral one; in the Doppler study, a decreased wave depth was identified, suggesting left renal hypoperfusion. With the clinical and laboratory manifestations described, the patient was diagnosed with TTP. The PLASMIC score was 7 (Table 1). He received fresh frozen plasma, renal replacement therapy, methylprednisolone cycles, human immunoglobulin, and rituximab (Table 1). Due to persistent severe thrombocytopenia, it was decided to initiate plasma exchange (PEX), and the patient received 14 sessions. Although the patient received platelet transfusion to compensate for the platelet deficit, he did not respond well.
Table 1 Laboratory test results during hospitalization
| Hospital admissions | 1st | 2nd | 3rd | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Department | Medicine | Medicine | Dermatology | PICU | ||||||
| Hospitalization day | 1 | 1 | 1 | 1* | 3+ | 11 | 16 | 23 | 30 | 37 |
| Treatment | MTP+Ig/Rtmb/PD | MTP/PEX/PD | MTP/Rtmb/PEX/HD | PDN/Rtmb/PEX/HD | PDN/Rtmb/HD | PDN/HD | ||||
| Hematology results | ||||||||||
| Hemoglobin (g/dL) | 13.3 | 11.3 | 11.6 | 10.6 | 8 | 10 | 7.8 | 9.1 | 7.8 | 8.4 |
| Leukocytes (103/mL) | 18.9 | 10 | 16.4 | 13.4 | 9.2 | 17.4 | 11.6 | 5.4 | 2.2 | 5.3 |
| Platelets (103/mL) | 528 | 606 | 170 | 19 | 14 | 68 | 40 | 32 | 99 | 51 |
| Prothrombin time (s) | 12.8 | 12.4 | 13 | 16 | 13 | 12.4 | 13.1 | 13.5 | — | 13.8 |
| INR | 1.1 | 1.0 | 1.1 | 1.4 | 1.1 | 1 | 1.1 | 1.1 | — | 1.2 |
| Thromboplastin time (s) | 29.6 | 28.5 | 34 | 44 | 29 | 33.5 | 37.2 | 43.7 | — | 42.7 |
| Thrombin time (s) | 17 | 17 | 18.7 | 27 | 23 | 21 | 19.8 | 25.8 | — | 24 |
| Fibrinogen (mg/dL) | 581.3 | 664 | 432 | 300 | 335 | 230 | 253.4 | 226.6 | — | 164.1 |
| D-dimer (mg/L) | 1.6 | 2.0 | 4.32 | 6.8 | 12.2 | — | — | 3.6 | — | |
| Biochemical results | ||||||||||
| Ferritin (ng/mL) | 1016 | 1030 | — | 344 | 23943 | 3254 | — | — | — | — |
| C3 (mg/dL) | 101 | 120 | — | — | — | — | — | — | ||
| C4 (mg/dL) | 24 | 32 | 28 | — | — | — | — | — | — | |
| Lactate dehydrogenase (U/L) | 1317 | 890 | 1862 | — | 10,562 | 2279 | 1780 | 754 | 494 | — |
| Urea (mg/dL) | 18 | 23 | 16 | 49 | 173 | 177 | 245 | 84 | 54 | 57 |
| Creatinine (mg/dL) | 0.4 | 0.32 | 0.34 | 1.23 | 4 | 3.6 | 4.14 | 1.9 | 1.8 | 1.8 |
| GOT (U/L) | 34 | 50 | 48 | 233 | 360 | — | — | 32 | — | — |
| GPT (U/L) | 17 | 49 | 9 | 61 | — | — | — | 42 | — | — |
| CPK (mg/dL) | 44 | 248 | — | 1883 | — | — | 31 | — | — | |
| CPK-MB (U/L) | 25 | 14 | 26 | — | 102 | — | — | 12 | — | — |
| C-reactive protein (mg/dL) | 4.6 | 8.17 | 4.76 | 5.9 | 6 | 2.6 | — | 2.2 | — | 12.8 |
*PLASMIC score: platelet count < 30,000/mL: 1; hemolysis: 1; no active cancer: 1; no solid organ or stem cell transplantation: 1; no diarrhea: 1; INR < 1.5: 1; creatinine < 2.0 mg/dL: 1 (score 7). Corticosteroid onset: MTP (1 g) + Ig 5% (30 g) for 5 days. Then it was decreased to MTP (125 mg) every 6 hours for 5 days and MTP (125 mg) every 8 hours for 5 days, then moved to PDN (1 mg/kg) equivalent dose in dexamethasone. Initiation of Rtmb (375 mg/m2/dose each week*4 doses). (+) Indication of fresh frozen plasma (10 cc/kg/dose every 8 hours).
CPK, creatine phosphokinase; GOT, aspartate aminotransferase; GPT, alanine aminotransferase; HD, hemodialysis; Ig, immunoglobulin; MTP, methylprednisolone; PD, peritoneal dialysis; PDN, prednisone; PEX, plasma exchange: received 14 cycles; Rtmb, rituximab.
The patient presented sensorium deterioration during the PICU stay, and an intracerebral hemorrhage was detected one month after the last PEX session in a CT scan. The patient evolved unfavorably with a fatal outcome in the second month of hospitalization. Gene sequence analysis and deletion/duplication test of 13 genes (ADAMTS13, C3, CD46, CD55, CD59, CFB, CFH, CFI, DGKE, INF2, MMACHC, PLG, THBD) for atypical hemolytic uremic syndrome and thrombotic microangiopathy were negative.
Discussion
Acquired TTP has been described as a clinical presentation associated with COVID-19 in adults6. However, since TTP in children is rare3, only a few cases have been reported in children7.
On our patient’s first hospital admission, he was diagnosed with the multisystem inflammatory syndrome (MIS-C), for which he received MIS-C therapy. He was readmitted to the hospital four months later with multiple organ damage, probably of autoimmune origin, triggered by SARS-COV-2 post-infection.
After PEX, our patient underwent ADAMTS13 dosing. This procedure delivers the deficient enzyme (ADAMTS13) and eliminates autoantibodies that inhibit ADAMTS13 activity. In some cases of acquired TTP with ADAMTS13 deficiency, antibodies may be undetectable, probably related to the lack of sensitivity of the available methods9 and the sensitivity of anti-ADAMTS13 antibodies of IgM or IgA type10, or due to altered synthesis or secretion of ADAMTS1311. Therefore, the result was interpreted in normal ranges.
Additionally, the patient presented features of MAHA associated with TMA in the clinical context of anemia, reticulocytosis, schistocytosis, elevated lactate dehydrogenase, undetectable haptoglobin, hemoglobinuria, and severe thrombocytopenia.
The PLASMIC score discriminates between PTT and other TMA, such as atypical uremic syndrome, reliably assesses the likelihood of a severe deficit of ADAMTS13 activity in patients with TMA and could help improve the accuracy of clinical assessment and be beneficial when ADAMTS13 testing is unavailable12,13. Gupta et al. reported that the PLASMIC score could also be a good predictor to identify pediatric patients with an ADAMTS13 activity less than 10%14.
On admission to the PICU, a PLASMIC score of 7 we obtained in the patient (platelet count < 30,000/μL, hemolysis, no active cancer, no solid organ or stem cell transplantation, no diarrhea, INR < 1.5, creatinine < 2.0 mg/dL). Therefore, the PLASMIC score predicted ADAMST13 deficiency and high suspicion of TTP and guided the management of PEX according to its score.
RT-PCR for SARS-CoV-2 was negative on readmission, but serologic testing was positive for SARS-CoV-2 IgG, similar to a 14-year-old male with a previous infection with SARS-COV-2 who developed a TTP with a favorable evolution after PEX sessions7.
The association of other viruses, such as human immunodeficiency virus, human lymphotropic lymphoma virus-1, hepatitis C, dengue, influenza, cytomegalovirus, human herpesvirus 8, human herpesvirus 6, and parvovirus B19, with the acute development of TTP has been described15. The late development of this complication, however, is unclear. Oka and Nohgawa described a case of acquired TTP associated with EBV reactivation in a 37-year-old healthy male who recovered spontaneously without any treatment16. As the mechanism is still under study, further clinical reports and studies are needed.
In the PICU, management was performed with hemodynamic support, corticosteroids, immunoglobulin, rituximab, and fresh frozen plasma. The patient showed a gradual but ineffective response while waiting for the initiation of PEX, which began 11 days after the diagnosis of TTP. It has been shown that the use of immunosuppressive therapy together with PEX improves the therapeutic response and decreases the days of PEX, avoiding the complications of this procedure17.
During PEX, a partial response was evidenced with a decrease in MAHA and partial clinical improvement of the patient. Unfortunately, due to the lack of resources needed to maintain PEX, the patient only received 14 cycles, and the target platelet count > 150 x 103/μL on two control measurements, or more days as a cut-off point for completion of this procedure, was not achieved (Table 1). PEX is used in patients with a presumptive or confirmed clinical diagnosis of TTP. It is used in all patients because, if left untreated, they progress to neurologic deterioration or renal failure. Mintz et al. have described the use of up to two cycles of PEX in patients with TTP in a clinical trial. In this study, of seven patients with a second cycle of PEX, four achieved remission18. Another report described up to 25 daily sessions of PEX without complications and with a favorable evolution7. A recent study on the use of PEX in a PICU in Chile reported complications related to the apheresis circuit, hypotension, anaphylaxis, or transfusion-related acute lung injury19. Although the mean number of PEX sessions in this study was lower than that in our patient because the pathologies evaluated were different, no hemorrhagic complications were reported19.
The delay in the initiation of treatment, the premature interruption of PEX, and the suspicion of recurrent acquired TTP were the possible causes of the patient’s poor response to treatment, with severe renal and neurological compromise. Analysis of the disease course suggested TMA diagnosis that was partially treated with corticosteroids, immunomodulatory therapy with immunoglobulin, rituximab, and PEX. The genetic panel to rule out hereditary TTP, complement-associated hemolytic uremic syndrome, and alterations of vitamin B12 metabolism tested negative, confirming acquired TTP.
One of the limitations of this report is the insufficient evidence in the literature to conclude that the immune response to SARS-CoV-2 caused acquired TTP. Further studies are required to confirm a causal relationship between these two conditions.
In conclusion, the presentation and clinical course of acquired TTP probably associated with SARS-CoV-2 was severe. Early diagnosis and prompt initiation of combination therapy are essential for a better prognosis.











 text new page (beta)
text new page (beta)


