Introduction
Chronic autoimmune thyroiditis (CAT) or Hashimoto's thyroiditis is the most frequent cause of acquired hypothyroidism and goiter in countries where iodine intake is adequate. A prevalence ranging from 1.2-3% has been reported in the pediatric age, being more frequent in adolescent females, with a mean age of presentation between 10 and 12 years1-8.
CAT pathophysiology is characterized by infiltration of the thyroid gland by T and B lymphocytes resulting in cytotoxicity, apoptosis, and release of pro-inflammatory cytokines and antibodies directed against thyroid antigens such as thyroglobulin (Tg-Ab), thyroid peroxidase enzyme (TPO-Ab), thyroid-stimulating hormone (TSH) receptor, sodium-iodine transporter, and pendrin3,5,9.
CAT is a disease of multifactorial origin resulting from the combination of environmental factors in genetically predisposed subjects. An increased risk has been described in patients with HLA-B*46:01, HLA-A*02:07, and HLA-DRB4 haplotypes and with polymorphisms in the CTLA-4, CD40, PTPN22, and IL2R genes9.
Environmental factors associated with CAT development include a diet high in iodine, hepatitis C virus and herpes virus type 6 infection, smoking, radiation, and the use of drugs such as lithium, tyrosine kinase inhibitors, amiodarone, interferon-alpha, and alemtuzumab3,5,9,10.
Some studies have found an increased prevalence of CAT in overweight patients, and those with CAT and obesity have high antithyroid-antibody levels. However, the direction of causality of this association is unclear1,9,10. In one aspect, weight gain is usually one of the most characteristic symptoms of hypothyroidism. Conversely, overweight and obesity cause an increase in leptin levels, which through the hypothalamic POMC/CART (pro-opiomelanocortin/cocaine- and amphetamine-regulated transcript) pathway stimulates the synthesis of thyrotropin-releasing hormone (TRH)3,9,11,12. Furthermore, it has been proposed that leptin also has immunomodulatory functions and that overweight produces a systemic inflammatory state that may favor the appearance of autoimmune diseases1,10,13,14.
This study aimed to determine the frequency of overweight and obesity in children and adolescents with chronic autoimmune thyroiditis and its distribution according to thyroid function at diagnosis and to evaluate whether there are differences in the levels of Tg-Ab, TPO-Ab, TSH, and thyroid hormones between patients with overweight, obesity, and normal-weight. Moreover, the changes in body mass index (BMI) at 6 and 12 months of follow-up were studied, comparing the variations between those who started treatment with levothyroxine at the first visit and those who did not receive pharmacological treatment regarding their initial thyroid function.
Methods
We conducted an observational, longitudinal, retrospective study in which we reviewed the records of patients aged 2 to 18 years with a diagnosis of CAT seen in the Endocrinology service of the Instituto Nacional de Pediatría (National Institute of Pediatrics, INP) in Mexico City from January 2016 to January 2019. A case of CAT was defined as any patient with antibodies against thyroglobulin or thyroid peroxidase (Tg-Ab and TPO-Ab, respectively) and alterations in thyroid function tests or presence of goiter. Patients with systemic autoimmune disease, patients with treatments or pathologies associated with an increased risk of CAT (Down syndrome, Turner syndrome, type 1 diabetes mellitus, celiac disease, and history of neck radiation), as well as individuals with excess weight caused by medications (glucocorticoids) or systemic diseases were excluded.
A total of 78 cases met the inclusion criteria. Demographic data, anthropometric data (weight and height), thyroid profile, Tg-Ab, TPO-Ab levels (measured by immunochemiluminescence), requirement or not of treatment, and levothyroxine dose at diagnosis and 6 and 12 months of follow-up were collected. Patients were classified according to thyroid function at diagnosis using the following criteria:
Hypothyroidism: Thyroid hormones below the normal lower limit or a TSH ≥ 20 mIU/L.
Subclinical hypothyroidism: TSH values > 4 but < 20 mIU/L and thyroid hormones within normal ranges.
Euthyroidism: TSH ≤ 4 mIU/L and thyroid hormones within laboratory reference ranges.
Hyperthyroidism: Thyroid hormones above the normal upper limit or a TSH ≤ 0.4 mIU/L.
BMI was calculated, and the Z-score and percentiles of BMI and height were estimated using the Center for Disease Control and Prevention (CDC) growth charts.
Nutritional status was classified according to the Endocrine Society criteria: overweight, BMI between 85th to 94th percentile; obesity, BMI above 95th percentile15,16.
Short stature was defined as a height-for-age and gender Z-score ≤ 2 SD.
Regarding antibodies directed against thyroid antigens, the laboratory upper detection limit is 3000 IU/mL for Tg-Ab and 1000 IU/mL for TPO-Ab. Therefore, higher values are reported as > 3000 IU/mL and > 1000 IU/mL, respectively. On this basis, we decided to analyze antibody titer as a categorical variable; TPO-Ab titers were classified into three groups: low (< 500 IU/mL), intermediate (500-999 IU/mL), and high (≥ 1000 IU/mL). Also, Tg-Ab titers were classified into low (< 500 IU/mL), intermediate (500-2999 IU/mL), and high (≥ 3000 IU/mL).
Statistical analysis was conducted using the SPSS program. Descriptive statistics were performed to characterize the sample and estimate the frequency of overweight and obesity in the study population and the frequency of thyroid function alterations at diagnosis. ANOVA and Kruskal-Wallis tests were performed to compare TSH and thyroid hormone levels and Χ2 tests to compare antibody titers according to baseline thyroid function. Pearson's Χ2 test was used to assess the distribution of excess weight according to baseline thyroid function. A two-factor repeated-measures ANOVA test was used to assess the change in BMI percentile at 6 and 12 months after diagnosis. Statistical significance was considered at a p-value < 0.05.
Results
Of the 78 cases included in the study, 70 were females (89.7%) and eight males (10.3%), with an 8.7:1 ratio. The mean age at diagnosis was 10.8 ± 3.01 years, with a minimum age of 3 years 4 months. A total of 52.6% of patients showed puberty signs at the time of presentation. In addition, a family history of thyroid pathology was reported in 25.6% of the individuals.
The most frequent reasons for consultation were the presence of goiter and symptoms of hypothyroidism, although 8% of the patients went for evaluation to address overweight or obesity. During the first consultation, 19.2% of the patients were short according to standard population parameters; the median height Z-score was -0.57 (-5.24 to 1.74).
Regarding thyroid function at diagnosis, 38 patients had hypothyroidism (48.7%), 16 had subclinical hypothyroidism (20.5%), 17 had normal thyroid function (21.8%), and 7 had hyperthyroidism (9%). Table 1 summarizes the clinical and biochemical characteristics of the patients according to baseline thyroid function.
Table 1 Clinical and biochemical features according to thyroid function at diagnosis
| Euthyroidism (n = 17) | Subclinical hypothyroidism (n = 15) | Hypothyroidism (n = 39) | Hyperthyroidism (n = 7) | p* | |
|---|---|---|---|---|---|
| Age (years) | 11.5 ± 2.8 | 11.5 ± 1.9 | 9.9 ± 3 | 12.4 ± 3.7 | 0.056 |
| Age <10 years | 5 (29.4%) | 4 (26.7%) | 21 (53.8%) | 1 (14.3%) | 0.073 |
| Prepuberal | 5 (31.2%) | 5 (33.3%) | 24 (61.5%) | 2 (28.6%) | 0.072 |
| F:M | 7.5:1 | 14:1 | 6.8:1 | 6:1 | 0.540 |
| Height Z-score | -0.47± 0.84 | -0.46 ± 0.59 | -1.31 ± 1.7 | -0.77 ± 1.23 | 0.111 |
| BMI (kg/m2) | 19.2 (15.6-38.7) | 17.5 (13.6-21.8) | 18.4 (13.9-35.9) | 22.03 (14.4-24.9) | 0.225 |
| BMI Z-score | 0.76 (-0.6 - +2.65) | -0.11 (-1.74 - +1.02) | 0.84 (-1.88 - +2.4) | 0.99 (-1.14 - +1.31) | 0.0251 |
| TSH (mUI/L) | 2.69 (0.55-3.97) | 5.26 (4.1-15.6) | 75 (12.3-75) | 0.01 (0.004-0.17) | 0.000 |
| FT4 (ng/dL) | 1.21 ± 0.11 | 1.05 ± 0.24 | 0.55 ± 0.25 | 1.65 ± 0.75 | 0.000 |
| TG-Ab (%) | 64.3% | 78.6% | 62.5% | 100% | 0.277 |
| < 500 UI/mL | |||||
| 500-2999 UI/mL | 35.7% | 7.1% | 28.1% | 0% | |
| ≥ 3000 UI/mL | 0% | 14.3% | 9.4% | 0% | |
| TPO-Ag (%) | 0.226 | ||||
| < 500 UI/mL | 60% | 37.5% | 23.5% | 40% | |
| 500-999 UI/mL | 0% | 7.1% | 17.6% | 20% | |
| ≥ 1000 UI/mL | 40% | 57.1% | 58.8% | 40% |
*Obtained by analysis of variance and Kruskal-Wallis test according to the distribution of the data.
Obtained by χ2 test.
1Post hoc analysis showed differences between patients with subclinical hypothyroidism and hypothyroidism (p 0.029) and between those with standard thyroid profiles and subclinical hypothyroidism (p 0.045).
BMI, body mass index; F:M, female:male ratio; FT4, free thyroxine; TG-Ab, antibodies against thyroglobulin; TPO-Ag, antibodies against thyroid peroxidase; TSH, thyroid-stimulating hormone.
A statistically significant difference was found in the median BMI Z-score according to baseline thyroid function (p = 0.025). The post hoc analysis showed that BMI Z-score was lower in patients with subclinical hypothyroidism than in those with hypothyroidism (p = 0.029) and normal thyroid function (p = 0.045) (Figure 1).

Figure 1 Body mass index (BMI) Z-score according to thyroid function at diagnosis. According to the thyroid function at diagnosis, there is a statistical difference in BMI Z-score (p = 0.025 obtained by Kruskal-Wallis test for independent samples). Post hoc analysis showed differences between patients with subclinical hypothyroidism and hypothyroidism (p = 0.029) and between those with standard thyroid profiles and subclinical hypothyroidism (p = 0.045).
We found a frequency of overweight of 19.2% and obesity of 15.4% in the population studied, with a combined prevalence of overweight + obesity of 34.6%.
The frequency of overweight and obesity was different between groups, with a higher proportion of overweight in patients with hyperthyroidism, while obesity was more common in those with hypothyroidism (p = 0.021) (Table 2).
Table 2 Frequency of overweight and obesity concerning thyroid function at diagnosis
| Euthyroidism (n = 17) | Subclinical hypothyroidism (n = 15) | Hypothyroidism (n = 39) | Hyperthyroidism (n = 7) | |
|---|---|---|---|---|
| Normal weight* (%) | 9 (52.9%) | 14 (93.3%) | 24 (61.5%) | 4 (57.1%) |
| Overweight* (%) | 6 (35.3%) | 1 (6.7%) | 5 (12.8%) | 3 (42.9%) |
| Obesity* (%) | 2 (11.8%) | 0 (0%) | 10 (25.6%) | 0 (0%) |
*Classification of nutritional status according to the criteria of the Endocrine Society15
There is a significant difference in the frequency of normal weight, overweight and obesity in relation to thyroid function at diagnosis (p = 0.021 obtained by Pearson's χ2 test).
Eighty percent of patients with obesity at diagnosis presented hypothyroidism compared with 42% of the non-obesity group (p = 0.009). Patients in the obesity group had higher TSH levels (p = 0.009) and lower free plasma thyroxine (FT4) (p = 0.001) and triiodothyronine (T3T) (p = 0.003) than the overweight and normal-weight groups. No differences were found in Tg-Ab titers according to nutritional status (p = 0.97). Although patients with obesity presented more frequently low (< 500 IU/mL) and less frequently high TPO-Ab titers (≥ 1000 IU/mL) when compared with the overweight and normal-weight groups, this difference was not statistically significant (p = 0.338) (Figure 2).
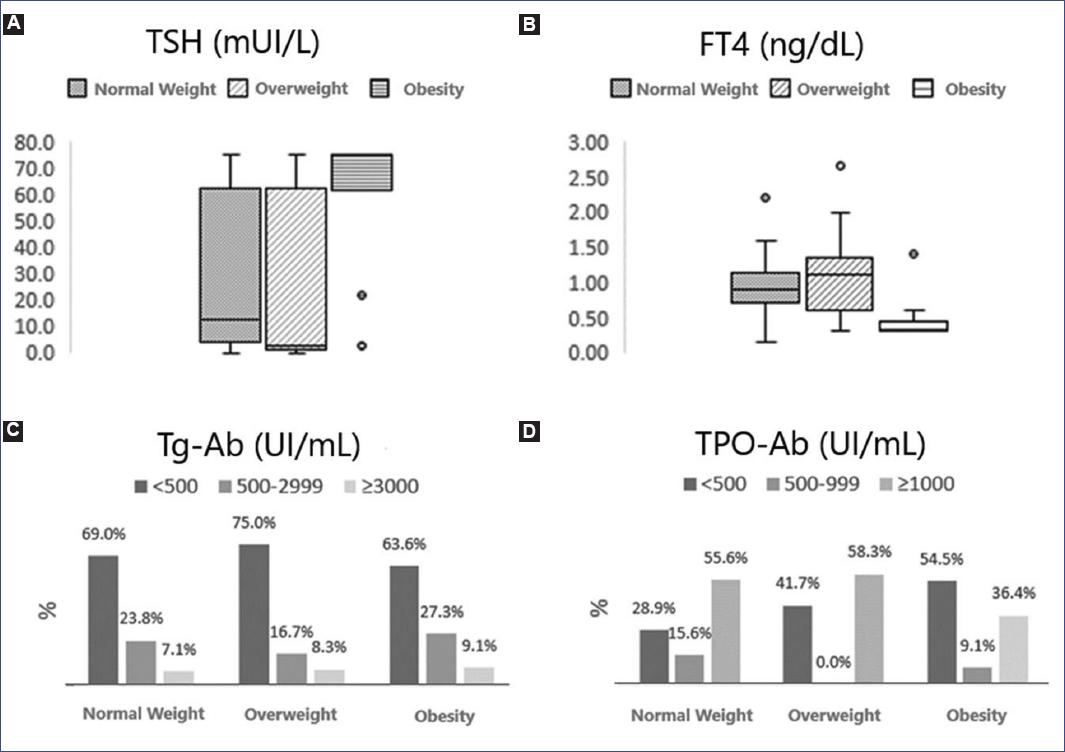
Figure 2 Biochemical profile at diagnosis according to nutritional status. A: patients with obesity presented higher TSH levels (p = 0.009 obtained by Kruskal-Wallis test). B: lower levels of FT4 (p = 0.001 obtained by Kruskal-Wallis test) than patients with normal weight and overweight. C: the Tg-Ab titer at diagnosis was similar between groups (p = 0.977 obtained by the Χ2 test). D: patients with obesity presented more frequently low titers (< 500 IU/mL) and less frequently high titers (≥ 1000 IU/mL) of TPO-Ab than patients with overweight and normal weight, the difference was not significant (p = 0.338 obtained by test Χ2).Ab, antibodies; FT4, free plasma thyroxine; Tg, thyroglobulin; TPO, thyroperoxidase enzyme.
Levothyroxine treatment was initially used in 61 patients: 39 with hypothyroidism, 13 with subclinical hypothyroidism, and nine with normal thyroid function. The mean initial dose of levothyroxine was 2.18 ± 0.65 mg/kg/day. Seventeen patients did not receive medical treatment, including seven patients with hyperthyroidism, two patients with subclinical hypothyroidism, and eight with standard thyroid function tests at diagnosis.
A repeated-measures ANOVA test was performed, showing a statistically significant decrease in BMI percentile at 6 months and 12 months (p = 0.016). However, no significant difference was observed between those who received initial treatment with levothyroxine and the rest of the patients (p = 0.456). A subgroup analysis was performed according to thyroid function at diagnosis and the received management, but no statistically significant differences were found in BMI percentile changes (p = 0.625) (Figure 3).
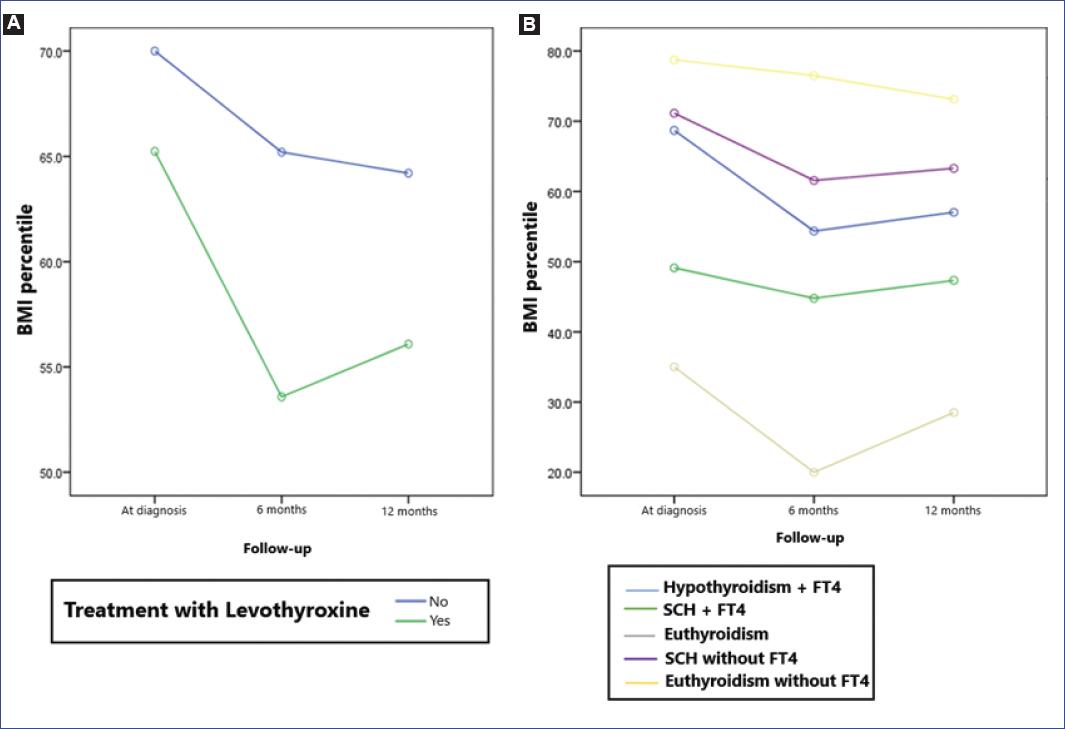
Figure 3 A: changes in body mass index (BMI) percentile during follow-up according to treatment. Repeated measures analysis of variances showed a decrease in BMI percentile at 6 and 12 months after diagnosis (p = 0.016); however, no significant difference was observed between those who received initial treatment with levothyroxine and the rest of the patients (p = 0.456). B: BMI percentile changes according to treatment and thyroid function at diagnosis. No significant difference was found when comparing patients according to thyroid function at diagnosis and treatment received (p = 0.625).FT4, free plasma thyroxine; SCH, subclinical hypothyroidism.*This analysis does not include patients with hyperthyroidism at diagnosis. Treatment with levothyroxine was initiated in 61 patients: 39 with hypothyroidism, 13 with subclinical hypothyroidism, and nine with normal thyroid function. Two patients with subclinical hypothyroidism and eight with normal thyroid function did not receive pharmacological treatment.
Discussion
In pediatrics, most CAT cases are diagnosed in females during adolescence. In accordance with other authors, our cohort shows greater prevalence of TCA in females (89.7% vs. 10.3%) and the appearance of secondary sexual characteristics in more than half of the cases at the time of diagnosis5,6,17,18.
Regarding different pediatric case series, patients with CAT usually present with normal thyroid function (21-63%) or subclinical hypothyroidism (19-47%)4,17,18. This study shows that obesity is associated with higher levels of TSH and lower levels of thyroid hormones. We need to emphasize that our hospital is a tertiary care center, where complex and mostly referral cases are treated. Therefore, a selection bias in our sample could be possible, increasing the frequency of hypothyroidism at diagnosis.
Due to the significant increase in overweight and obesity and their complications in the pediatric population worldwide, it is essential to have timely detection of both pathologies. Our observations (19.2% prevalence of overweight and 15.4% of obesity) reflect the current situation in our country, where 20% and 14% of children between 5 and 19 years of age show overweight and obesity, respectively19,20. In addition, our cohort of patients with a diagnosis of CAT showed a higher obesity rate in those with hypothyroidism at diagnosis.
In the medical literature, a bidirectional relationship between excess weight and autoimmune thyroid disease has been proposed. First, hypothyroidism may favor weight gain by reducing the basal metabolic rate; second, obesity and increased leptin levels play an immunomodulatory and pro-inflammatory role that triggers autoimmunity. Furthermore, the excess weight alone can elevate TSH levels even in the absence of autoimmunity markers, thus increasing the controversy between the cause-effect role of each player1,3,9-14,21.
Other authors have reported an increased risk of hypothyroidism in individuals with overweight and obesity. For example, a recent meta-analysis by Song et al. that included 22 studies of children and adults with excess weight found a relative risk of hypothyroidism of 3.1 in patients with obesity, while the risk of subclinical hypothyroidism was reported to be 1.722. In the present study, we found that 80% of patients with CAT and obesity presented hypothyroidism at diagnosis compared with 33% of patients with overweight and 47% of subjects with normal weight.
The factors that determine the biochemical pattern in CAT at diagnosis are not fully understood; however, it has been suggested that younger age and prepubertal status at presentation may be associated with the presence of hypothyroidism23. In this study, we observed that hypothyroidism was found in younger patients at the time of diagnosis and more frequently in the prepubertal stage, although no statistical significance was observed. Our findings indicate that obesity is associated with hypothyroidism as an initial biochemical pattern in patients with autoimmune thyroiditis; the same effect was not observed in patients with overweight.
Valea et al. compared TSH and thyroid hormone levels in a group of adults with CAT and classified them according to their BMI, showing higher TSH levels and lower levels of T3 and T4 in patients with excess weight1. As observed in adults, the group of patients with obesity in our study presented higher TSH levels and lower levels of free T3T and FT4.
Other authors have reported higher levels of TPO-Ab in patients with overweight and obesity1,22. On the contrary, in our study, patients with obesity presented TPO-Ab levels in lower ranges than those with normal weight and overweight, although the difference was not significant. One of the main limitations of this analysis was the laboratory's upper detection limit that did not allow analyzing antibody levels as a quantitative variable.
Another important finding was the significant decrease in BMI percentile at 6 and 12 months in the studied individuals, which was similar between groups regardless of treatment and thyroid function. Treatment with levothyroxine in patients with CAT without hypothyroidism is not indicated; however, it is used in patients with significant goiter or frankly elevated or steadily rising anti-thyroid antibody titers in many centers. Although an increased risk of progression to hypothyroidism has been described in patients with high titers of anti-thyroid antibodies, and treatment with levothyroxine has been shown to decrease titers in some studies, no clinical benefit from this practice has been demonstrated8,24. Our findings indicate that levothyroxine does not improve body composition in patients with CAT. Therefore, the presence of overweight or obesity should not be used as a parameter to decide the initiation of levothyroxine treatment.
Weight loss is likely to be observed in all groups during follow-up, as hygienic-dietary modifications are part of the treatment offered to every patient with CAT in our clinic, reinforcing the importance of hygienic dietary modifications in the comprehensive treatment of patients with CAT.
In addition to cardiometabolic complications classically associated with excess weight, thyroid disorders, including CAT, appear to be related to overweight and obesity.
It is known that patients with overweight and obesity have a higher risk of presenting antibodies against thyroid antigens. This study shows that obesity is associated with high levels of TSH and low levels of thyroid hormones, and a high frequency of hypothyroidism as an initial biochemical pattern in children and adolescents with CAT. The use of levothyroxine in patients with chronic autoimmune thyroiditis does not have a significant effect on body composition and, therefore, the presence of overweight or obesity does not justify its use in patients without hypothyroidism. Although this observational study does not resolve the dilemma of the directionality of the effect, it reinforces and confirms a significant association between the two variables.
Further high-quality research is required to determine the direction of causality between excess weight and autoimmune thyroiditis and issue recommendations on thyroid function assessment in patients with obesity. Therefore, this study could be considered as an initial step in direction.











 text new page (beta)
text new page (beta)


