Services on Demand
Journal
Article
Indicators
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Boletín médico del Hospital Infantil de México
Print version ISSN 1665-1146
Bol. Med. Hosp. Infant. Mex. vol.68 n.1 México Jan./Feb. 2011
Artículo original
A scoring system to predict superinfections in high-risk febrile neutropenic children with cancer
Hugo Paganini,1 Juliana Caccavo,1 Clarisa Aguirre,1 Sandra Gómez,1 Pedro Zubizarreta2
1 Infectious Diseases Department
2 Oncology and Hematology Department, Hospital de Pediatría Prof. Dr. Juan P. Garrahan, Buenos Aires, Argentina
Correspondence to:
Hugo Paganini, MD Combate de los Pozos 1881 (1245)
Buenos Aires, Argentina Tel: 54.11.43083400,
Ext: 1751 Fax: 54.11.43085325
E-mail: hpaganini@yahoo.com.ar
Received for publication: 26-07-10
Accepted for publication: 22-11-10
Abstract
Background. No scoring system has been published to date to assess the risk of superinfections (SI) for high-risk children with febrile neutropenia (HRFN).
Methods. SI diagnoses during or 1 week after initiating antibiotic therapy in HRFN children were evaluated. Eight hundred and forty-nine episodes of febrile neutropenia (FN) were included in a prospective study to evaluate a scoring system designed to identify SI.
Results. In the derivation set (566 episodes), 17% had SI. A multivariate analysis identified the following significant SI-related risk factors: acute lymphoblastic leukemia-acute myeloid leukemia (ALL-AML, OR, 1.87; 95% CI, 1.13-3.10), central venous catheter (OR, 2.11; 95% CI, 1.23-3.62), and febrile episode occurring within 10 days after chemotherapy (OR, 1.86; 95% CI, 1.09-3.15). A SI scoring system could be built: 1 point for ALL-AML, 1 point for the presence of a central venous catheter, and 1 point for the febrile episode occurring within 10 days after chemotherapy. If patients collected 3 points, then their risk of SI was 25.8%. With 2 points the risk was 16.7%, and with one minimum score of 1 point, their risk was 10.9%. The sensitivity to predict SS was 100% and its negative predictive value (NPV) was 100%. In the validation set (283 episodes), 49 (17%) children had SI. For children with scores > 0, the scoring system yielded a sensitivity of 100%, and a NPV of 100% for predicting SI.
Conclusions. The use of a SI score for HRFN patients was statistically validated by these results. A better initial predictive approach may allow improved therapeutic decisions for these children.
Keywords: superinfections, secondary infections, febrile neutropenia, children, cancer.
INTRODUCTION
During the last decade, significant progress was achieved in the comprehensive approach to diagnosis and treatment of infections during neutropenia in children with cancer under chemotherapy.1 Intensive new chemotherapy schedules, the massive use of intravenous devices and the major use of antimicrobial therapy or prophylaxis deepened the complexity of this approach.1,2
Likewise, a very judicious progress has been made in the identification of severe infection-related risk factors in febrile neutropenia (FN).3-6 Several studies proved the importance of the categorization of FN episodes in high or low risk.6-9 The type and status of baseline malignant disease, the presence of high-risk infectious foci (i.e., face orperianal cellulitis, enteritis or respiratory distress), the presence of comorbidity, bone marrow depression, the febrile episode occurring a short time after chemotherapy administration, and high CRP serum levels are the most important risk factors.1,3,10 The application of these criteria has produced a positive impact in terms of the rational use of antimicrobial therapy and the improvement of mortality rates of children with FN. Death related to FN has been minimized, now being < 5% in the majority of reported series in Argentina and worldwide, with worse outcome in high-risk patients.1,5,10,11
High-dose chemotherapy and intensive bacteria selection induced by antimicrobial therapies have contributed to longer neutropenia periods and higher exposure to undergo secondary infections or superinfections (SI) during the course of the same FN episode.12 There is scarce reported information on this theme, particularly in pediatrics. Reported incidence of SI in adults occurs in between 12% and 25% of patients and is a major cause of mortality in patients with FN.12-15
The use of a scoring system to define risk factors is of vital importance because through the analysis of simple clinical variables the onset of severe infectious complications or death may be predicted. Mortality scores in adults16 and children17 with FN have been reported. But so far, no scoring system based on risk factors leading to SI has been reported.
With the objective of defining a scoring system to predict the onset of SI and to validate it in a different population of children with high-risk FN, a prospective study was conducted. Previously, risk factors predisposing to SI, clinical features and outcome of SI were all analyzed.
METHODS
From September 2005 to March 2008 a prospective study was conducted. It was comprised of two steps. During the first step (Step A or definition of the derivation set, from September 2005 to December 2006), a risk score to develop SI was elaborated. During the second step of the study (Step B or validation step, from March 2007 to March 2008), the scoring system was statistically proven and validated.
All consecutive cancer patients hospitalized for high-risk FN were included. The study was conducted following the guidelines of the Declaration of Helsinki and was approved by our institutional ethics committee.
Inclusion criteria were 1) children < 18 years old with high-risk FN after chemotherapy for primary malignant disease, 2) absolute neutrophil count (ANC) < 500/mm3, and 3) one episode of fever > 38.5°C or two recordings > 38.1°C within 24 h. Patients undergoing bone marrow transplantation were excluded.
The following data were collected from each patient at onset: age, gender, type and stage of the baseline malignant disease, prediction of duration of neutropenia, intravascular devices, antibiotic delivery during the previous month, infection focus, and use of granulocytic colony stimulant factors (G-CSF). Further data registered for analysis were microbiological findings, therapy employed, and final outcome.
The FN episode was considered as high risk supported by the following evidence-based criteria: 1) severe comor-bidity (i.e., incoercible bleeding, refractory hypoglycemia and hypocalcaemia, hypotension, altered mental status, renal insufficiency (estimated glomerular filtration rate of < 50% normal for age, hypoglycemia); 2) respiratory failure; 3) poor clinical condition; 4) anal, facial, pericathe-ter, or oral cellulitis, enteritis, sepsis; 5) gingivitis and/or, mucositis; 6) advanced baseline malignant disease.4,5,7,9,10
Blood and urine cultures and a chest X-ray were taken at onset in each case. In case of skin infection, enteritis or pharyngitis, microbiological samples were also obtained. If the patient had a long-term intravenous device, blood samples from each catheter lumen were taken for quantitative cultures. After initial assessment the patient was categorized as high or low risk.
High-risk patients remained hospitalized for treatment and close observation. Therapy was stopped whenever the child remained > 72 h without fever and showed and absolute neutrophil count > 100/mm3.
Renal failure was defined as a fall of 50% of the glomerular filtration rate according to patient age. Liver impairment was defined as a serum alanine aminotransfe-rase level >4 times normal orbilirubin > 3 mg/dL. Sepsis, septic shock, and multiorgan failure were defined as previously reported.18 Metabolic data were analyzed based on previously reported recommendations.19
The advanced stage of baseline malignant disease was stated if there was a bone marrow disease compromise, disease recurrence, second tumor, high-dose chemotherapy, or associated genetic abnormality.
SI was defined as any infectious or febrile episode not present when initial detection of a FN occurred within the period of antimicrobial treatment or within 7 days after completing this therapy.12
Treatment was assumed as successful when the FN episode resolved without a new need of hospitalization within 7 days alter discharge. The end-point variable was the presence of SI.
Data from episodes with SI were compared with those without during both phases of the study episodes (definition of the derivation set and validation step). Data analysis was performed using the Statistical Package for Social Sciences (SPSS) v.11.0. Descriptive data were expressed as percentages, medians, and ranges or as mean and standard deviation. Associations between potential risk factors and bacteremia were analyzed through biva-riate and multivariate methods. Statistical significance was calculated using chi-square or Fisher exact test for nominal variables and Student t tests or Wilcoxon rank-sum test for numerical variables. All independent variables that yield p < 0.10 in the bivariate analysis were included as covariables in the multivariate analysis (logistic regression, forward stepwise method; p in = 0.05; p out = 0.10). Risk is reported as the relative risk (RR) for bivariate analyses and adjusted odds ratio (OR) for logistic regression analyses. Statistical significance is reported as p and 95% confidence interval (95% CI). Model and score performance are expressed as sensitivity, specificity, predictive values and overall accuracy. All p values < 0.05 were regarded as significant.
RESULTS
During the accrual study period, 848 episodes of FN in 628 pediatric cancer patients were included, 143 (17%) of whom developed SI.
Superinfections
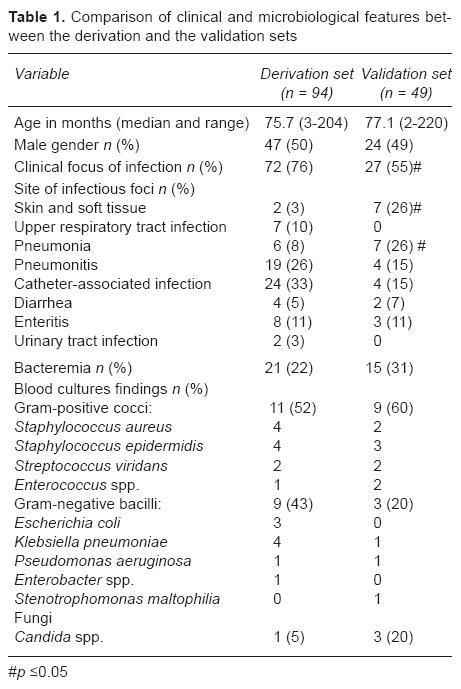
During Step A, where the derivation set was defined, 94 (17%) episodes of SI were diagnosed, and during the validation Step B, 49 (17%) episodes were detected (p = NS). The median time to SI onset was 9.3 days (range: 1-25 days). There were no significant differences in SI onset between the study phases (8.1 vs. 10.6 days for Step A and B, respectively) (p = NS). It could be stated that patients included in the derivation set showed with more frequency a clinical focus of infection. They had lower levels of skin and soft tissue infections and pneumonia than those included in the validation set (p < 0.05). The incidence of bacteremia was similar for both groups (22% vs. 31% in the derivation set and the validation set, respectively, p = NS). Gram-positive cocci were more common, coagulase-negative Staphylococci being the predominant microorganism for both groups of patients. Children with SI included in the derivation set showed a higher incidence of Gram-negative bacilli infections (p = NS). Candida spp. infections were more frequent in the validation set (p = NS) (Table 1).
Step A: Defining the Derivation Set
During this period, 566 episodes of FN in 432 patients were included, of whom 94 developed SI (17%). Median age of patients included in this phase was 60 months and did not differ from patients included in the validation set (84 months, p = NS).
No statistically significant differences in demographic and clinical features were observed in the FN episodes included in both phases of the study. During both periods of the study, leukemia, and particularly acute lymphoblastic leukemia (ALL), was the most common malignant disease. Twenty-five percent of these patients were undergoing the induction phase of leukemia therapy, and most of them had severe neutropenia (ANC < 100/mm3). Two thirds of patients had a central venous catheter and most showed a clinical focus of infection at onset. Mortality was low in both phases of the study (2%) (Table 2).
A bivariate analysis was conducted to investigate the risk factors to develop SI. It could be stated that the children with SI were < 1 year of age (RR: 1.74; 95% CI: 1.12-2.69). Leukemia was the most common baseline disease (RR: 1.95; 95% CI: 1.27-2.98). There was a higher rate of presence of a central venous catheter (RR: 2.21; 95% CI: 1.33-3.37) and shorter time since the last chemotherapy administration (6.4 vs. 8.4 in patients with and without SI, respectively, p < 0.001). When this last variable was split, it could be determined that a higher risk of developing SI appeared when the time elapsed from the last chemotherapy was < 10 days (RR: 1.72; 95% CI: 1.09-2.70) (Table 3).
All variables that proved to be statistically significant (p < 0.20) were taken into account to perform the mul-tivariate analysis. The model includes three significant variables as independently associated wit SI: acute leukemia as malignant baseline disease (OR: 1.87; 95% CI: 1.13-3.10), presence of central venous device (OR: 2.11; 95% CI: 1.23-3.62), and time elapsed since the last chemotherapy administration < 10 days (OR: 1.86; 95% CI: 1.09-3.15) (Table 4).
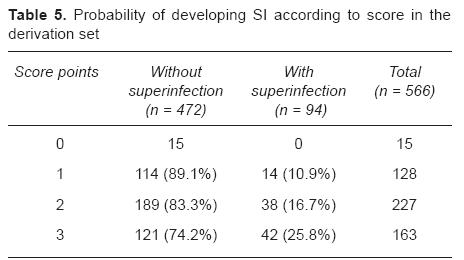
The score to predict SI development was built based on these three variables. Given the fact that their statistical weight (beta coefficient and adjusted odds ratio, Table 4) was very similar and that this would simplify the use of the score in everyday practice, one point was assigned in the score to each significant variable (leukemia as baseline disease, presence of central venous device, and time elapsed since last chemotherapy administration < 10 days). Patients with only one point of the score had an estimate risk of 10.9% of developing SI, with 2 points: 16.7%, and summing up 3 points: 25.8%. Contrariwise, patients with a score of 0 seemed to have no risk to develop SI (Table 5). This cut-point of the score was significantly correlated with SI development (p ≤ 0.0001, chi square). All patients with SI had a score > 0 and no patient with a score of "0" points developed SI (sensitivity and negative predictive value of 100%).
Step B: The Validation Phase
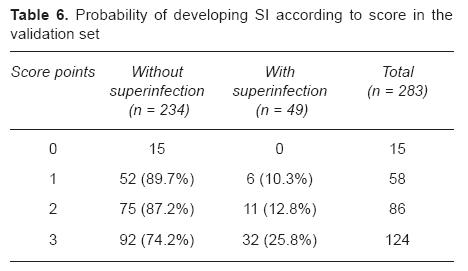
During this phase, 283 FN episodes in 196 patients were included. When the score defined by the derivation set was applied to the validation set, a highly significant association between the score and the presence of SI was observed (p < 0.0001, chi square). Incidence of SI increased with the score value (10.3% with 1 point, 12.8% with 2 points and 25.8% with 3 points, Table 6). Again, all patients with SI had a score > 0 (sensitivity 100%; 95% CI: 92.7-100%), and no patient with a score of "0" points developed SI (negative predictive value 100%; 95% CI: 79.6%-100%).
DISCUSSION
Risk assessment allows the classification FN patients in high and low risk, adapting therapies and allowing new treatment modalities for low-risk patients, which are cost-saving and improve their quality of life or more aggressive and precocious approaches for the high risk subset of patients.20-24 This last group of patients develops subsequent secondary infections or SI, which are a frequent problem with high morbidity and mortality rates.12-15 In spite of the importance of this subject, very few studies have addressed the matter and none in children. SI were studied secondarily in clinical trials addressing the treatment of FN where the efficacy of antimicrobial agents was compared.12
The reported incidence of SI in adults was between 12% and 24%,12-15 results in accordance with ours (17%). This somewhat wide variation in the incidence of SI may be due to different features of the populations selected for the studies.
SI usually appear after the first 5 days of therapy. Only a small proportion of them take place before that time.12 Median time for SI onset in our study was 9.3 days, similar to reported international evidence where the average was 10 days.12-15
The microbial agents responsible for SI vary according to studies. Nucci et al reported a high incidence of fungi isolations (33%) in their patients.13 However, in the series of Arkova et al. > 50 bacteriological isolations in patients with secondary infections (50%) were caused by gram-positive bacteria, and in the second place fungi were found in 42% of the isolations.12 In our pediatric series, gram-positive bacteria were more frequently found in blood cultures. Staphylococcus epidermidis was the most common, followed by S. aureus. In third place were the fungi. We could document a higher incidence of Klebsiella spp. in children with SI. This agent was not frequently reported in other series. This may be displaying a particular epidemiological context in our institution. In a previous study on intranosocomial infections carried out in our Hospital, Klebsiella spp. was a highly prevalent pathogen.25
The incidence of identification of a clinical focus of infection in our patients was high (65%) in comparison with that reported by Arkova et al (30%).12 The most frequent clinical foci of infection were respiratory, in accordance with data reported by these authors in a study including 1720 adult patients with FN.12
Infections associated with the presence of central venous catheters followed in terms of incidence in our setting. In the multivariate analysis it could be determined that patients with AML or ALL on induction, with central intravenous catheter and chemotherapy delivered before 10 days from the onset of a FN episode, were all significant risk factors to develop SI.
Onset of SI is in close relation with the duration of severe neutropenia.12,13 In patients receiving more intensive chemotherapy, this condition is always present. In our study, patients undergoing induction of remission therapy for acute leukemia displayed all risk factors associated with developing SI. Other authors reported the association of acute leukemia and SI in adults.12,13
Santolaya et al. reported that a short time elapsed since the last chemotherapy administration is a predictive factor to develop SI in children.26 This was a major risk factor to develop SI in our setting. We could further split this variable to define that a time < 10 days was statistically significant to predict these infections.
The use of intravenous catheters is associated with the onset of fungemia and bacteremia in patients with FN.27,28 Nucci et al. detected that the use of central venous catheters was a risk factor to develop SI in a group of 3 3 adult patients with FN, 46 of whom developed secondary infections.13 Likewise, Arkova et al. could state that the presence of a central venous catheter was a risk factor to develop SI in a multivariate analysis of their series.12 We were able to observe the same findings in our setting.
SI was reported to have a high mortality rate. In data reported by Arkova et al., mortality was 5.4% in the subset of patients with SI and 1.4% for those patients who did not develop secondary infections.12 Other series showed higher rates, even up to 24%.12-15 In our study, 14% of children who underwent SI died, whereas mortality rate of patients without SI was only 2%.
Several authors studied the risk factors to acquire severe infections or to die as a result of the infection.1 Different scores were put forward to assess the risk of mortality in adult cancer patients with FN admitted to Intensive Care Units.16 Recently, we designed and tested a score to predict mortality in children with FN. Its application implied a major clinical advantage because the recognition of simple variables may lead to an appropriate and reproducible risk categorization of these patients and subsequently optimize the therapeutic approach.17 To the best of the authors' knowledge, there have been no scores reported up to now to assess the risk of developing SI in children with FN.
The scoring system defined in the present study was able to identify FN children in risk of developing SI with a high sensitivity and a high negative predictive value, both of which are necessary conditions in a screening tool for clinical practice. The variables introduced in the score are easy to obtain (acute leukemia under induction therapy, presence of a central venous device, and < 10 days elapsed since the last chemotherapy administration), easy to apply and reproduce results in children with FN. Its validation with a significant number of patients gave a solid statistical support to justify its safe usage in FN pediatric cancer patients.
It may be proven that SI is a fairly common event in high-risk FN children with cancer therapy. SI are generally associated with a clinical focus of infection (usually respiratory or enteric infections). It is strongly associated with acute leukemia under induction therapy, presence of an intravenous device, and < 10 days elapsed since the last chemotherapy administration. In our setting Klebsiella spp. is frequently found in blood cultures. SI implied high morbidity rates, long hospitalizations, frequent admission in ICU, longer need of parenteral antibiotics, and higher mortality.
The judicious application of this score may permit the early detection of SI, allowing a faster therapeutic intervention and eventually improving the associated morbidity and mortality rates.
REFERENCES
1. Orudjev E, Lange BJ. Evolving concepts of management of febrile neutropenia in children with cancer. Med Pediatr Oncol 2002;39:77-85. [ Links ]
2. Cordonnier C, Buzyn A, Leverger G, Herbrecht R, Hunault M, Leclercq R, et al. Epidemiology and risk factors for gram-positive coccal infections in neutropenia: toward a more targeted antibiotic stratategy. Clin Infect Dis 2003;36:149-158. [ Links ]
3. Paesmans M. Risk factors assessment in febrile neutropenia. Int J Antmicrob Agents 2000;16:107-111. [ Links ]
4. Rackoff WR, Gonin R, Robinson C, Kreissman SG, Breitfeld PB. Predicting the risk of bacteremia in children with fever and neutropenia. J Clin Oncol 1996; 14:919-924. [ Links ]
5. Paganini H, Bologna R, Debbag R, Casimir L, Gómez S, Ro-sanova M, et al. Fever and neutropenia in children with cancer in one pediatric hospital in Argentina. Pediatr Hematol Oncol. 1998; 15:405-413. [ Links ]
6. Talcott JA, Finberg R, Mayer RJ, Goldman L. The medical course of cancer patients with fever and neutropenia: clinical identification of a low-risk subgroup at presentation. Arch Inter Med 1988; 148:2561-2568. [ Links ]
7. Talcott JA, Siegel RD, Finberg R, Goldman L. Risk assessment in cancer patients with fever and neutropenia: a prospective, two-center validation of a prediction rule. J Clin Oncol 1992; 10:316-322. [ Links ]
8. Klastersky J, Paesmans M, Rubenstein RB, Boyer M, Elting L, Feld R, et al. The Multinational Association for Supportive Care in Cancer risk index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol 2000;18:3038-3051. [ Links ]
9. Paganini H, Rodriguez-Brieshcke T, Zubizarreta P, Latella A, Firpo V, Fernández C, et al. Criteria of low risk of mortality in children with neutropenia and fever during cancer chemotherapy. Medicina (B Aires) 2001;61:63-66. [ Links ]
10. Hughes WT, Armstrong D, Bodey G, Bow EJ, Brown AE, Calandra T, et al. 2002 Guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 2002;34:730-751. [ Links ]
11. Pizzo PA, Robichaud KJ, Wesley R, Commers JR. Fever in the pediatric and young adult patient with cancer. A prospective study of 1001 episodes. Medicine (Baltimore) 1982;61:153-165. [ Links ]
12. Akova M, Paesmans M, Calandra T, Viscoli C, International Antimicrobial Therapy Group of the European Organization for Research and Treatment of Cancer. A European Organization for Research and Treatment of Cancer-International Antimicrobial Therapy Group study of secondary infections in febrile, neutropenic patients with cancer. Clin Infect Dis 2005;40:239-245. [ Links ]
13. Nucci M, Spector N, Bueno AP, Solza C, Perecmanis T, Bacha PC, et al. Risk factors and attributable mortality associated with superinfections in neutropenic patients with cancer. Clin Infect Dis 1997;24:575-579. [ Links ]
14. Feld R, Goodman PJ, Higgins B, De Pauw BE, Deresinski S, Donnelly JP, et al. Prognostic factors for the development of superinfections in febrile neutropenic cancer patients (abstract 1695). 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy, 11-14 October 1992, Anaheim, CA. [ Links ]
15. Serra P, Santini C, Venditti M, Mandelli F, Martino D. Superinfections during antimicrobial treatment with betalactam-aminoglycoside combinations in neutropenic patients with hematologic malignancies. Infection 1985;13(suppl 1 ):S115-122. [ Links ]
16. Guiget M, Blot F, Escudier B, Antoun S, Leclercq B, Nitenberg G. Severity-of-illness scores for neutropenic cancer patients in an intensive care unit: which is the best predictor? Do multiple assessment times improve the predictive value? Crit Care Med 1998;26:488-493. [ Links ]
17. Paganini H, Aguirre C, Puppa G, Garbini C, Javier RG, Ensinck G, et al. A prospective, multicentric scoring system to predict mortality in febrile neutropenic children with cancer. Cancer 2007;109:2572-2579. [ Links ]
18. Sáez-Llorens X, McCracken GH Jr. Sepsis syndrome and septic shock in pediatrics: current concepts of terminology, pathophy-siology, and management. J Pediatr 1993;123:497-508. [ Links ]
19. Lacy CF, Armstrong LL, Godman MP, Lance LL. Drug Information Handbook. Hudson, OH: Lexi-Comp; 1999. [ Links ]
20. Paganini H, Sarkis CM, De Martino MG, Zubizarreta PA, Casimir L, Fernández C, et al. Oral administration of cefixime to lower risk of febrile neutropenic children with cancer. Cancer 2000;88:2848-2852. [ Links ]
21. Paganini H, Rodriguez-Brischcke T, Zubizarreta P, Latella A, Firpo V, Casimir L, et al. Oral ciprofloxacin in the management of children with cancer with lower risk febrile neutropenia. A randomized controlled trial. Cancer 2001 ;91:1563-1567. [ Links ]
22. Mullen CA, Petropoulos D, Roberts WM, Rytting M, Zipf T, Chan KW, et al. Outpatient treatment of fever and neutropenia for low risk pediatric cancer patients. Cancer 1999;86:126-134. [ Links ]
23. Rolston KV. New trends in patient management: risk-based therapy for febrile patients with neutropenia. Clin Infect Dis 1999;29:515-521. [ Links ]
24. Paganini H, Gomez S, Ruvinsky S, Zubizarreta P, Latella A, Fraquelli L, et al. Outpatient, sequential, parenteral-oral antibiotic therapy for lower risk febrile neutropenia in children with malignant disease. A single-center, randomized, controlled trial in Argentina. Cancer 2003;97:1775-1780. [ Links ]
25. Paganini H, Rodriguez-Brieshcke T, Casimir L, Seu S. Risk factors for nosocomial bacterial infection in children: a case-control study. Medicina (B Aires) 1999;59:43-48. [ Links ]
26. Santolaya ME, Alvarez AM, Becker A, Cofré J, Enríquez N, O'Ryan M, et al. Prospective, multicenter evaluation of risk factors associated with invasive bacterial infection in children with cancer, neutropenia and fever. J Clin Oncol 2001; 19:3415-3421. [ Links ]
27. Raad II, Bodey GP. Infectious complications of indwelling vascular catheters. Clin Infect Dis 1992;15:197-208. [ Links ]
28. Wenzel RP. The mortality of hospital-acquired bloodstream infections: need for a new vital statistic? Int J Epidemiol 1988;17:225-227. [ Links ]














