The more frequently accepted strategy for the closure of complex atrial septal defects (ASDs) includes the use of one ASD device to close the largest defect, followed by another atrial septal defect (ASD), patent foramen ovale (PFO), or cribriform device to close the remaining smaller defects, but this requires assessment on a case-by-case basis1-3. This study aimed to report our experience in closing complex ASDs implanting pfm Nit Occlud® ASD-R (Fig. 1 A and B) and PFO devices focusing on technical success and acute and mid-term outcomes.
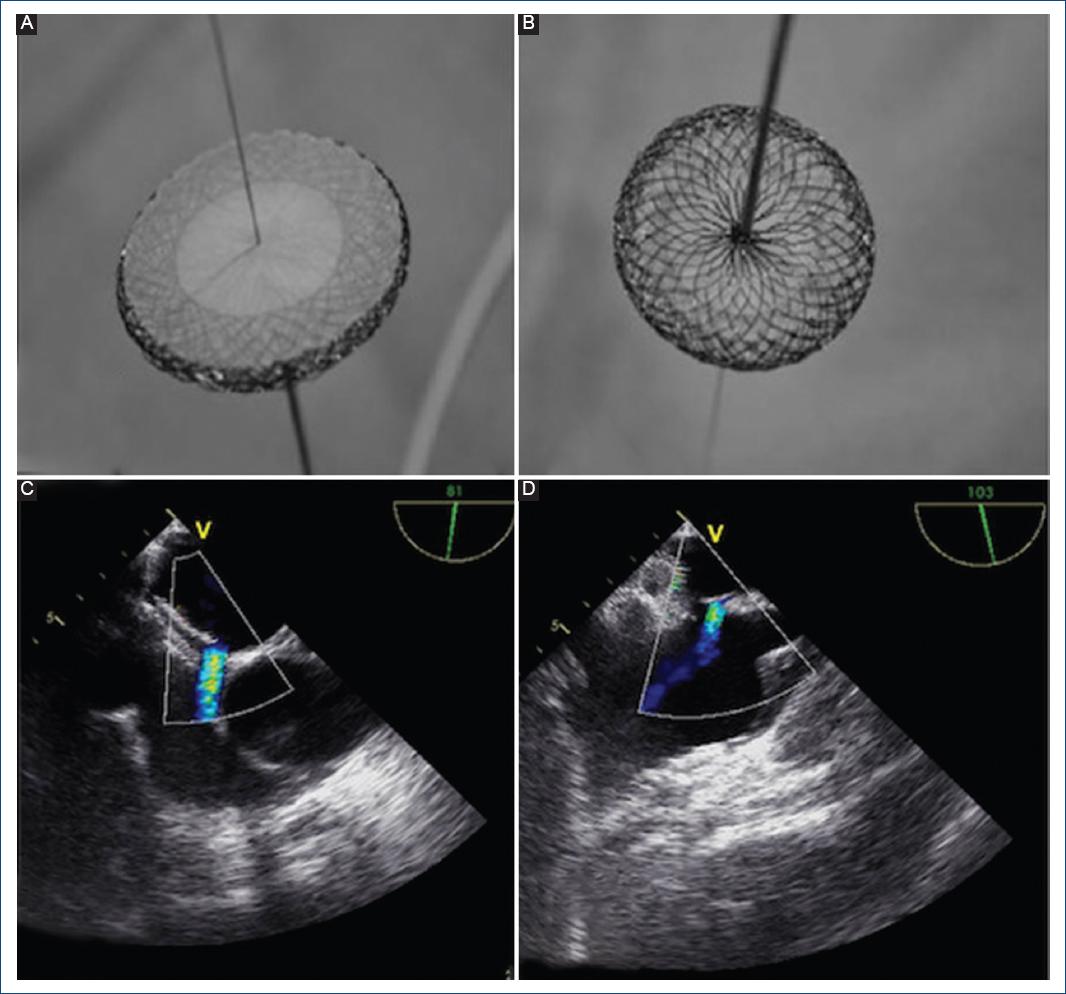
Figure 1 A: premounted Nit Occlud ASD-R device with its left atrial disc and. B: right atrial disc. The release mechanism constituted by the "locking wire" crossing the entire device and the proximal pusher are visualized. C: transesophageal Doppler color echocardiogram imaging showing a residual defect after the PFO device was released (case 1). D: transesophageal Doppler color echocardiogram identifying a residual defect proximal to the superior vena cava (case 2).
Case 1
A 52-year-old man was referred following an event of hemiparesis and aphasia. Transesophageal echocardiography (TEE) showed a PFO and a small ASD. A repeated TEE evaluation during cardiac catheterization showed a multifenestrated and aneurysmal atrial septum and a 4 mm high-risk PFO near a small ostium secundum ASD with a weak posterior rim. With the aim of closing both defects, a 30 mm Nit-Occlud PFO was deployed. After releasing this device, the atrial septum was not completely covered and a residual shunt of 12 mm through the ASD was still visualized (Fig. 1C). Finally, an 18 mm Nit-Occlud ASD-R device (oversized to offer stability) was implanted. TEE images demonstrated rectification of the atrial septum and adequate position of both devices without residual shunting or impingement on intracardiac structures.
Case 2
A 37-year-old man was diagnosed with an ostium secundum ASD after complaining of dyspnea, fatigue, and palpitations. A complex ASD was identified under TEE visualization, which showed a large multi fenestrated ostium secundum ASD over an aneurysmal septum. At least four defects (12 mm, 8 mm, 4 mm, and 3 mm) were identified. A sizing balloon placed in one of the inferior defects was measured in 4 mm, while the superior defect was balloon sized up to 12 mm. First, the multi fenestrated aneurysm was cannulated in the mid portion and a 30 mm diameter Nit Occlud PFO device was deployed, achieving atrial septum rectification and successful closure of the three smaller defects. However, a 12 mm residual defect proximal to the superior vena cava was still identified by echocardiography Doppler color (Fig. 1D). The residual defect was completely closed, implanting a 14 mm Nit-Occlud ASD-R device.
Case 3
A 64-year-old male was diagnosed with an ostium secundum ASD after syncope work-up. TEE showed two large and distant ASDs (a superior defect measuring 16 mm with an hypoplastic aortic rim, and a 12 mm inferior defect) associated with severe right ventricle enlargement (Fig. 2A and B). The distance between both defects was 14 mm. Appropriate rim visualization avoided the need of balloon sizing and left to right shunting was demonstrated by Doppler color echocardiography (Fig. 2A). Under TEE guidance, a 20 mm Nit-Occlud ASD-R was deployed within the superior and larger defect followed by the implantation of an additional 16 mm in size Nit-Occlud ASD-R device, which was deployed into the inferior defect to achieve complete closure (Fig. 2C and D).
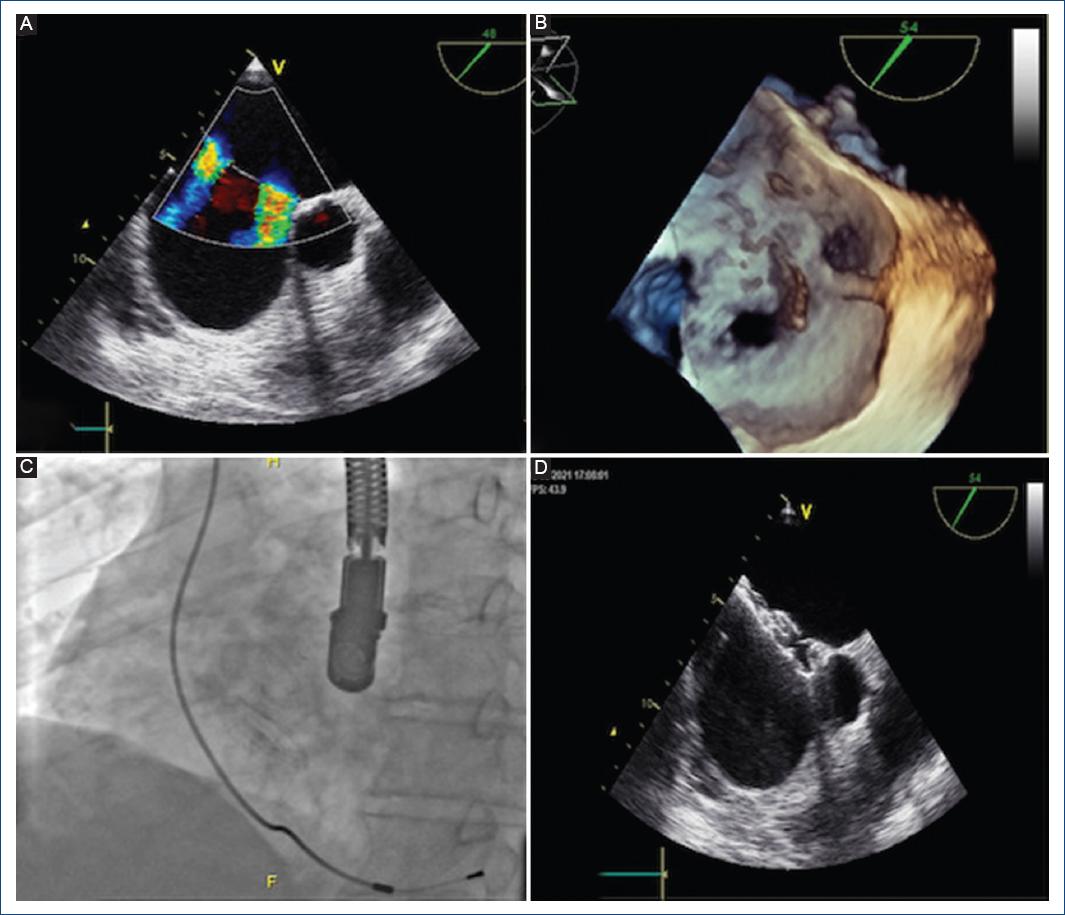
Figure 2 A: transesophageal echocardiogram imaging showing two ostium secundum type ASDs and B: 3-D transesophageal echocardiogram displaying the same complex atrial defect (Case 3). C: fluoroscopy in anterior-posterior view of both devices in situ and D: transesophageal echocardiogram showing both devices after final release (case 3).
Case 4
A 35-year-old woman with a history of percutaneous closure of a large atrial septal defect with a 24 mm Nit-Occlud ASD-R device four years before was diagnosed with a residual ASD defect of 10 mm and persistent right side chamber enlargement. A TEE showed an in situ ASD device with no residual shunt and an inferiorly located moderate to large size residual ASD measuring 12.7 mm. During a repeated intervention, a sizing balloon was inflated up to 15 mm within the residual defect, which was located inferior and posterior to the previous device and near the inferior vena cava. This residual defect had a deficient posterior rim (< 5 mm). A 16 mm Nit-Occlud ASD-R device was deployed in the residual defect reaching complete closure.
Discussion
In the last 15-25 years, percutaneous device implantation has emerged as an attractive, safe, and effective alternative to surgical treatment in the management of the ostium secundum ASD4. The presence of complex defects or the coexistence of a PFO and an additional atrial septal defect causing interatrial shunting is uncommon, but not rare5. When planning a percutaneous closure in such anatomical variants, a detailed septal evaluation is crucial to delineate the closure strategy. 3D color Doppler echocardiography and 3D printed models have been recently proposed to better plan the intervention in such cases increasing the success rate6. 2D TEE was the method of choice for control in the catheterization laboratory, especially if the rims of the interatrial septum are not flaccid; conversely, if the defect is more complex, oval or multiple, 3D TEE can provide an advantage in anatomical assessment and device selection7. In the context of appearance of additional defects, the decision of closure implanting a second device was based on the size of the residual defect and the consequences of significant shunting. The presence of a residual shunt after PFO closure was associated with an increased incidence of recurrent stroke or transient ischemic attack compared with complete closure (HR 3,05 CI 1,65-5,62)8. Although several studies have reported closure using multiple and different devices, there are no previous published series reporting occlusion implanting two Nit Occlud® devices (pfm Medical, Cologne, Germany). The Nit-Occlud ASD-R is a double-disc, self-expandable, self-centering, and low profile device, easy to position with a simple locking mechanism that allows for recapturing and repositioning several times before release. The visualization both on echocardiography and fluoroscopy is optimal. The delivery catheter is flexible, minimizing the tension on the device before final release, which is accomplished in a simple and predictable way due to its snare-like and tension-free mechanism. There are some advantageous characteristics of this device. The peculiar "reverse configuration" of the left atrial disc tightly fixes the implant to the left atrial aspect of the interatrial septum offering a secure anchoring mechanism, minimizing the risk of "pulling- through" during implantation or inadvertent embolization after release.
In our cohort of four patients, in three of them two devices were implanted during the initial intervention for closing more than one defect. In the remaining patient, a staged approach was performed due to a discovery of a large residual shunt, which was identified during follow-up after the initial procedure. At the time of discharge, all patients received aspirin 100 mg/day during 6 months. The need of continued antiaggregation after 6 months was indicated according to the neurology team.
Farhaj et al. reported on 150 patients presenting mASDs undergoing closure using different techniques and devices achieving optimal outcomes. The immediate complete closure rate was 61.3% and 6-month complete closure rate was 77.2%9. In view of these outcomes, selection of patients is probably the most important step in the whole process and it is important to determine whether the implantation of more than one occluder or oversizing a single device results in a higher risk for complications.
The more frequently described strategy includes the use of one ASD device to close the largest defect, followed by an additional cribriform type device to close the remaining defect8.
In our experience, we use a less conventional strategy: in two patients, we implanted a large PFO device with the intention to occlude all the defects; however, due to the presence of an aneurysmal atrial septum, a second ASD device was needed to achieve complete closure. In the remaining two patients, two ASD devices were used to close both distant defects (Table 1).
Table 1 Patient's defect types and device selection
| Patient | Defect type | First device | Residual shunt | Second device |
|---|---|---|---|---|
| #1 | PFO and ASD | 30 mm Nit-Occlud PFO | 12 mm | 18 mm Nit-Occlud ASD-R |
| #2 | Multiple ASD's (> 4 defects) | 30 mm Nit-Occlud PFO | 12 mm | 14 mm Nit-Occlud ASD-R |
| #3 | 2 ASD's | 20 mm Nit-Occlud ASD-R | 14 mm | 16 mm Nit-Occlud ASD-R |
| #4 | 2 ASD's (two-stage closure) | 24 mm Nit-Occlud ASD-R | 15 mm | 16 mm Nit-Occlud ASD-R |
During follow-up (median 1.4, range: 0.7-2.5), all the patients remain well with no evidence of residual shunts, erosion, or arrhythmias.
This report describes the clinical performance of more than one Nit-Occlud device (ASD-R and PFO) implanted for transcatheter closure of mASDs. In this small cohort of patients with several atrial defects which required decisions on a case-by-case basis, the use of these devices was feasible, safe, and effective resulting in adequate immediate- and short-term outcomes.
Technical success and closure rate were high, and there were no instances of immediate- or short-term complications.











 text new page (beta)
text new page (beta)


