COVID-19 mRNA vaccines have been associated with the development of myocarditis, specifically in young men after the administration of the second dose, with a low rate of 1 case/10 000 vaccinated people1.
We present the case of a 28-year-old male patient without the previous medical history referring chest pain episodes for the past 3 days. He received the second dose of BNT162b2 vaccine against COVID-19 4 days before. Electrocardiogram showed 1mm ST-segment elevation in lateral and inferior leads (Fig. 1) and high-sensitivity cardiac troponin T (hs-cTnT)) was 1470 ng/L (< 14 ng/L). Normal left ventricle (LV) ejection fraction without wall motion abnormalities (WMA) was noted in echocardiogram. Acute COVID-19 infection was ruled out by negative SARS-CoV-2 polymerase chain reaction test, chest X-ray was normal (Fig. 1).
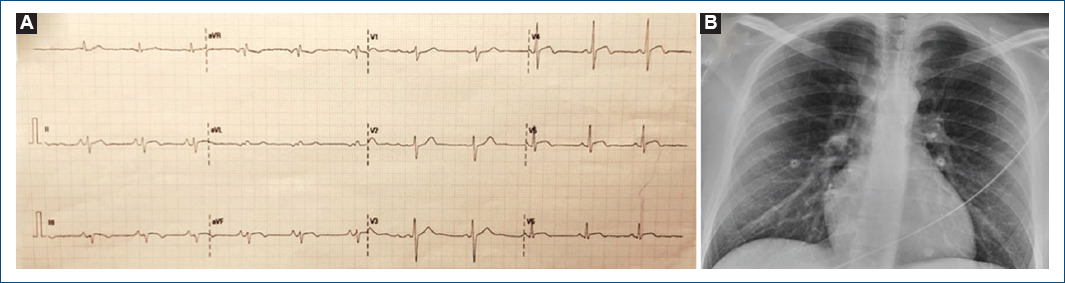
Figure 1 A: electrocardiogram showing 1 mm ST-segment elevation in lateral and inferior leads. B: normal chest X-ray.
The patient was admitted and remained asymptomatic requiring no treatment. The peak value of hs-cTnT (2200 ng/L) was reached the day 5 after vaccination. Given its low yield, no serological tests for cardiotrophic viruses were ordered. Within the first 24 h, cardiac magnetic resonance imaging was performed, and mapping sequences showed increased T2 values in inferior and inferolateral basal segments (67 ms and 63 ms; normal < 60 ms) indicating myocardial oedema (Fig. 2); native T1 was also increased in inferior basal segment (1130 ms, normal < 1050 ms, Fig. 2). Late gadolinium enhancement with subepicardial and intramyocardial pattern was observed in the region with edema, and in mid-inferior and mid-inferolateral segments (Fig. 3). LV showed normal systolic function and no WMA. The final diagnosis was acute myocarditis in relation with mRNA vaccine against COVID-19. The patient was discharged after 5 days without complications, and he has not presented any events in the subsequent 4 months.
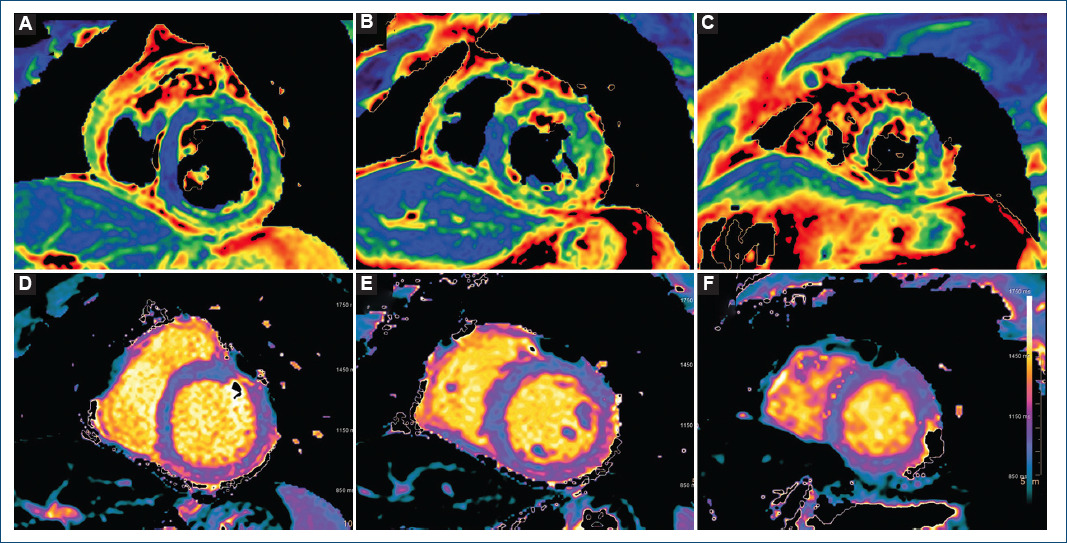
Figure 2 Advanced tissue characterization with cardiac magnetic resonance imaging: T2 mapping sequences. A: basal short axis, note the increased values in inferior and inferolateral segments. B: mid short axis. C: apical short axis. T1 mapping sequences. D: basal short axis, note the increased value in the inferior segment. E: mid short axis. F: apical short axis.
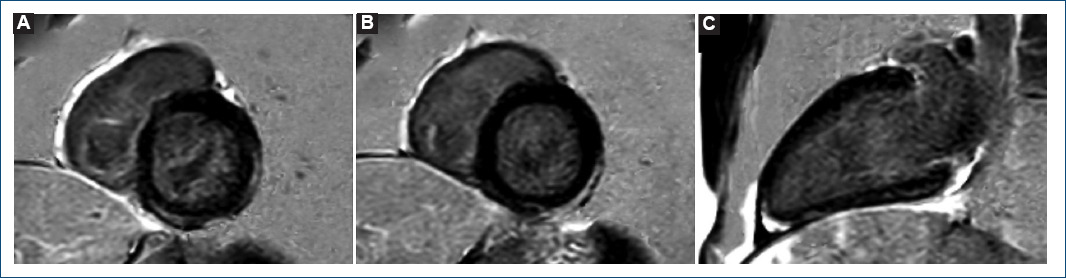
Figure 3 Late gadolinium enhancement with cardiac magnetic resonance imaging. phase-sensitive inversion recovery sequences. Note the subepicardial and intramyocardial pattern. A: basal short axis. B: mid short axis. C: 2-chamber long axis.
Albeit relative uncommon, physicians must be aware of this adverse event of COVID-19 vaccination, but keeping in mind its undoubtedly favorable benefit-risk profile1-3.











 text new page (beta)
text new page (beta)


