Introduction
Traditional cardiovascular risk factors (CVRFs) are well known for their association with the presence and prognosis of coronary artery disease (CAD)1. Although multiple risk scores were developed to predict CAD with CVRF, new biomarkers play an important role in cardiovascular prognosis. It was reported that plasma levels of N-terminal pro-brain natriuretic peptide (NT-proBNP)2 and high-sensitivity C-reactive protein (hsCRP)3 are related to cardiovascular events. There is a link between these biomarkers and the inflammatory condition and atherosclerosis as the cornerstone of CAD is indeed an inflammatory disease4.
Cyclophilins (Cyps), a subfamily of immunophilins of peptidyl-prolyl cis/trans isomerases, have been implicated in various cellular processes and involved in oxidative stress5. Some of them, such as CypA6, CypB7, and CypD8, have been associated with atherosclerotic disease. It has been published that high serum CypC levels are a possible novel biomarker for diagnosing CAD, with a good correlation using a cutoff point of 17.5 pg/mL9.
Serum CypA levels are significantly higher in patients with CAD in proportion to the severity of disease10. Moreover, CypA levels 1 month after acute myocardial infraction have an impact on the prognosis11. These findings suggest that Cyps play a continuous role in the CAD and they could be sensitive follow-up biomarkers. Since CypCs have been rarely studied in this field, we analyzed the serum CypC levels of CAD patients from baseline to 6 and 12 months for a better understanding of its behavior during the follow-up in atherosclerosis. This study is aimed at investigating the changes in serum CypC levels and assess if the cutoff point of 17.5 pg/mL keeps the ability to discriminate CAD throughout the 12-month follow-up.
Methods
Population study
The study included a total of 125 subjects (40 patients with acute CAD, 40 patients with chronic CAD, and 45 control volunteers) that were enrolled following the same protocol reported in our previous study9. Acute CAD was defined as unstable angina, non-ST-segment myocardial infarction (STEMI), or STEMI according to the current European Society of Cardiology (ESC) Practical Clinical Guidelines12,13. Chronic CAD was defined as a clinically stable syndrome without an increase in myocardial biomarkers, as defined in the ESC guidelines14. The institutional and regional ethics committee approved the study (Reference: 2016/508, Approval date: December 19, 2016) according to the principles outlined in the Helsinki Declaration. Informed oral and written consent was given by all the subjects participating in this study.
Blood sampling protocol and Cyps measurements
Peripheral blood was obtained from subjects and analyzed as previously described9. The blood was centrifuged (3000 rpm, 10 min at 4°C) and supernatants were collected and stored at −80°C until Cyps analysis. After thawed at room temperature, these supernatants were used to measure CypC levels using an ELISA kit. Absorbance measurements were taken using a microplate reader at 450 and 540 nm. Samples were always run in duplicate. The measurement range was 23.5-1.500 pg/mL for CypC. Serum levels below the lower limit of quantitation were undetectable and were therefore considered as 0 pg/mL for statistical analysis. The intra- and inter-assay coefficients of variation of the ELISA kits were < 10%. No cross-reactivity was observed between Cyp antibodies. The human cyclophilin C ELISA kit (CSB-EL018473HU) was obtained from Cusabio.
Baseline measurements
The electronic medical history was reviewed to obtain all clinical data relative to patients. Clinical characteristics and laboratory values, including NT-proBNP and hsCRP, were collected.
Statistical analysis
SPSS 24 for Windows was used for the statistical analysis. Categorical variables were presented as percentages and continuous variables were presented as means ± SEM. Kolmogorov–Smirnov (with Lilliefors correction) was first performed as a normality test. Statistical significance in qualitative variables was calculated using the Chi-square test. Continuous variables with normal distribution were compared between two groups using the Student’s t-test (including Levene’s test); otherwise, the non-parametric Mann–Whitney U-test was used. Differences between three groups were calculated using the ANOVA test. Receiver operating characteristic (ROC) curves were generated to assess the sensitivity and specificity of CypC.
Results
The study included 125 subjects (40 patients with acute CAD, 40 patients with chronic CAD, and 45 control volunteers) with a mean age 57.8 ± 14.5 years and 72.8% were male. Patients with CAD had more CVRF as compared to the controls. In the results of the laboratory tests, white blood cells, neutrophils, monocytes, hemoglobin, and glucose were significantly increased in CAD patients. Comparing acute to chronic CAD, a significant difference was seen in only CVRF (active smoker 37.5% vs. 17.5%; p = 0.045). The clinical characteristics of the sample are given in table 1. CypC levels (mean ± SD) were analyzed at baseline, at 6 months, and at 12 months (Fig. 1). The mean follow-up was 64.76 ± 22.16 months. NT-proBNP and hsCRP were measured in 74 CAD patients (37 chronic CAD and 37 acute CAD), with a mean of 463 ± 1699 pg/mL for NT-proBNP and 3.20 ± 6.46 mg/L for hsCRP, and no significant differences between the two groups.
Table 1 Demographic and clinical characteristics
| Variables | Controls (n = 45) | Coronary artery disease | p | ||
|---|---|---|---|---|---|
| Chronic (n = 40) | Acute (n = 40) | Between three groups | Chronic CAD versus acute CAD | ||
| Gender (male) | 51.1% (23) | 82.5% (33) | 87.5% (35) | < 0.001 | 0.531 |
| Age | 45.6 ± 1.5 | 65.2 ± 1.8 | 64.2 ± 1.9 | < 0.001 | 0.713 |
| LVEF (%) | -- | 54.2 ± 10.3 | 55.6 ± 8.7 | -- | 0.528 |
| Cardiovascular risk factors | |||||
| Hypertension | 6.7% (3) | 60.0% (24) | 47.5% (19) | < 0.001 | 0.262 |
| Dyslipidemia | 13.3% (6) | 77.5% (31) | 67.5% (27) | < 0.001 | 0.317 |
| Active smoker | 4,5% (2) | 17.5% (7) | 37.5% (15) | 0.001 | 0.045 |
| Diabetes | 2.2% (1) | 35% (14) | 20% (8) | < 0.001 | 0.248 |
| Family history of CAD | 4.4% (2) | 12.5% (5) | 17.5% (7) | 0.768 | 0.531 |
| Medications | |||||
| ASA | 0% (0) | 77.5% (31) | 15% (6) | < 0.001 | < 0.001 |
| Clopidogrel | 0% (0) | 12.5% (12) | 7.5% (3) | 0.04 | 0.712 |
| Statins | 11.1% (5) | 75% (30) | 42.5% (17) | < 0.001 | 0.003 |
| Number of coronary artery vessels with significant stenosis | 0.219 | ||||
| 0 | 0 (0%) | 2.5% (1) * | 0% (0) | < 0.001 | |
| 1 | 0 (0%) | 35% (14) | 47.5% (19) | ||
| 2 | 0 (0%) | 22.5% (9) | 30% (12) | ||
| 3 | 0 (0%) | 40.5 (16) | 22.5% (9) | ||
| Type of coronary artery revascularization | 0.405 | ||||
| None | 100% (45) | 7.5% (3) | 2.5% (1) | < 0.001 | |
| PCI | 0% (0) | 82.5% (33) | 92.5% (37) | ||
| CABG | 0% (0) | 10% (4) | 5% (2) | ||
| Complete coronary artery revascularization | |||||
| Yes | 0% (0) | 40% (16) | 70% (28) | < 0.001 | 0.007 |
| Laboratory parameters | |||||
| Total cholesterol (mg/dL) | 201.14 ± 26.98 | 149.7 ± 33.06 | 177.05 ± 41.55 | < 0.001 | 0.006 |
| LDL (mg/dL) | 120.77 ± 24.44 | 81.85 ± 26.32 | 108.0 ± 34.17 | < 0.001 | 0.001 |
| HDL (mg/dL) | 57.77 ± 15.28 | 41.15 ± 7.91 | 36.53 ± 7.95 | < 0.001 | 0.024 |
| TG (mg/dL) | 107.17 ± 40.16 | 133.78 ± 45.91 | 164.0 ± 69.69 | <0.001 | 0.053 |
| CRP (mg/dL) | 0.49 ± 0.5 | 8.33 ± 11.64 | 43.45 ± 45.31 | 0.001 | 0.050 |
| ALT (U/L) | 22.06 ± 2.38 | 33.14 ± 19.47 | 33.32 ± 22.75 | 0.029 | 0.973 |
| WBC (number/µL) | 6632.35 ± 320.45 | 7305,25 ± 1958.07 | 10383.50 ± 4732.36 | < 0.001 | < 0.001 |
| Lymphocytes (number/µL) | 2139.39 ± 113.98 | 2030.75 ± 788.78 | 1982.50 ± 1167.70 | 0.806 | 0.829 |
| Neutrophils (number/µL) | 3757.58 ± 293.36 | 4347.5 ± 1543.06 | 7462.50 ± 4238.39 | < 0.001 | < 0.001 |
| Monocytes (number/µL) | 548.48 ± 23.08 | 641.75 ± 222.98 | 730.00 ± 315.58 | 0.010 | 0.153 |
| Hemoglobin (g/dL) | 13.88±1.30 | 14.33 ± 1.54 | 14.65 ± 1.54 | 0.068 | 0,326 |
| Platelet | 229147±52606 | 188250.0 ± 55290.72 | 221925.0 ± 77988.62 | 0.013 | 0.029 |
| Glucose (mg/dL) | 90.5±32.4 | 120.28 ± 48.55 | 110.8 ± 19.97 | 0.002 | 0.259 |
ALT: alanine aminotransferase; ASA: acetylsalicylic; BNP: brain natriuretic peptic; CABG: coronary artery bypass graft; CAD: coronary artery disease; CRP: C-reactive protein; HDL: high-density lipoprotein; LDL: low-density lipoprotein; LVEF: left ventricular ejection fraction; n/p: not apply; PCI: percutaneous coronary intervention; TG: triglyceride; WBC: white blood cell.

Figure 1 Serum CypC values (mean) along 6- and 12-month follow-up. CypC: cyclophilin C; CAD: coronary artery disease.
CypC levels were significantly higher in CAD patients than in the controls: 32.42 pg/mL ± 3.71 versus 9.38 pg/mL ± 1.51 (p < 0.001), but there were no differences between the acute and chronic CAD groups (34.28 pg/mL ± 5.77 vs. 30.56 pg/mL ± 4.73; p = 0.620). CypC ≥ 17.5 pg/mL was present in 72.5% of acute CAD cases, 57.5% of chronic CAD cases, albeit only in 11.1% of controls. We assessed several differences in the clinical characteristics and CVRF in CAD patients between CypC < 17.5 pg/mL and CypC ≥ 17.5 pg/mL. CypC ≥ 17.5 pg/mL was associated with an older age (64.0 ± 1.6 vs. 52.8 ± 1.8 years; p < 0.001), hypertension (48.2% vs. 27.5%; p = 0.017), dyslipidemia (69.6% vs. 36.2%; p < 0.001), and a higher number of coronary arteries with significant stenosis (91.1% vs. 42%; p < 0.001).
We collected the CypC levels from all patients, except 2 patients (1.6%) at 6 months and 7 patients (5.6%) at 12 months. A significant predictive value of serum CypC levels was found during the 6- and 12-month follow-up. The area under the curve (AUC) (c-statistic) calculated was 0.85, with a significant value (p < 0.001) of CypC as a predictor of CAD at baseline. The AUC was 0.76 at 6 months and 0.89 at 12 months, persisting as a good predictor of CAD (p < 0.001) (Fig. 2).
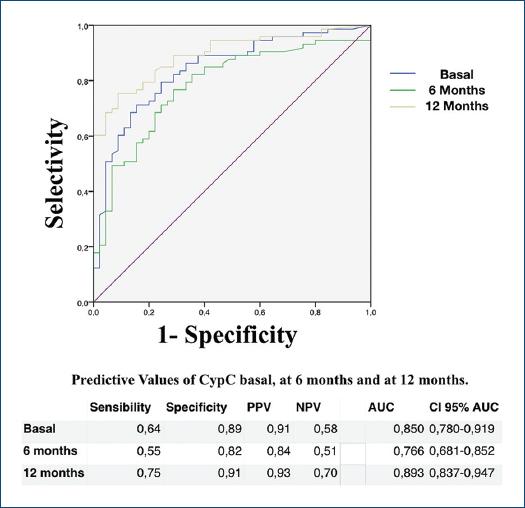
Figure 2 Area under the curve of CypC ≥ 17.5 pg/mL at baseline, at 6 months, and at 12 months. AUC-COR: area under the curve; 95% CI AUC: 95% confidence interval area under the curve; NPV: negative predictive value; PPV: positive predictive value.
The analysis of CypC levels during the period evidenced that it gradually increased in the CAD group (30.63 pg/mL ± 3.77 at baseline, 38.70 pg/mL ± 6.41 at 6 months [p = 0.25], and 47.27 pg/mL ± 5.65 at 12 months [p = 0.007]). CypC did not rise in the control group over 12 months (9.4 pg/mL ± 1.5 baseline vs. 9.0 pg/mL ± 1.1; p = ns).
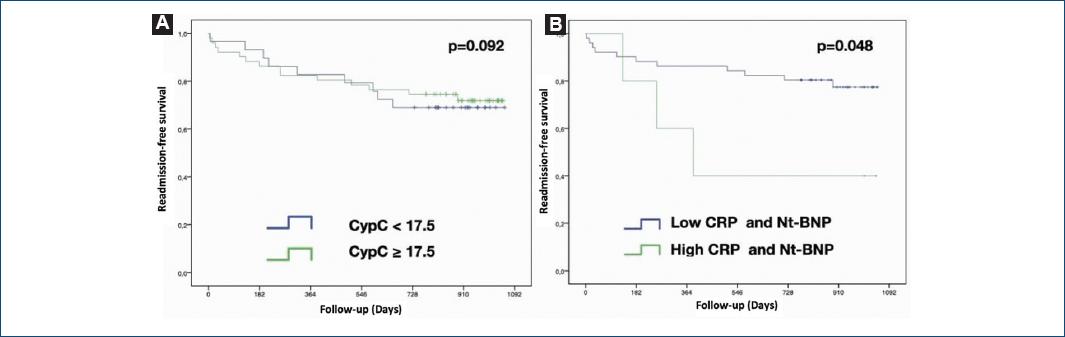
Seventeen of the 80 patients with CAD were admitted with acute coronary syndrome (ACS), 11 patients in the first 12 months, and 6 after that. Four patients were admitted for heart failure, three of them in the first 12 months of follow-up. Patients who experienced a cardiovascular event in the follow-up had 3 times higher CypC levels than those who did not (29.02 pg/mL ± 6.39 vs. 79.96 pg/mL ± 22.18; p = 0.029). Furthermore, in CAD patients, CypC increased more in those who had a second cardiovascular event during the 12-month follow-up, although this was not significant probably due to the lack of events (CypC 23.0 pg/mL ± 6.6 baseline vs. CypC 83.5 pg/mL ± 24.1; p = ns). The CypC cutoff of 17.5 pg/mL did not prove effective in predicting events in patients with CAD (Table 2). Only when considering ACS or HF together did we observe 23.2% (13 patients) in the CypC ≥ 17.5 pg/mL group versus 10.1% (7 patients) in the CypC < 17.5 pg/mL group (p = 0.047). Nevertheless, CypC persisted as a good diagnostic tool to discriminate between patients with and without CAD during the follow-up.
Table 2 CypC cutoff of 17.5 pg/mL and cardiovascular events during follow-up
| Events | Acute CAD | Chronic CAD | ||||
|---|---|---|---|---|---|---|
| CypC ≥ 17.5 | BNP > 300 | PCR > 2.3 | CypC ≥ 17.5 | BNP > 300 | PCR > 2.3 | |
| ACS | ||||||
| Yes | 17.9% (5) | 10.0% (1) | 0% | 26.1% (6) | 27.3% (3) | 60.0% (10) |
| No | 82.1% (23) | 90.0% (9) | 100% (11) | 73.9% (17) | 72.7% (8) | 40.0% (4) |
| HF | ||||||
| Yes | 7.1% (2) | 20.0% (2) | 18.2% (2) | 4.3% (1) | 9.1% (1) | 10.0% (1) |
| No | 92.9% (26) | 80.0% (8) | 81.8% (9) | 95.7% (22) | 90.9% (11) | 90.0% (9) |
ACS: acute coronary syndrome; BNP: brain natriuretic peptide; CAD: coronary artery disease; CypC: cyclophilin C; CRP: C-reactive protein; HF: heart failure.
Considering CypC as a predictor of any cardiovascular event (ACS and/or HF) in the follow-up, we did not observe any statistical significance, but there was a tendency to difference.
Kaplan–Meier curves showed significant differences in the cardiovascular event prediction during the follow-up using a hsCRP cutoff point of 2.3 mg/L and a NT-proBNP cutoff point of 300 pg/mL. In this regard, plasma levels of hsCRP > 2.3 mg/L plus NT-proBNP > 300 pg/mL combined were significant predictors of cardiovascular events during the follow-up in CAD patients with levels of CypC > 17.5 pg/mL (p = 0.048) (Fig. 3).
Discussion
The major findings of our study are that serum CypC levels were a good biomarker of CAD as have been published9 and that this correlation persisted over the 12-month follow-up. The previous studies correlated higher plasma CypA levels with CAD and it had a prognostic impact to predict mortality, readmission, and the need for coronary artery revascularization11 CypA is secreted from vascular smooth cells in response to oxidative stress15 and it has been reported that CypA may also be secreted from macrophages, lymphocytes, and platelets16,17. As it has been shown that CypA levels are increased proportionally to CAD severity10, there is no information about serum CypC levels and their role in CAD overtime.
Serum hsCRP and uric acid levels have been correlated with complex CAD, mainly with the Syntax score18. Furthermore, serum hsCRP levels on admission in patients with ACS could predict the severity and complexity of coronary atherosclerosis together with multivessel CAD, left ventricular ejection fraction, and troponin levels19. A retrospective analysis of 2.867 consecutive patients who underwent percutaneous coronary intervention for stable CAD evaluated the association between baseline hsCRP and both all-cause and cancer deaths, concluding that elevated baseline hsCRP was significantly associated with cancer mortality in patients with stable CAD20. A recent meta-analysis concluded that, comparing high to low serum levels of hsCRP in the general population, the relative risk was significantly higher (1.25 for cancer-related mortality, 2.03 for cardiovascular mortality, and 1.75 for all-cause mortality) in the highest level group21.
Other meta-analyses showed that elevated circulating interleukin-6 levels were independently associated with a risk of cardiovascular and all-cause mortality in the general elderly population, considered as over 60s22. Several biomarkers, such as hsCRP and NT-proBNP, have been correlated with increased global mortality in the general population and they are strong predictors of cardiovascular events in patients with stable CAD20,23,24.
However, CypC, as an endoplasmic reticulum cyclophilin, plays a major role in the inflammation and cellular oxidation status by the regulation of redox homeostasis in the endoplasmic reticulum25. These findings may be related to atherosclerotic disease, promoting macrophage release of pro-inflammatory cytokines in the vascular wall and endothelial dysfunction. Cyps have not been adequately studied in chronic CAD scenarios. We considered that CypC could play a key role in CAD, from the acute phase of the disease to the last phases in the follow-up of these patients.
We highlighted that serum CypC levels were high in patients with CAD and they remained high over the 12-month follow-up and could be a novel biomarker in these patients. There was no significant association with cardiovascular events, because of the small number of patients, but serum CypC levels were generally higher in patients suffering cardiovascular events during this time. In addition, the combination of different cardiovascular risk biomarkers in CAD patients could be a good option for monitoring the risk during the follow-up. In our study, the combination of high levels of CypC > 17 pg/mL, added to high levels of hsCRP > 2.3 mg/L plus NT-proBNP > 300 pg/mL, was significant predictors of cardiovascular events at 12 months in these CAD patients.
However, our study has limitations related to the small sample size, so further studies with a larger sample size are needed to support the results obtained in the current study.
Conclusions
Our data demonstrated that serum CypC levels increase during the follow-up in CAD patients. CypC could have a role as a novel biomarker in CAD patients, with a possible prognostic value in combination with other biomarkers (hsCRP and NT-proBNP). Thus, further analyses with more patients may show the possible prognostic value of CypC in CAD patients.











 text new page (beta)
text new page (beta)