Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Archivos de cardiología de México
On-line version ISSN 1665-1731Print version ISSN 1405-9940
Arch. Cardiol. Méx. vol.82 n.1 Ciudad de México Jan./Mar. 2012
Investigación clínica
The TIMI risk score for STEMI predicts in-hospital mortality and adverse events in patients without cardiogenic shock undergoing primary angioplasty
La escala de riesgo TIMI para infarto del miocardio con elevación del segmento ST predice mortalidad y eventos adversos intrahospitalarios en pacientes sin choque cardiogénico sometidos a angioplastía coronaria
Héctor González-Pacheco,1 Alexandra Arias-Mendoza,1 Amada Álvarez-Sangabriel,1 Úrsulo Juárez-Herrera,1 Félix Damas,1 Guering Eid-Lidt,2 Francisco Azar-Manzur,1 Carlos Martínez-Sánchez.1
1 Coronary Care Unit. Instituto Nacional de Cardiología Ignacio Chávez. Mexico City. Mexico.
2 Catheterization Laboratory. Instituto Nacional de Cardiología Ignacio Chávez. Mexico City. Mexico.
Corresponding author:
Héctor González Pacheco.
Juan Badiano N° 1. Colony Sección XVI, Z P. 14080,
Tlalpan, Mexico City.
Phone: (52 55) 5485 2219.
Fax: (52 55) 5485 2219.
E-mail: hectorglezp@hotmail.com
Received on October 6, 2010;
Accepted on June 28, 2011.
Abstract
Introduction: Patients with ST elevation acute myocardial infarction (STEMI) comprise a heterogeneous population with respect to the risk for adverse events. Primary percutaneous coronary intervention (PCI) has shown to be better, mainly in high-risk patients.
Objective: The purpose of this study was to determine if the Thrombolysis in Myocardial Infarction (TIMI) risk score for STEMI applied to patients undergo primary PCI identifies a group of patients at high risk for adverse events.
Methods: We identifed patients with STEMI without cardiogenic shock on admission, who were treated with primary PCI. The TIMI and CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications) risk scores were calculated to determine their predictive value for in hospital mortality. Patients were divided into two groups according to their TIMI risk score, low risk being 0-4 points and high risk ≥5 points, and the frequency of adverse events was analyzed.
Results: We analyzed 572 patients with STEMI. The c-statistics predictive value of the TIMI risk score for mortality was 0.80 (p=0.0001) and the CADILLAC risk score was 0.83, (p=0.0001). Thirty-two percent of patients classifed as high risk (TIMI ≥5) had a higher incidence of adverse events than the low-risk group: mortality 14.8% vs. 2.1%, (p=0.0001); heart failure 15.3% vs. 4.1%, (p=0.0001); development of cardiogenic shock 10.9% vs. 1.5%, (p=0.0001); ventricular arrhythmias 14.8% vs. 5.9%, (p=0.001); and no-refow phenomenon 22.4% vs. 13.6%, (p=0.01).
Conclusions: The TIMI risk score for STEMI prior to primary PCI can predict in hospital mortality and identifes a group of high-risk patients who might develop adverse events.
Key words: Primary percutaneous coronary intervention; ST elevation acute myocardial infarction; Score Risk; Mexico.
Resumen
Introducción: Los pacientes con infarto agudo del miocardio con elevación del segmento ST (IAM CEST), son una población heterogénea por lo que toca al riesgo de eventos adversos. La intervención coronaria percutánea (ICP) primaria mostró ser mejor, principalmente en los pacientes de riesgo alto.
Objetivo: La propuesta de este estudio fue determinar si la escala de riesgo de trombólisis en infarto del miocardio (TIMI) para IAM CEST, aplicado a los pacientes sometidos a ICP primaria, identifica a grupos de pacientes de riesgo alto de eventos adversos.
Métodos: Se identificaron a pacientes con IAM CEST sin choque cardiogénico al ingreso, quienes fueron tratados con ICP primaria. Se calcularon las escalas de riesgo TIMI y CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications), para determinar su valor predictivo de mortalidad intrahospitalaria. Los pacientes se dividieron en dos grupos de acuerdo a su escala de riesgo TIMI, riesgo bajo con 0-4 puntos y riesgo alto con ≥5 puntos, se analizó la frecuencia de eventos adversos.
Resultados: Se analizaron 572 pacientes con IAM CEST. El valor predictivo del estadístico C de la escala de riesgo TIMI para mortalidad fue de 0.80 (p=0.0001), y la escala de riesgo CADILLAC fue de 0.83, (p=0.0001). El 32% de los pacientes clasificados como riesgo alto (TIMI ≥5), tuvo una alta incidencia de eventos adversos comparada con el grupo de riesgo bajo: la mortalidad 14.8% vs. 2.1%, (p=0.0001); falla cardiaca 15.3% vs. 4.1%, (p=0.0001); desarrollo de choque cardiogénico 10.9% vs. 1.5%, (p=0.0001); arritmias ventriculares 14.8% vs. 5.9%, (p=0.001), y fenómeno de no reflujo 22.4% vs. 13.6%, (p=0.01).
Conclusiones: La escala de riesgo TIMI para IAM CEST, previo a ICP primaria puede predecir mortalidad intrahospitalaria e identificar a un grupo de pacientes de riesgo alto, los cuales pueden desarrollar eventos adversos.
Palabras clave: Intervención coronaria percutánea primaria; Infarto agudo del miocardio con elevación del segmento ST; Escala de riesgo; México.
Introduction
Reperfusion therapy, either pharmacological or mechanical, is indicated in patients with ST elevation acute myocardial infarction (STEMI) with duration of less than 12 hours. The superiority of primary percutaneous coronary intervention (PCI) over fibrinolysis has been demonstrated in several studies:1–3 primary PCI has better results if there is a catheterization laboratory and interventional cardiologist available and if the procedure can be done within 90 minutes of the patient arriving at the hospital. However, it has been observed that the benefit of primary PCI is different in each group of patients and the benefit is greatest in those at high risk.4 Thus, risk stratification prior to intervention has great clinical importance to identify this group of patients at higher risk and to optimize their therapeutic management.
The risk scores applied to patients who are treated exclusively with primary PCI have reported favorable results.5–7 The risk score developed in the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) study, which includes clinical and angiographic variables in patients undergoing primary PCI, is the most accurate for predicting 30 day and one year mortality. Risk stratification using the Thrombolysis in Myocardial Infarction (TIMI) risk score for STEMI is a simple assessment based on clinical data at the time of patient arrival at the hospital.8
We hypothesized that the TIMI risk score applied to patients with STEMI without cardiogenic shock who undergo primary PCI predicts in hospital mortality and also identifies a group of patients at high risk of developing other adverse events.
Methods
The information for the analysis was obtained prospectively from the database of the Coronary Care Unit of the National Institute of Cardiology in Mexico City, covering the period from October 2005 to February 2010. The information included demographic data, risk factors, angiographic characteristics, procedures, and in hospital course. We analyzed all patients who met the criteria for acute myocardial infarction with an ST segment elevation >1mm in ≥2 continuous leads or left bundle branch block and who were scheduled for primary PCI. We excluded those who at admission had cardiogenic shock and analyzed only those who underwent primary PCI. The TIMI risk score was calculated for each patient using the variables obtained at admission according to the published criteria8 listed in Table 1. Mortality during hospitalization was calculated according to the risk score.
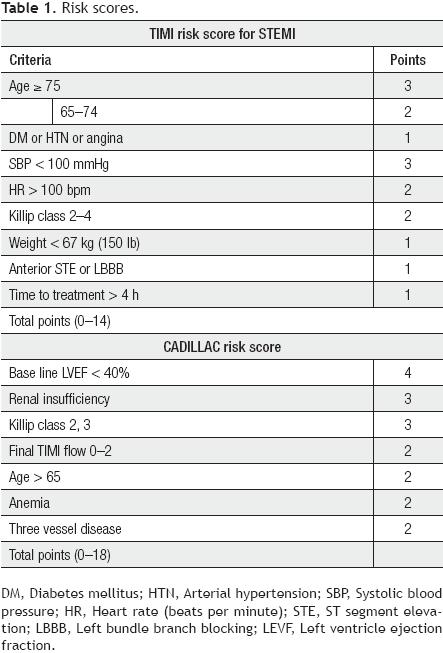
The CADILLAC risk score was calculated with the variables published in this study7 (Table 1). However, since left ventriculography is not routinely performed during primary PCI in our hospital, the ejection fraction of the left ventricle was taken from echocardiography performed at 24 to 48 hours postprocedure.
Patients were classifed as low risk if their TIMI score was 0–4 and as high risk if their TIMI score was ≥5. In each group, we analyzed the frequency of adverse events during hospital care, including mortality, reinfarction, stroke, heart failure, cardiogenic shock, ventricular arrhythmias, and the presence of the no refow phenomenon. More than one adverse event could be present in one patient.
Statistical analysis
Analysis was performed with the statistical package SPSS 13. The continuous and discrete variables were expressed as mean and standard deviation (SD). Differences were analyzed with Student's t test to compare two variables and continuous or discrete analysis of variance (ANOVA) when comparing more than two variables. The categorical variables were expressed as frequencies and percentages and compared with chi-square (χ2) or Fisher's exact test, depending on the frequency of expected events. Results are reported as the two-tailed odds ratio (OR) with 95% confdence interval (CI). Differences were considered significant at a p value of less than 0.05. The TIMI and CADILLAC risk stratification scales were compared using receiver operating characteristic (ROC) curves for their ability to predict the end point of mortality and a value greater than 0.75 for the area under the curve was considered significant.
Results
Data were obtained from a total of 662 patients with STE-MI, who were taken to the catheterization laboratory to undergo primary PCI. Ninety patients were excluded: 19 with cardiogenic shock at admission and 71 because the procedure was not performed (anatomy was not favorable, normal coronary fow, no significant lesions, or the presence of a large-load thrombus). We analyzed a total of 572 patients whose baseline characteristics are shown in Table 2. The average age of the population was 57.9 ± 11.6 years and 84.6% were men; 30.1% had a previous history of diabetes and 50.3% a history of hypertension. With respect to cardiac function, 19.3% of the patients were in Killip–Kimball class 2–3 and the mean ejection fraction measured by echocardiography was 50.1 ± 10.3%; 19.8% of patients had an ejection fraction <40%.

The distribution of patients according to TIMI score was as follows: 0 points, 25 patients (4.4%); 1 point, 89 patients (15.6%); 2 points, 116 patients (20.3%); 3 points, 80 patients (14%); 4 points, 79 patients (14.8%); 5 points, 68 patients (11.9%); 6 points, 45 patients (7.9%); 7 points, 34 patients (5.9%); and ≥ 8 points, 36 patients (6.2%).
The overall in hospital mortality was 6.1% and its frequency relative to the TIMI risk score is shown in Figure 1. The areas under the ROC curve for the mortality related to TIMI and CADILLAC risk scores are shown in Figure 2.

The TIMI risk score was highly predictive of in hospital mortality with a c-statistics of 0.800 (95% CI 0.71-0.88, p<0.0001), which was comparable with the results of the CADILLAC score, which in the same population gave a c-statistics of 0.831 (95% CI 0.750.91, p<0.0001).
Patients were classifed as low risk with a TIMI score of 04 (n=389, 68%) and high risk with a TIMI score ≥5 (n=183, 32%). All variables included in the TIMI risk score were present with significantly greater frequency in the high-risk group (Table 3). Adverse events that occurred in both groups during hospitalization are shown in Table 4. We observed that mortality was eight-fold higher in the high-risk group than in the low-risk group (14.8% vs. 2.1%; OR 8.2, 95% CI 3.66–18.54, p=0.0001). Other adverse events also occurred more frequently in the high-risk group: heart failure (15.3% vs. 4.1%, p=0.0001), development of cardiogenic shock (10.9% vs. 1.5%, p=0.0001), ventricular arrhythmias (14.8% vs. 5.9%, p<0001), and development of the no-refow phenomenon (22.4% vs. 13.6%, p=0.01). The incidence of reinfarction and stroke was low and there were no significant differences between both groups. There was no difference between the two groups for stent placement (92.2% vs. 87.8%, p=0.11), but the use of inhibitors of glycoprotein (Gp) IIb/IIIa antagonists (75.8% vs. 66.1%, p=0.01), angiotensin-converting enzyme inhibitors (90.2% vs. 78.1%, p=0.0001) and beta blockers (62.2% vs. 44.3%, p=0.0001) was less frequent in the high-risk group.

Discussion
A potentially relevant issue in the treatment of patients with STEMI is that this population is highly heterogeneous regarding their risk of adverse events. Thus, their correct stratification becomes essential to evaluate their prognosis and to take accurate therapeutic decisions. An ideal risk score must be useful, simple and fast to apply to predict prognosis at short and long range.9,10
The TIMI risk score for STEMI is a clinical stratification calculated with data obtained at hospital presentation that can easily classify patients into low and high risk. It was developed using data from patients treated with thrombolytic therapy in a randomized trial and predicts mortality at 30 days. This risk score revealed that about 20% of patients were at higher risk of death.8 The analysis was subsequently validated in an unselected patient population in the National Registry of Myocardial Infarction—3 and showed a strong predictive value for mortality in patients treated with thrombolytic therapy (c-statistics = 0.79).11
In our study of 572 patients who underwent primary PCI, 32% (n=183) were stratifed as high risk (TIMI risk score ≥5) before the procedure. Thune et al. in the DANish trial in Acute Myocardial Infarction-2 (DANAMI-2) strati-fed patients by TIMI risk score; 25% of patients were high risk (TIMI ≥5). At three years of follow-up, it was clear that the benefit of primary PCI over thrombolytic therapy in reducing mortality was restricted to high-risk patients (25% mortality for PCI vs. 32.6% for fibrinolysis, p=0.02) with no significant difference in low-risk patients (8% for PCI vs. 5.6% for fibrinolysis, p=0.11).4 Lev et al. without identifying a high-risk group, reported that stratification with the TIMI risk score in patients undergoing primary PCI predicts mortality and major adverse cardiac events (death, myocardial infarction, target vessel revascularization).12
We applied the TIMI risk score for STEMI in a group of patients without cardiogenic shock, who underwent primary PCI and showed that an increase in TIMI risk score is associated with increased frequency of in hospital death and has a high predictive value for mortality that is comparable with of the CADILLAC risk score in the same group of patients (TIMI c-statistics = 0.80 and CADILLAC c-statistics = 0.83). The CADILLAC risk score reportedly has a better predictive value for mortality at 30 days and one year, but differs from other primary angioplasty risk scores because it includes angiographic parameters such as the presence of three-vessel disease and final TIMI fow, as well as the left ventricle ejection fraction determined by ventriculography. The progress achieved in reducing in hospital mortality in patients with STEMI increases the importance of predicting other postprocedural complications, that may have a strong influence on patient outcomes. In the meta-analysis of Keeley et al. overall mortality and other adverse events such as nonfatal reinfarction, stroke, and bleeding occurred less frequently in the group undergoing primary PCI than in those undergoing throm-bolysis.3 Kent et al. documented that the greatest benefit of primary PCI is found in high-risk patients.13 Negasso et al. reported a decision-tree structure prognostic classification for acute myocardial infarction undergoing PCI to predict in hospital complications after intervention, four important variables at the time of presentation were identifed: cardiogenic shock, heart failure, age, and diabetes.14 Although the TIMI risk score was developed to predict mortality, an important clinical implication of this study is that it identifes a group of high-risk patients (TIMI risk ≥5), who not only have a mortality rate eight-times higher than the low-risk group (TIMI risk <4), but also have an increased frequency of in hospital adverse events such as heart failure (p=0.0001), development of cardiogenic shock (p=0.0001), ventricular arrhythmias (p=0.001), and no-refow phenomenon (p=0.01), which may have a bearing on the poor hospital outcome seen in this group of patients. In our results, there was no difference between high and low-risk groups in the incidence of reinfarction and stroke.
The no-refow phenomenon has been reported in 25% of patients undergoing primary PCI, the predictors for its development are the presence of diabetes, advanced age, Killip class >2, previous stroke, and the duration of ischemia.15,16 In the present study, we report an overall frequency of 16.4%, with significantly higher prevalence in the high-risk group than in the low-risk group (22.4% vs. 13.6%, p=0.01). Although the high-risk group presented all the risk factors mentioned above, it has been observed that suboptimal reperfusion may be present in a large proportion of patients despite the achievement of TIMI 3 fow. This has been reported to be principally caused by the no-refow phenomenon and distal embolization,17 which led to consider Gp IIb/IIIa antagonists as adjunct therapy. In the meta-analysis by De Luca et al. of patients with STEMI undergoing primary PCI, there was a significant relationship between the risk profle and the benefit of adjunct Gp IIb/IIIa antagonists in reducing mortality at 30 days.18 In our group of analyzed patients, the frequency of using Gp IIb/IIIa antagonist was lower in the high-risk group (66.1% vs. 75.8%, p=0.01) and the lack of embolectomy, which has demonstrated benefits, in our series may have influenced the incidence of the no-refow phenomenon.
We are aware of the relationship between the presence of the no-refow phenomenon and other complications such as increased incidence of fatal arrhythmias and heart failure in patients with STEMI. Most patients developed cardiogenic shock during hospitalization19 and Lindholm et al. have reported that primary PCI does not prevent its development20 In our series, cardiogenic shock developed overall in 26 patients (4.5%) and was significantly more frequent in the high-risk group than in the low-risk group (10.9% vs. 1.5%, p=0.0001). It would be important to identify this group of at-risk patients, as has been done for patients receiving thrombolytic therapy,21 so that preventive measures could be implemented in an attempt to prevent the development of cardiogenic shock.
Conclusions
The TIMI risk score applied to STEMI patients without cardiogenic shock, undergoing primary PCI, identifies a group of patients at high-risk not only for higher in hospital mortality, but also for other adverse events such as the no-refow phenomenon, heart failure, development of cardiogenic shock, and ventricular arrhythmias.
Acknowledgment
We appreciate the secretarial staff of the Coronary Care Unit, Leticia Casiano and Benita Medrano, for their valuable cooperation in the preparation of this manuscript.
References
1. Van de Werf F, Ardissino D, Betriu A, et al. Management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2003;24:28–66. [ Links ]
2. Fibrinolytic Therapy Trialists' (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomized trials of more than 1000 patients. Lancet 1994;343:311–322. [ Links ]
3. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361:13– 20. [ Links ]
4. Thune JJ, Hoefsten DE, Lindholm MG, et al. Simple risk stratification at admission to identify patients with reduced mortality from primary angioplasty. Circulation 2005;112:2017–2021. [ Links ]
5. Addala S, Grines CL, Dixon SR, et al. Predicting mortality in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention (PAMI risk score). Am J Cardiol 2004;93:629–632. [ Links ]
6. De Luca G, Suryapranata H, Arnoud WJ, et al. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty. Implications for early discharge. Circulation 2004;109:2737–2743. [ Links ]
7. Halkin A, Singh M, Nikolsky E, et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. J Am Coll Cardiol 2005;45:1397–1405. [ Links ]
8. Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction, a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000;102:2031–2037. [ Links ]
9. Rizo G, Ramirez JI, Perez D, et al. Valor predictivo de muerte y complicaciones intrahospitalarias de los modelos de estratificación de riesgo en pacientes con infarto miocárdico agudo. Rev Fed Arg Cardiol 2011;40:57-64. [ Links ]
10. Zapata G. Predicción temprana del riesgo en el Infarto agudo de miocardio: una difícil tarea de todos los días. Rev Fed Arg Cardiol 2011;40:1-2. [ Links ]
11. Morrow DA, Antman EM, Person L, et al. Application of TIMI risk score for ST elevation myocardial infarction in the National Registry of Myocardial Infarction 3. JAMA 2001;286:1356–1359. [ Links ]
12. Lev EI, Kornowski R, Vaknin-Assa H, et al. Comparison of the predictive value of four different risk scores for outcomes of patients with ST-elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol 2008;102:6–11. [ Links ]
13. Kent DM, Schmid CH, Lau J, et al. Is primary angioplasty for some as good as primary angioplasty for all? Modeling across trials and individual patients. J Gen Intern Med 2002;17:887–894. [ Links ]
14. Negassa A, Monrad ES, Srinivas VS. A simple prognostic classification model for postprocedural complications after percutaneous coronary intervention for acute myocardial infarction (from the New York State Percutaneous Coronary Intervention Database). Am J Cardiol 2009;103:937–942. [ Links ]
15. Morishima I, Sone T, Okumura K, et al. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol 2000;36:1202–1209. [ Links ]
16. Jaffe R, Charron T, Puley G, et al. Microvascular obstruction and the no-refow phenomenon after percutaneous coronary intervention. Circulation 2008;117:3152–3156. [ Links ]
17. De Luca G, Van't Hof AW, Ottervanger J P, et al. Unsuccessful reperfusion in patients with ST-segment elevation myocardial infarction treated by primary angioplasty. Am Heart J 2005;150:557–562. [ Links ]
18. De Luca G, Navarese E, Marino P. Risk profile and benefits from Gp IIb/IIIa inhibitors among patients with ST-segment elevation myocardial infarction treated with primary angioplasty: a meta-regression analysis of randomized trials. Eur Heart J 2009;30:2705–2713. [ Links ]
19. Babaev A, Frederick PD, Pasta DJ, et al .Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2005;294:448–454. [ Links ]
20. Lindholm MG, Boesgaard S, Thune JJ, et al. Percutaneous coronary intervention for acute MI does not prevent in hospital development of cardiogenic shock compared to fibrinolysis. Eur J Heart Fail 2008;10:668–674. [ Links ]
21. Hasdai D, Califf RM, Thompson TD, et al. Predictors of cardiogenic shock after thrombolytic therapy for acute myocardial infarction. J Am Coll Cardiol 2000;35:136–143. [ Links ]














