Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos de cardiología de México
versión On-line ISSN 1665-1731versión impresa ISSN 1405-9940
Arch. Cardiol. Méx. vol.76 no.1 Ciudad de México ene./mar. 2006
Investigación básica
Comparison of 18FDG PET with thallium SPECT in the assessment of myocardial viability. A segmental model analysis
Comparación de 18FDG PET con talio SPECT en la evaluación de la viabilidad mocárdiaca. Un modelo de análisis segmentario
Erick Alexánderson,*** Alejandro Ricalde,** José Luis Romero–Ibarra,** Aloha Meave***
* Department of Nuclear Cardiology, Instituto Nacional de Cardiología "Ignacio Chávez", Mexico City, Mexico.
** PET–Cyclotron Unit, Medical School, Universidad Nacional Autónoma de México. Mexico, City.
*** Department of Cardiovascular Magnetic Resonance, Instituto Nacional de Cardiología "Ignacio Chávez", Mexico City, Mexico.
Address:
Erick Alexanderson
Department of Nuclear Cardiology, Instituto Nacional de Cardiología "Ignacio Chávez"
(INCICH, Juan Badiano No. 1, Col. Sección XVI, Tlalpan, México, D.F.)
Business telephone number: (0052) (55) 56232299
Home telephone number: (0052) (55) 54241026
Fax number: (0052) (55) 5623
E–mail: alexanderick@yahoo.com
Recibido: 20 de septiembre de 2005
Aceptado:
Summary
Background: In patients with myocardial infarction and left ventricular dysfunction, the evidence of myocardial viability is primordial. There are some methods to detect the presence of myocardial viability, 201 thallium reinjection SPECT protocol represents the most common radioisotopic technique to evaluate it. Positron emission tomography (PET) using FDG is considered the gold standard. The aim of this study was to compare globally and by segments the value of both techniques in the detection of viable myocardium.
Methods: Twenty–three consecutive patients with previous myocardial infarction and left ventricular dysfunction were studied. All of them underwent into a SPECT perfusión scan and a FDG PET study to asses myocardial viability. Each study was performed in less than one week between the other. For the analysis, the myocardium was divided into 17 segments. A visual semi–quantitative analysis was carried out according to the following score indicating radiotracer uptake: O = normal to 4 = absent. Myocardial viability was defined as the presence of normal, mildly or moderately reduced radiotracer uptake. The scores obtained by PET were compared to those obtained in SPECT. A statistical analysis was performed using the SPSS v. 10.
Results: 391 segments were analyzed. PET detected viability in 130 segments that had been defined as non–viable by SPECT. No differences in the analysis by vascular territories were found. Thirty percent of the segments that were defined as non viable by SPECT were viable by PET, meanwhile only 1% of the segments detected viable by SPECT were considered non viable with PET.
Conclusions: FDG PET study represents a better technique to detect myocardial viability, compared to thallium reinjection SPECT protocol. By this study we have demonstrated that more of 3 of each 10 studies may be diagnosed as non viable where viability is present.
Key words: Myocardial viability. Positron emission tomography. PET. Myocardial perfusión. SPECT.
Resumen
Antecedentes: La detección de la viabilidad miocárdica en pacientes con infarto de miocardio y disfunción del ventrículo izquierdo resulta indispensable. Existen varios métodos que permiten identificar viabilidad miocárdica, la técnica SPECT con protocolo reinyección con talio 201 representa la técnica radioisotópica más comúnmente empleada para evaluarla. Sin embargo, la tomografía por emisión de positrones (PET) usando fluorodeoxiglucosa es considerada hoy el "estándar de oro". El objetivo del presente trabajo es comparar en forma global y segmentaria el valor de ambas técnicas en la detección de miocardio viable.
Método: Se estudiaron 23 pacientes con antecedente de infarto de miocardio y disfunción ventricular izquierda, a todos se les realizó un SPECT de perfusión y un estudio PET–FDG para identificar viabilidad. Los estudios fueron realizados al menos con una semana de diferencia. Para su análisis se utilizó el modelo de 17 segmentos. Las imágenes se evaluaron mediante un análisis visual semicuantitativo de acuerdo a la escala de 0 = captación normal a 4 = ausencia de captación. Se definió viabilidad miocárdica como la presencia de captación de radiotrazador normal, leve o moderadamente reducida. Los resultados obtenidos con ambas técnicas fueron comparados y analizados en forma estadística con el programa SPSS v. 10.
Resultados: Se analizó 391 segmentos. PET detectó viabilidad en 130 segmentos que el SPECT definió como no viables. No se encontraron diferencias estadísticamente significativas al analizarlos por territorio vascular. El 30% de los segmentos que fueron definidos como no viables por el SPECT fueron reconocidos como viables por PET, mientras que sólo 1% de los segmentos detectados como viables por el SPECT fueron considerados como no viables con PET
Conclusiones: El PET–FDG representa una mejor técnica para detectar viabilidad, comparada con el protocolo SPECT reinyección con talio. Mediante este estudio se demostró que más de 3 de cada 10 estudios evaluados por SPECT pueden ser diagnosticados como no viables donde existe viabilidad.
Palabras clave: Viabilidad miocárdica. Tomografía por emisión de positrones. PET. Perfusión miocárdica. SPECT.
Introduction
The recognition of myocardial viability has important therapeutic and prognostic implications in patients with coronary artery disease and left ventricular dysfunction.12 In patients with chronic ischemic cardiomyopathy, the presence of hibernating myocardial tissue represents regions at risk of infarction, potentially contributing to the development or exacerbation of heart failure.3,4 During the present decades, the management of patients with coronary artery disease and left ventricular dysfunction was limited to medical therapy; nonetheless, this strategy has been associated with a high mortality rate (15–60%).5 The revascularization of dysfunctional (asinergic) segments showing viability in patients with coronary artery disease and left ventricular dysfunction has been associated to improved survival and New York Heart Association (NYHA) class. An improvement in global as well as regional left ventricular function has also been described.1,6 The identification of myocardial viability, in patients with coronary artery disease and left ventricular dysfunction is useful in risk stratification, for it allows the prediction of a group of patients that may benefit the most from a revascularization procedure.3 The outcome from revascularization depends on the presence as well as the amount of hibernating myocardium related to dysfunctional segment.7 201T1 SPECT (stress–redistribution–reinjection or late redistribution images) has been the most employed imaging technique for the assessment of myocardial viability in patients with coronary artery disease and left ventricular dysfunction.8,9 PET imaging has demonstrated viability in almost half of myocardial regions with reversible perfusión defects on 201T1 SPECT.3 This non–invasive technique allows the assessment of cellular metabolism, associated with the presence of viable myocites, by obtaining images with great resolution, with the advantage of low radiation exposure for the patient. Dilsizian et al12 and Marin–Neto et al13 compared 201T1 SPECT and 18FDG PET for the detection of myocardial viability, reporting 72% and 78% correlation between these methods, respectively. Various studies12–17 assessing myocardial viability with 18FDG PET have been published. Image analysis in these studies has been made by dividing the myocardium in different segments. No studies have been performed in Mexico to compare 201Tl SPECT and 18FDG PET for the detection of myocardial viability. The aim of this study was to compare globally and by segments the value of both techniques in the detection of myocardial viability.
Material and methods
Twenty–three patients were studied. All patients included in the study had a history of myocardial infarction, proved by electrocardiogram; coronary artery disease by coronary angiography; and left ventricular dysfunction (ejection fraction (EF) < 50%). Unstable angina, valvulopathies, congenital heart disease, and blood glucose higher than 120 mg/dL at the time of the study were considered as exclusion criteria. Poor quality images from either 18FDG PET or 201T1 SPECT were used as elimination criteria.
All patients underwent a 201T1 stress–redistribution–reinjection SPECT protocol. To induce a pharmacologic stress, dypiridamole was administered intravenously at a dose of 0.56 mg/kg over 4 minutes. During infusion, regular electrocardiographic and blood pressure monitoring was performed. 201T1 (3mCi) was injected at minute 7, and stress images acquisition was started 5 minutes later. Redistribution images were obtained 4 hours later. Additional 1.5 mCi of 201T1 were injected, images were acquired 3 hours later (reinjection). None of the patients had taken xanthine–containing drugs nor caffeine at least 24 hours previous to the study.
Image acquisition was performed with a dualhead SPECT gamma camera Millennium VG Hawkeye (General Electric, Milwaukee, Wisconsin) equipped with a high resolution collimator, 75 photomultiplier tubes, 0.64 cm width iodide crystals, and an Entegra processing system. Thirty–two projections (25 seconds/projection) were acquired by continuously rotating the camera through a 180° semicircular orbit (45° RAOLPO). A 20% energy window centered at 60–80 KeV, and a second 10% energy window centered at 167 KeV were employed. A 64 x 64–pixel matrix was used.
A 18FDG PET study was performed to assess cardiac metabolism. Radiotracer was produced at the Cyclotron–UNAM. Subsequent to an overnight fast, a loading dose of 50 g of glucose was administered to every patient, after capillary glucose determination. Thirty minutes later, 10 mCi of 18FDG were injected intravenously. Image acquisition was performed 40 minutes after injection of the tracer, for a period of 40 minutes. A transmission scan was obtained to correct emission images for photon attenuation. PET imaging was performed on a full ring, whole–body system ECAT EXACT HR+ (Siemens/CTI, Knoxville, TN, USA) in a 2–dimensional mode. This system covers an axial field–of–view of 14.5 cm by collecting 63 transverse slices with 4 mm slice thickness, with an in–plane resolution of 4.5–5.8 mm in transverse direction and 4.9–8.8 in axial direction.18
All patients underwent to both studies within a mean period of 11 days (1–22 days). During this time, none of the patients presented acute coronary syndromes or were admitted to the hospital for cardiovascular reasons. All patients signed a written informed consent. All images acquired in both studies (SPECT and PET) were reconstructed in short–axis, vertical long axis and horizontal long–axis. For the analysis, the myocardium was divided into 17 segments.19 A visual semiquantitative analysis was made according to the following score related to the qualitative radiotracer uptake: 0 = normal to 4 = absent.8 Images were assessed by consensus of two experienced observers, unaware of the clinical data of each patient.
For either SPECT (reinjection) or PET images, myocardial viability was defined as the presence of normal (0) to moderately reduced (2) radiotracer uptake for each analyzed segment. Besides, the relationship between perfusión and metabolism (SPECT–PET) was classified as: normal (perfusion–metabolism preserved); match (diminished perfusión and metabolism); mismatch (diminished perfusión and preserved metabolism) or reverse mismatch (preserved perfusión and diminished metabolism).1
Statistical analysis was performed using SPSS v. 10. Data are expressed as mean values ± standard deviation (SD). Correlations were obtained for each segment and vascular territory.
Results
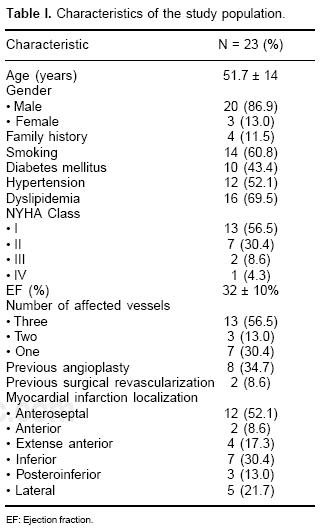
We studied twenty–three consecutive patients with history of myocardial infarction and left ventricular dysfunction (EF: mean = 32%; SD:10); 20 men and 3 women; mean age: 54 years (SD 14). Principal characteristics of the study population are described in Table I. Dislipidemia (n = 16, 61.5%) and smoking (n = 14, 53.8%) were the most prevalent coronary risk factors; 10 patients (43.4%) had diagnosis of non–insulin dependent diabetes mellitus; most (n = 16, 69.5%) of them were in NYHA class I. The most prevalent myocardial infarction localization was anteroseptal, in 12 patients (52.1%). Fourteen patients (60.8%) presented triple–vessel disease, as documented by coronary angiography. A total of 391 segments were analyzed, 127 were classified as normal, 67 matched, 182 mismatched and 15 reverse mismatched segments. Correlation between methods for each segment is shown in Figure 1. Differences in the analysis were found among both methods. No differences were found regarding the score assigned by SPECT and PET in the analysis by vascular territory between diabetic vs non–diabetic patients. Differences were encountered between the number of segments detected as non–viable by SPECT and PET, most of the segments considered non–viable by SPECT were found viable with PET (Fig. 1).

Among the 391 segments analyzed, 205 segments (52.4%) were detected as viable by PET as well as SPECT, 130 segments (33.3%) were defined as viable by PET and as non–viable by 201T1 SPECT, 5 segments (1.2%) were defined as viable by SPECT and as non–viable by PET and the rest of the segments (13.1%) did not show viability neither by PET nor by SPECT (Table II).

Discussion
Nuclear medicine, using 201T1 SPECT (stressre–distribution–reinjection) has been the most used method to evaluate myocardial viability.20 FDG PET study has been reported as the better technique for the evaluation of myocardial viability previously to the revascularization therapy. However, because of the poor availability of this technique, a comparison between both techniques has not been done in our country before. The importance of the detection of myocardial viability is funded in the fact that patients with myocardial infarctions and left ventricular dysfunction with viability demonstrated by PET may be benefited from a revascularization procedure and patients without viability demonstrated may not.21 In the present study, we compared 201T1 SPECT and 18FDG for the detection of myocardial viability in patients with coronary artery disease and left ventricular dysfunction.
Among the 391 analyzed segments, we found more segments that showed perfusion/metabolism mismatch than perfusion/metabolism match (127 F567). A bad correlation (r < 0.7) between SPECT and PET was observed in the left anterior descendent artery and in the right coronary artery territories when vascular territories were analyzed. Using the segmental model, we showed a low correlation (p < 0.50) between both techniques in 5 of the 7 segments assigned to the LAD territory (all except basal anterior and basal anteroseptal) in 3 of the 5 segments assigned to the RCA territory (all except basal inferoseptal and mid inferoseptal) and in 2 of the 5 segments assigned to the LCx (basal inferolateral and mid inferolateral), which means that PET detected more viable segments than SPECT did (11 of 17 segments analyzed) and that these differences are statistically significant.
In this study, 18FDG PET detected more viable segments (30%) than 201T1 SPECT. It means that 3 of each 10 patients may be diagnosed as without myocardial viability when in fact there is viability present.
Comparing this study to the one reported by Bonow et al,22 in the present study a higher percentage of viable segments were founded, however patients at Bonow et al study had a lower mean EF than ours (27% vs 32%) which may explain this difference.
The results of this study are in agreement with those reported by Burt et al,23 who compared both methods in 20 patients with coronary artery disease and left ventricular dysfunction, and reported that in 23.3% of the segments that had been classified as non–viable by SPECT, PET detected viability.
A poor myocardial radiotracer uptake has been described in diabetic patients, with a subsequent poor image quality and lower accuracy of this technique.23 The use of the "euglycemic hyperinsulinemic clamp" has been reported to improve PET accuracy in diabetic patients.24 However, in our study, no differences between SPECT and PET scores were found in the analysis by vascular territories in diabetic vs non–diabetic patients, even in the absence of the euglycemic clamp method.
Limitations of the study: This study did not include the follow up of patients that were revascularized in agreement with the PET or SPECT results. However, several studies have shown that 18–FDG PET as well as Tl–201 SPECT are accurate methods for the prediction of postrevascularization improvement of ventricular function. Bonow et al20 reported a good correlation between qualitative and quantitative image analysis, in the present study only semiquantitative analysis was performed. We should also take an account the issue of limited clinical sample, we only included 23 patients. Trials that include a larger number of patients should be done in order to confirm the results founded in this study.
Conclusions
The recognition of myocardial viability has important therapeutic and prognostic implications in patients with coronary artery disease and left ventricular dysfunction.1,2
PET constitutes an advanced technique that has high sensitivity in detection of myocardial viability. The segmental model analysis could be useful to recognize in precise form the specific number of viable segments that can improve after a coronary revascularization procedure. Based on the results of this study, 18FDG PET detects a higher number of viable segments in comparison with 201T1 SPECT justifying its use in patients with coronary artery disease and left ventricular dysfunction.
Acknowledgements
We wish to thank the physicist Adolfo Zárate–Morales, and the chemist Efrain Zamora for their help in the radiotracer production, as well as the nuclear medicine technicians Luis Osorio and Isabel Porras who helped us with the acquiring and processing of data.
References
1. Marwick TH: The viable myocardium: epidemiology, detection and clinical implications. Lancet 1998; 351: 815–19. [ Links ]
2. Bonow RO: Identification of viable myocardium. Circulation 1996; 94: 2674–80. [ Links ]
3. Stillman AE, Wilke N, Jerosch–Herold M: Myocardial viability. Radiol Clin North Am 1999; 37(2): 361–78. [ Links ]
4. Wijns W, Vatner S, Camici P: Hibernating myocardium. N Engl J Med 1998; 339(3): 173–81. [ Links ]
5. Maddahi J: The use of positron emission tomography in management of patients with ischemic cardiomyopathy. Adv Card Surg 1996; 7: 163–88. [ Links ]
6. Perrone–Filardi P, Chiariello M: The Identification of myocardial hibernation in patients with ischemic heart failure by echocardiography and radionucleide studies. Prog Cardiovasc Dis 2001; 43(5): 419–32. [ Links ]
7. Ordoubadi F, Beatt KJ, Spyrou N: Efficacy of coronary angioplasty for the treatment of hibernating myocardium. Heart 1999; 82: 212–16. [ Links ]
8. Alexánderson E, Varguez V, Bialostozky D, Arroyo A, Alexánderson G, Victoria D: Valoración simultánea de la perfusión y la viabilidad miocárdica a través del estudio de dos isótopos (Talio reposo/MIBI esfuerzo). Experiencia inicial en México y América latina. Arch Inst Cardiol Mex 1997; 67: 106–13. [ Links ]
9. Dilsizian V, Rocco TP, Freedman NM, Leon MB, Bonow RO: Enhanced detection of ischemic but viable myocardium by the reinjection of thallium after stress–redistribution imaging. N Engl J Med 1990; 323(3): 141–6. [ Links ]
10. Dutka DP, Camici PG: The contribution of positron emission tomography to the study of ischemic heart failure. Prog Cardiovasc Dis 2001; 43(5): 399–418. [ Links ]
11. Bax JJ, Wijns W, Cornel JH, Visser FC, Boersma E, Fioretti PM: Accuracy of currently available techniques for prediction of functional recovery after revascularization in patients with left ventricular dysfunction due to chronic coronary artery disease: comparison of pooled data. J Am Coll Cardiol 1997; 30: 1451–60. [ Links ]
12. Dilsizian V, Perrone–Filardi P, Arrighi JA, Bacharach SL, Quyyumi AA, Freedman NM, et al: Concordance and discordance between stress–redistribution–reinjection and rest–redistribution thallium imaging for assessing viable myocardium. Comparison with metabolic activity by positron emission tomography. Circulation 1993; 88(3): 941–52. [ Links ]
13. Marin–Neto JA, Dilsizian V, Arrighi JA, Perrone–Filardi P, Bacharach SL, Bonow RO: Thallium scintigraphy compared with 18–F–fíuorodeoxyglucose positron emission tomography for assessing myocardial viability in patients with moderate versus severe left ventricular dysfunction. Am J Cardiol 1998; 82: 1001–7. [ Links ]
14. Eitzman D, Al–Aouar ZR, Kanter HL: Clinical outcome of patients with advanced coronary artery disease after viability studies with positron emission tomography. J Am Coll Cardiol 1992; 20:559–65. [ Links ]
15. Brunken RC, Mody FV, Hawkins RA, Nienaber C, Phelps ME, Schelbert HR: Positron emission tomography detects metabolic viability in myocardium with persistent 24–hour single–photon emission tomography 201Tl defects. Circulation 1992; 86(5): 1357–69. [ Links ]
16. Pagano D, Bonser RS, Townend JN, Ordoubadi F, Lorenzoni R, Camici PG: Predictive value of dobutamine echocardiography and positron emission tomography in identifying hibernating myocardium in patients with postischaemic heart failure. Heart 1998; 79: 281–8. [ Links ]
17. Kuhl HP, Beek AM, van der Weerdt AP, Hofman MB, Visser CA, Lammertsma AA, et al: Myocardial viability in chronic ischemic heart disease: comparison of contrast–enhanced magnetic resonance imaging with (18)F–fiuorodeoxyglucose positron emission tomography. J Am Coll Cardiol 2003; 41(8): 1341–8. [ Links ]
18. Adam L, Zaers J: Performance evaluation of the whole–body PET scanner. Proc IEEE 1997; 2: 1270–4. [ Links ]
19. Cerqueira M, Weissman N, Dilsizian V, Jacobs A, Kaul S, Laskey W, et al: Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002; 105: 539–42. [ Links ]
20. Maddahi J, Shelbert H, Brunken R, Di Carli MF: Role of thallium–201 and PET imaging in evaluation of myocardial viability and management of patients with coronary artery disease and left ventricular dysfunction. Journal of Nuclear Medicine 1994; 35(4): 707–15. [ Links ]
21. Di Carli MF, Davidson M, Little R, Khanna S, Mody FV, Brunken RC, et al: Value of metabolic imaging with positron emission tomography for evaluating prognosis in patients with coronary artery disease and left ventricular dysfunction. American Journal of Cardiology 1994; 73(8): 527–33. [ Links ]
22. Bonow RO, Dilsizian V, Cuocolo A, Bacharach SL: Identification of viable myocardium in patients with chronic coronary artery disease and left ventricular dysfunction. Comparison of thallium scintigraphy with reinjection and PET imaging with 18–F–fluorodeoxyglucose. Circulation 1991; 83(1): 26–37. [ Links ]
23. Burt RW, Perkins OW, Oppenheim BE, Schauwecker DS, Stein L, Wellman HN: Direct comparison offluorine–18–FDG SPECT, fluorine–18–FDG PET and rest thallium–201 SPECT for detection of myocardial viability. J Nucl Med 1995; 36(2): 176–9. [ Links ]
24. Marinho NVS, Keogh BE, Costa DC, Lammerstma AA, Ell PJ, Camici PG: Pathophysiology of chronic left ventricular dysfunction. New insights from the measurement of absolute myocardial blood flow and glucose utilization. Circulation 1996; 93: 737–44. [ Links ]
25. Knutti MJ, Nuutila P, Ruotsalainen U: Euglycemic hyperinsulinemic clamp and oral glucose load in simulating myocardial glucose utilization during positron emission tomography. J Nucl Med 1992; 33: 1255–62. [ Links ]














