Introduction
Treatment adherence is essential to prove the effectiveness of nutrition and pharmacological intervention trials. Compliance with health care _I treatments of people affected with chronic and degenerative diseases is related to patients' morbidity and mortality rates, and it is one of the main challenges for health care specialists (Estrela, Alves, Gomes & Isosaki, 2017).
Treatment compliance can be estimated by subjective and objective methods (Estrela et al, 2017). In the subjective methods, information about adherence is collected through interviews, questionnaires, phone calls, or record formats from the patient, a family member, or a health care professional. The subjective methods are commonly used due to their simplicity and low cost, but their primary disadvantage is the information bias produced unintentionally or deliberately by the person who informs. On the other hand, the objective methods usually quantify metabolites or drugs linked with the intervention. Laboratory blood tests are the most common in pharmacological and nutrition trials, but they may be expensive and invasive. In specific populations, invasive methods' use generates desertion in longitudinal studies (Anghel, Farcas & Oprean, 2019).
Recently, the use of stable isotopes as tracers in health research is emerging and has been used to assess dietary lifestyles in the context of different pathologies (O'Brien, 2015; Patel et al., 2014). An example is the quantification of carbon 13 (13C) and Nitrogen 15 (15N) in human blood serum and used as a dietary intake marker. An alternative to avoid invasiveness of blood sampling procedures is to measure 13C enrichment through breath testing. After the oral ingestion of a substrate labelled with 13C, the 13CO2/12CO2 ratio is measured in the exhaled air. Through this quantification, it is possible to establish inferences related to different physiological processes in the organism. The 13C-glucose breath test has been used previously by our crew to assess insulin resistance, metabolic syndrome, and other obesity-related disorders (Maldonado-Hernández et al, 2016; Salas-Fernández, Maldonado-Hernández, Martínez-Basila, Martínez-Razo & Jasso-Saavedra, 2015).
The breath test has several advantages over other sampling techniques: it is a non-invasive method, breath samples do not need further processing before performing analyses and are stable at room temperature in the absence of light up to 8 months (Keller et al, 2021). Considering these benefits, we hypothesized that the 13C-glucose breath test could be a valid method to assess adherence to treatment in clinical trials.
This preliminary study aims to develop and validate a breath test method to evaluate adherence to a nutritional intervention of soybean/corn starch snack enriched with a micro-dose of 13C-glucose, by quantifying the 13CO2 recovery in exhaled air. This research is part of a clinical trial that is currently being conducted to evaluate the effect of the soybean/corn snack on various outcomes related to the metabolic disorders of overweight and obesity.
Materials and Methods
This pilot trial was accomplished in the Escuela Nacional de Ciencias Biológicas (ENCB) of the Instituto Politécnico Nacional (IPN) in Mexico City, Mexico. The study protocol complied with the World Medical Association Declaration of Helsinki (last amended by the 64th World Medical Association General Assembly, Fortaleza, Brazil, October 2013) regarding the ethical conduct of human subjects research. The Ethics Committee of the mentioned institution approved the protocol (CEI-SH-004-2018).
Study procedures
Volunteers assented to participate in the study by a signed informed consent. Exclusion criteria were current chronic disease or soy intolerance, or both. Clinical history, weight, and height data were obtained. Body surface area (BSA) was calculated according to Mosteller's equation "defined as in Eq. 1" (Mosteller, 1987):
(Eq. 1.)
Participants were previously instructed to collect their breath samples in Exetainer tubes (Labco Ltd., High Wycombe, UK), blowing lightly through a standard straw. On the first day of the study, subjects collected an initial breath sample to establish a baseline. In the following days, breath samples were taken daily in the morning, before the consumption of the first portion of the snack, in duplicate at the same time for two weeks. The sampling time was recorded. Study subjects were randomly assigned by Microsoft Excel software into groups A, B and C. People in group A consumed the snack daily (14 consecutive days, representing 100% of the treatment), group B ate the snack five days a week (from Monday to Friday, for a total of 10 days, representing 70% of the treatment) and group C, consumed it, three days a week (Monday, Wednesday and Friday, for a total of 6 days, representing 50% of the treatment), this is shown in Table I. The indicated days, subjects consumed 100 g/day of the snack, dividing 50 g during the morning and 50 g in the evening. Subjects were asked not to modify their conventional diet.
Table I Snack consumption intervention design.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | S | M | T | W | T | F | S | S | M | T | W | T | F | |
| G A | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| G B | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||
| G C | ● | ● | ● | ● | ● | ● |
All study subjects begin on Saturday (S) and go through all the week: Sunday (S), Monday (M), Tuesday (T), Wednesday (W), Thursday (T), Friday (F). G: Group.
Snack preparation
Soybean (Glycine max L.) seeds and white corn (Zea mays) starch were purchased from the central market in Iztapalapa, Mexico City. The snack was prepared at the IPN laboratory. The first step was to clean soybean seeds from impurities and ground (BAUER mill model 148-2). The fat of soy flour was then removed with n-Hexane 95% ACS in the following proportions: 1 part offlour: 3 parts of hexane, in continuous shaking for 12 hours, and then dried for another 12 hours (POLINOX tray dryer). Fat-free soy flour and corn starch were mixed with water in a traditional blender, adding 100 mg of U-13C-glucose (Cambridge Isotope Laboratories, Inc., Andover, MA, USA) dissolved in 280 mL of water per kilogram of mixed flour to reach 29% of moisture (12 h before extrusion). The raw material was extruded using a single screw extruder of the Centro de Investigación en Ciencia Aplicada y Tecnología Avanzada (CICATA) IPN, Mexico City, Mexico. The final composition to achieve the recommended FDA's soy protein intake (FDA, 1999) was: 25.0±1.09%, soluble fiber 2.76±0.19%, insoluble fiber 11.56±0.49% (data expressed as the mean value of triplicate ± standard deviation), total dietary fiber 14.32%. The final amount of U-13C-glucose in the snack was 10 mg/100 g of product.
Breath CO2 analysis
Breath CO2 analysis was performed in an isotope ratio mass spectrometer Delta VAdvantage (Thermo Scientific™ Bremen, Germany) coupled with a universal inline gas introduction and preparation system Thermo Scientific™ GasBench II of the Laboratorio de Isotopía, Gerencia de Geotermia, INEEL, Morelos, Mexico. A non-normalized CO2 lab-tank was used as a reference gas. Samples were measured once, and the average of five peaks was used to calculate δ13C in the CO2 of breath. The standard deviation for CO2 reference gas peaks was consistently less to 0.06 %o. The breath test results were calibrated to zero and expressed as δ13C PDB (Pee Dee Belemnite, the calcium carbonate standard), this is the isotopic notation from the next equation (Eq. 2).
Eq. 2, Craig, (1957):
Area Under de Curve (AUC) for δ13C PDB values was calculated with the Riemann trapezoid method from day 0 to day 14 (Weideman, 2002).
Statically analysis
Statistical analysis was performed with SPSS software (version 26 SPSS Inc., Chicago, IL). The study subjects' general characteristics are shown as the mean ± standard deviation; a one-way ANOVA test with Tukey post-hoc analysis was used to determine statistical significance among study groups. AUC for δ13C PDB is reported as the median [minimum, maximum]; comparison between groups A, B, and C was made with a Mann-Whitney U-test. According to the intervention groups, a Spearman correlation analysis was used to establish the association between the median values of δ13C PDB and the treatment intensity. Finally, a Receiver-Operator Characteristic (ROC) curve was constructed with a confidence interval of 95% to determine a cut-off point to assess compliance with treatment through the 13C-glucose breath test. Sensitivity and specificity were calculated. A p-value <0.05 was considered as a threshold to establish statistical significance.
Results
Eleven adults (seven women and four males) with a mean age of 31.2 years and body mass index (BMI) of 24.3 kg/m2 participated in the study. Table II summarizes the subjects' general characteristics stratified by groups. Significant differences were obtained only for BMI when comparing groups, A and C. However, several characteristics between groups B and C show a statistical trend to significance: weight (p = 0.074), BMI (p = 0.066), and body surface area (p = 0.078).
Table II Anthropometric subject characteristics.
| Group A n=4 |
Group B n=3 |
Group C n=4 |
|
|---|---|---|---|
| Age [years] | 31.3 ± 6.1 | 25.0 ± 4.0 | 28.5 ± 4.7 |
| Height [m] | 1.7 ± 0.1 | 1.6 ± 0.0 | 1.7 ± 0.7 |
| Weight [Kg] | 67.8 ± 12.1 | 61.7 ± 5.4§ | 85.1 ± 14.3§ |
| BMI [Kg/m2] | 24.3 ± 2.7b | 24.3 ± 1.3§ | 29.6 ± 3.1b, § |
| BSA [m2] | 1.8 ± 0.2 | 1.7 ± 0.1§ | 2.0 ± 0.2§ |
Results are expressed as the mean value ± standard deviation. The same letters between columns show significant differences p ≤ 0.05 with Tukey's post hoc test.
§represents a statistical trend to significance.
Figure 1. illustrates the Delta Over Base (DOB) values (δ13C) for each study group through study time. The chart represents the behaviour of exhaled 13CO2, according to the intervention group. As expected, group A reported higher proportions of 13C in exhaled air during study time; however, the error bars overlap among study groups.
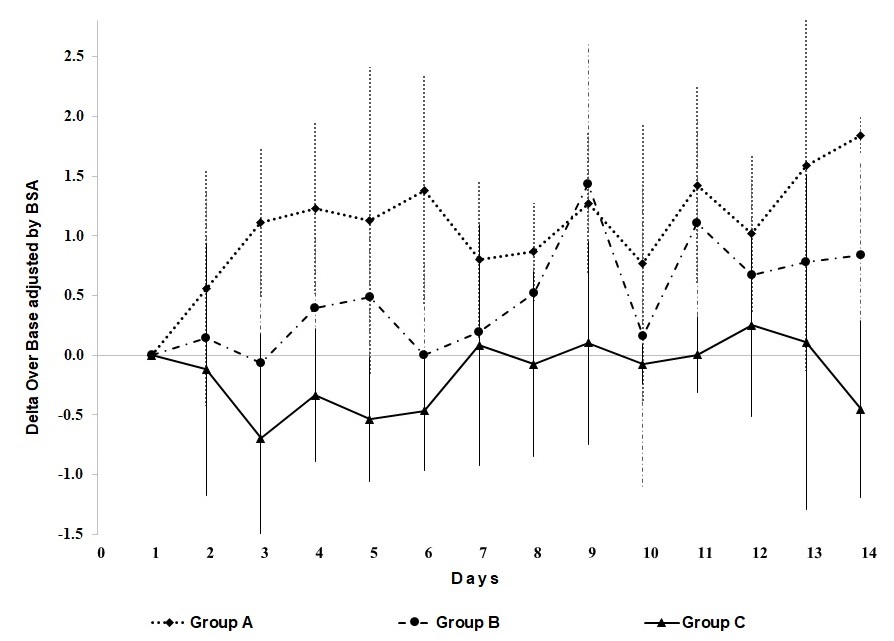
Figure 1 Variation of the delta over base δ13C in exhaled air according to treatment group through study time.
The whisker plots of AUC for δ13C PDB values, adjusted for BSA, are shown in Figure 2. The median of group A (12.4 [6.2, 25.3]) was significantly higher compared to that of group C (0.14 [-7.3, -1.4]). No differences were observed between the medians of groups B (8.8 [-0.1, 10.9]) and C. However, significant differences were found when groups A and B were got together and were compared with group C (p = 0.038). Furthermore, a significant negative association was found between δ13C PDB values and treatment intensity (Spearman rho = -0.708; p = 0.015).
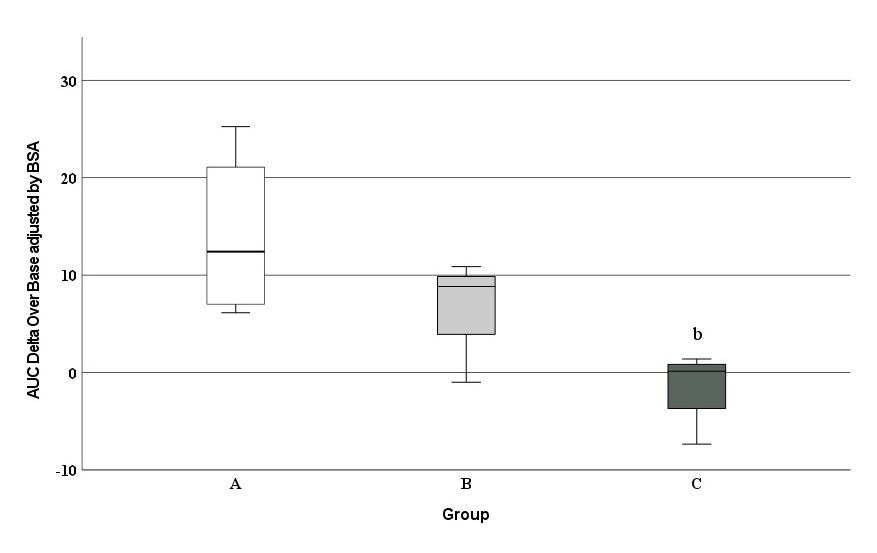
Figure 2 Comparison of the area under the curve (δ13C PDB values) between study groups. b Statistical Significance between groups A and C; p = 0.029.
Finally, a ROC curve analysis was computed to establish a cut-off point for the assessment with treatment compliance. A significant AUC value (0.89 CI 95% [0.68 to 1.0]; p = 0.038) was obtained (Figure 3). A cut-off ≤ 3.78 could identify subjects that complied less than 70% of the treatment (specificity of 100% and a sensitivity of 86%).

Figure 3 Receiver operating characteristic curve evaluating the sensitivity and specificity of the 13C-glucose breath test to define treatment compliance. State variable: 0 = who ate the snack >70% of the treatment (Groups A and B), 1 = who eat the snack ≤ 70% of the treatment (Group C). Area under the curve = 0.89 (0.62 - 1.00) p = 0.038.
Discussion
The results suggest that the 13C-glucose breath test could be a suitable method to assess compliance with treatments in nutritional intervention trials. The amount of 13C quantified in the exhaled air among study groups was directly linked to the assigned treatment intensity. Furthermore, a cut-off point ≤ 3.78 was proposed to identify compliance with the treatment of less than 70% with good diagnostic performance. A dose of 10 mg of U-13C-glucose per 100 g of the snack was adequate to achieve breath labelling and found differences among study groups. However, a smaller amount of labelled substrate could be used in more extended intervention studies where the accumulation of 13C-glucose in different tissues could compensate for a lower dosage.
A systematic review published by Hubbard, Elia, Holdoway & Stratton (2012), summarizes the treatment adherence of 46 published studies that supplied oral nutritional supplements (Hubbard et al., 2012). In most of the examined studies, treatment adherence was performed with subjective methods like interviews, questionnaires, phone calls, counting the number of bottles consumed, or using secondary anthropometric indexes. The authors reported mean overall compliance of 78% (that ranged from 37% to 100%) with similar levels of compliance between randomized (79%) and non-randomized clinical trials (71%). These results are consistent with the minimum threshold adherence proposed in this study (70%).
Concerning objective methods for treatment compliance assessment, information is scarce. Papada, Amerikanou, Forbes & Kaliora, (2019), evaluated the adherence to a Mediterranean diet in patients with Crohn's disease. A negative correlation was found (r = -0.4; p < 0.001) between a diet score and the Harvey-Bradshaw Index (a clinical index of Crohn's disease severity). Serum C-reactive protein also correlated inversely with the Mediterranean diet score (r = -0.268; p = 0.027). Interestingly, our results showed a strong correlation between δ13C PDB values and treatment intensity (rho = -0.708; p = 0.015), suggesting that the amount of 13C quantified in people's breath is closely related to the amount of snack consumed during the intervention. Some variations of the peaks like shown in Figure 1, could be explained by specific characteristics of the subjects, like: kind of diet, ingestion of food with more content of 13C, or physical activity; that is why, results are presented as AUC.
Indeed, a limitation of this study is its small sample size. The validity of the results obtained in the statistical analyses performed could be questioned for this reason. Nevertheless, our study's preliminary results encourage and suggest that the breath test could have a good diagnostic performance to assess treatment adherence. Despite the small sample size, statistical significance was achieved in most of the statistical tests performed. Therefore, we consider it convenient to carry out a complementary study with a larger population to confirm the usefulness of the 13C-glucose breath test to assess treatment adherence.
Conclusions
One of the primary biases of clinical trials is the lack of treatment adherence. Our results demonstrate that breath testing could be a good non-invasive alternative to evaluate compliance treatment adherence in intervention trials. Moreover, a micro-dose of U-13C-glucose (10 mg/100g soy-corn snacks per day) used as a tracer showed successful results about 13C appearance in exhaled air. A cut-off point of δ13C AUC PDB was computed to validate a minimum threshold adherence of 70% with good diagnostic performance. To the authors' knowledge, this is the first study that uses the 13C-glucose breath test to assess treatment compliance in nutritional intervention studies. In short, the 13C-glucose measure by a breath test could be a valid method to assess treatment adherence in intervention in clinical trials.
Author Contributions
Conceptualization: [Rosalva Mora-Escobedo], [Jorge Maldonado-Hernández], [Vanessa García-Rojas]; Methodology: [Jorge Maldonado-Hernández]; Formal analysis: [Jorge Maldonado-Hernández], [Vanessa García-Rojas]; Funding acquisition: [Rosalva Mora-Escobedo]; Investigation: [Vanessa García-Rojas]; Project administration: [Rosalva Mora-Escobedo], [Jorge Maldonado-Hernández]; Supervision: [Rosalva Mora-Escobedo], [Jorge Maldonado-Hernández]; Resources: [Georgina Izquierdo-Montalvo], [Eduardo San Martín-Martínez]; Data curation: [Dominic Ángel-Serrato], [Jorge Saúl Encarnación-Fernández]; Writing original draft: [Vanessa García-Rojas], [Jorge Maldonado-Hernández]; Writing, review and editing: [Mahendra P. Verma], [Alfonso Aragón-Aguilar], [Jorge Maldonado-Hernández], [Vanessa García-Rojas].
All authors declare not to have a conflict of interest.











 text new page (beta)
text new page (beta)


