Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Ingeniería, investigación y tecnología
On-line version ISSN 2594-0732Print version ISSN 1405-7743
Ing. invest. y tecnol. vol.11 n.3 Ciudad de México Jul./Sep. 2010
Hydrogen Sorption Properties of the Intermetallic Mg2Ni Obtained by Using a Simoloyer Ball Milling
Propiedades de ab–desorción de hidrógeno del intermetálico Mg2Ni obtenido empleando un molino de bolas Simoloyer
Martínez–Franco E.1, Klassen T.2, Jaramillo–Vigueras D.3 y Bormann R.4
1 GKSS, Research Center Geesthacht, Germany, CIITEC, Instituto Politécnico Nacional, Mexico City, E–mail: enmartinezf@ipn.mx
2 GKSS, Research Center Geesthacht, Germany, E–mail: Thomas.Klassen@hsu–hh.de
3 CIITEC, Instituto Politécnico Nacional, Mexico City, E–mail: djaramillo@ipn.mx
4 GKSS, Research Center Geesthacht, Germany, Department of Materials Science and Technology, Technical University Hamburg–Harburg, Eissendorfer Str. 42, E–mail: president@uni–bayreuth.de
Recibido: septiembre de 2008
Aceptado: junio de 2009
Abstract
Intermetallic Mg2Ni was produced from elemental powder blends by mechanical alloying in a batch scale using a rotary horizontal mill (Simoloyer). Fast hydrogenation kinetics are obtained: 2.2 wt.% of hydrogen is absorbed within 10 minutes at 300 °C. Hydrogen sorption kinetics were further improved by adding Pd (1 mol%) powder as a catalyst during ball milling. Crack formation and concomitant particle size reduction was observed by scanning electron microscopy after hydrogen cycling, which is attributed to internal stresses in the particles.
Keywords: High–energy ball milling (Simoloyer), hydrogen storage, particle size evolution.
Resumen
Se produjo por aleación mecánica el compuesto intermetálico Mg2Ni a partir de polvos elementales, empleando un molino de alta energía (producción por lotes) horizontal rotatorio comercialmente conocido como Simoloyer. Se observó que este compuesto presentaba una rápida absorción de hidrógeno, en donde se obtuvo 2.2 % peso de hidrógeno a 300 °C después de 10 minutos de proceso. La cinética de absorción de hidrógeno se mejoró al agregar 1 % mol de paladio durante la molienda. Se observaron por microscopía electrónica de barrido, grietas y disminución del tamaño de partícula por efecto de los ciclos de absorción–desorción de hidrógeno; y los cuales se atribuyeron a esfuerzos internos en las partículas.
Descriptores: molienda de bolas de alta energía (Simoloyer), almacenamiento de hidrógeno, evolución del tamaño de partícula.
Introduction
Mechanical alloying (MA) yields chemically homogeneous alloys (Benjamin, 1976), ( Benjamin et al., 1974), (Koch, 1989), (Bormann, 1977) with extremely fine crystallite sizes. The process is characterized by high–energy impact collisions of the milling tools, which lead to plastic deformation, cold–welding, and particle fracture which provides the driving force for microstructural refinement. Different techniques are available to conduct MA on the laboratory scale. However, few types of equipment have been designed to obtain larger quantities of powder for industrial production, such as horizontal rotary ball mills (Simoloyer model CM100s) (Zoz et al., 1996, 1999). The charge–discharge operations in this equipment can either take place under inert gas or vacuum to prevent oxidation. The milling chamber of this mill is designed such that allows vials cooling using either liquid or gas media. Magnesium and magnesium–based alloys have been widely studied as hydrogen storage materials by many research groups (Zaluzski et al., 1995), (Liang et al., 1999), (Zaluski, 1995), (Zaluski et al., 1999), (Kumar et al., 1995) because of their high hydrogen storage capacities by weight (7.6 wt.% of MgH2 and 3.6 wt.% of Mg2NiH4), long–term stability, full reversibility and low raw materials costs. A technological break through regarding reaction kinetics has been achieved by preparing nanocrystalline alloys by high–energy milling and by adding suitable catalysts, e.g., metal additives (Zaluzski et al., 1995), (Liang et al., 1999) or more recently, metal oxides (Oelerich et al., 2001), (Youp et al., 2002), (Inui et al., 2002). For example, Zaluski et al. (1995) used elemental Pd as catalyst and stored about 2.5 wt.% of hydrogen in Mg2Ni at 200°C/15bar in 10 minutes. For MgH2 with oxide catalysts, 80% of the theoretical capacity, i.e., 6.1 wt.% was obtained at 300°C/8 bar in 5 minutes (Oelerich, 2001). So far, all studies related to nanocrystalline hydrides were based on materials produced in small milling machines on the laboratory scale. For prototype purposes and technical applications, larger quantities are required. However, respective studies regarding upscaling of the powder production are still lacking.
Experimental procedure
Elemental powder blends (Mg JCPDF–35–0821, Ni JCPDF–04–0850) corresponding to the stoichiometric ratio for the intermetallic Mg2Ni phase were milled in a high–energy rotary ball mill (Simoloyer model CM01–2l, Zoz GmbH Germany). These results are al ready obtained and publication is available (Martínez et al., 2006). Different ball–to–powder mass ratios were used to evaluate efficiency of the mill system and samples without catalyst were stored for several months before measuring hydrogen sorption properties. Palladium powder (1 mol%) was added as a catalyst in a sample with a mass ratio of 10:1, for comparing hydrogen sorption kinetics with the powders without catalyst.
For measurements of hydrogen absorption kinetics, sample holders were sealed inside a glove box and attached to a hydrogen titration apparatus, which was specially designed (Schulz et al., 1999) for fast data acquisition.
Particle morphology during milling, as well as, after hydrogen charging and discharging was investigated by SEM (JEOL JSM–35CF). Contamination by oxygen and iron was quantified using energy disperse spectrometry (EDS) available in the SEM.
Results and discussion
Mechanical alloying using the Simoloyer ball mill
The XRD pat terns for different ball–to–powder mass ratios as a function of milling time are shown in figure 1. Peak broadening indicates microstructural refinement. The crystallite size of Mg2Ni is estimated between 4 and 11 nm using the Scherrer–formula (Cullity, 1978). Peaks corresponding to the intermetallic Mg2Ni (JCPDF–35–1225, mainly diffracted between 20°–22° and 40°–45°) are observed after different milling times depending on the mass ratio. Traces of Ni were found in all milling experiments and unreacted Mg was only found for a mass ratio of 50:1. These results show that Mg2Ni formation strongly depends on the mass ratio. Increasing the mass ratio considerably reduces the Mg2Ni synthesis time from 25 hours down to 4 hours using mass ratios of 10:1 and 100:1, respectively.
In all milling process the possibility exits of contamination due to wear of media (balls) and control process (atmosphere). Analysis of possible contaminants such chromium, iron and oxygen by using energy dispersive spectrometry (EDS) were carried out.
EDS measurements showed only the presence of iron and oxygen as is reported in table 1 for all ball–to–powder mass ratios. The numbers in brackets indicate the milling time of the sample. Average and corresponding deviations of all measurements are reported. Iron and oxygen contamination took place from the vial's erosion and air when samples were taken for characterization. Even though the milling time required for complete transformation into the Mg2Ni phase is much shorter for a mass ratio of 100:1, the highest iron contamination was detected in this sample. This is attributed to the smaller powder quantity and therefore, more impacts collisions of the balls. The oxygen contamination is similar (approximately 5 wt.%) for all specimens independently of the mass ratios, which indicates contamination due to handling for sampling to analysis.
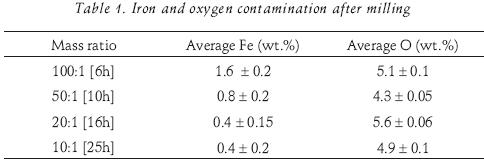
Hydrogen storage properties
Sorption properties of Mg2Ni without catalyst
The hydrogen reaction kinetics at 9 bar hydrogen pressure and 300°C of the intermetallic Mg2Ni obtained with different ball–to–powder mass ratios is shown in figure 2. Absorption kinetic is best for the mass ratio of 10:1 and 2.2 wt.% of hydrogen is absorbed within 10 minutes at 300°C. It is observed that increasing mass ratios hydrogen absorption kinetics is lower, with exception of the mass ratio of 50:1. According to our previously work (Martínez et al., 2006), if is taken into account only the iron content, the sample with mass ratio of 100:1 should present the lower kinetic and hydrogen capacity, but in the sample with mass ratio of 50:1 Mg–traces still present after milling which can easily forms an oxide layer which inhibits the reaction with the hydrogen.
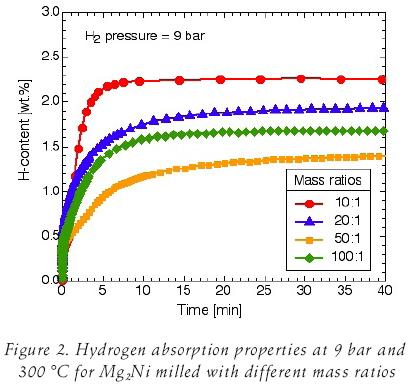
Due to its highest hydrogen absorption capacity, the powder milled with the mass ratio of 10:1 was used for further kinetics characterization. Absorption–desorption curves at different temperatures are shown in figure 3.
As expected, the best absorption kinetics are achieved at 300°C, taking about 10 minutes for completion. At lower temperatures, the absorption kinetics slow down and the maximum hydrogen storage is achieved only after 90 minutes at 250°C. There is no significant difference observed between desorption at 300°C and 250°C and is completed after 12 minutes for both temperatures.
Sorption properties of Mg2Ni with catalyst
The absorption and desorption curves of Mg2Ni+Pd (1 mol%) are presented in figure 4. Absorption kinetics were enhanced in comparison to the material without catalyst, and the total capacity (after 90 minutes) was about 3.0 wt.%, which is very close to the results obtained by Klassen et al. (1998) for similar material. Full desorption was achieved after 2 and 8 minutes for 300 and 250°C, respectively. Comparing with the results for Mg2Ni without catalyst, desorption kinetics was about five times faster at 300 °C. These results demonstrate the enhancement of the sorption properties by adding Pd as a catalyst. The catalytic effect of Pd suggested by Zaluski et al. (1999) is due to small Pd particles distributed on the metal surface. They process is explained by a "spill–over" phenomenon: hydrogen is easily chemisorbed i.e. dissociate, don the Pd–surface and migrates to the base storage material, i.e. Mg, which does not show easy chemisorption of hydrogen. Although no direct evidence is reported in this work, is suggested that high–energy ball milling by using a Simoloyer produces very fine Pd particles and/or disperses Pd–clusters on the powder surface and absorption–desorption kinetics are improved.
As was reported previously (Martínez et al., 2006) particle size decrease upon increasing milling time. The average particle size before milling was in the range of 40–50 µm, and reduces to 5–20 µm after 25 hours milling time when a ball–to–powder mass ratio of 10:1 is used. Figure 5 shows back scattered electron images of powders milled at 8 and 25 hours with a mass ratio of 10:1. While Mg–rich and Ni–rich areas can still be distinguished after 8 hours, the powder appears mostly homogeneous after 25 hours milling.
Figure 6 shows SEM pictures of the Mg2Ni with catalyst (mass ratio of 10:1) after the second absorption measurement and after six ab–desorption cycles. It is shown cracking of agglomerates and particles. We suppose that exists an effect of the internal stresses in particles built up during hydrogenation–dehydrogenation process (Mg2Ni+2H2 Mg2NiH4). Regarding to this effect, Inui and co–workers (2002) reported that for some materials, the particle size decreases during absorption–desorption cycling because of internal stresses caused by lattice expansion–contraction, which is a good agreement with our results. Although reticular parameters were not calculated, and considering cell expansion–contraction, is supposed that theoretically the cell volumes of an hcp (Mg2Ni) and fcc (Mg2NiH4) crystal structures are 311 and 278, respectively; therefore, distortion of cell is evident and our experimental results are also in good agreement with this consideration.
Mg2NiH4). Regarding to this effect, Inui and co–workers (2002) reported that for some materials, the particle size decreases during absorption–desorption cycling because of internal stresses caused by lattice expansion–contraction, which is a good agreement with our results. Although reticular parameters were not calculated, and considering cell expansion–contraction, is supposed that theoretically the cell volumes of an hcp (Mg2Ni) and fcc (Mg2NiH4) crystal structures are 311 and 278, respectively; therefore, distortion of cell is evident and our experimental results are also in good agreement with this consideration.
Cracks are formed and some smaller particles break loose, exposing fresh surfaces that are beneficial for improving the reaction with hydrogen. A sample with Pd as catalyst was cycled at 300°C/ 9 bar hydrogen pressure and results of the absorption process is shown in figure 7.
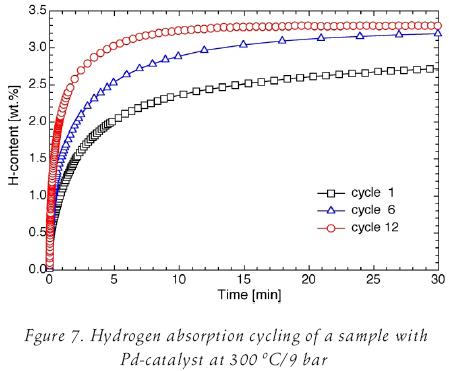
As is shown in figure 6, "fresh" surfaces generated during hydrogen cycling improves hydrogen absorption process (figure 7). Hydrogen content of 3.3 wt. % is obtained after 15 minutes in a sample with 12 cycles; which is near to the theoretical capacity, 3.6 wt. %, for the reaction Mg2Ni+2H2 Mg2NiH4.
Mg2NiH4.
Conclusions
1. Material stored in glass vial under argon atmosphere for several months (Mg2Ni without catalyst) shows fast absorption kinetics at 300°C (15 minutes for complete absorption), although its capacity is rather low (2.2 wt.%). Full desorption of Mg2Ni without catalyst is readily achieved at 250°C in about 10 minutes in vacuum.
2. Small amounts of Pd used as a catalyst (1 mol%) further improve the hydrogen sorption kinetics. Full desorption at 300°C was achieved after 2 minutes.
3. The average particle size decreases with increasing milling time, and a further decrease due to particle cracking was observed during cycling.
4. Hydrogen ab–desorption cycles produces internal stresses and generated new surfaces that improve hydrogenation process.
Acknowledgements
E. Martínez–Franco is grateful for receiving a fellowship by CONACyT–Mexico and DAAD–Germany, and would like to acknowledge M. Sc. Emma Morales (GKSS) for assistance in SEM operation. Authors are grateful for financial support from ICyTDF/SIP–IPN for this research.
References
Benjamín J.S. Scientific American. Vol. 234. 1976. Pp. 40–48. [ Links ]
Benjamin J.S., Volin T.E. Metallurgical Transactions A. Vol. 5. 1974. Pp. 1929–1934. [ Links ]
Koch C.C. Material Synthesis by Mechanical Alloying. Annual Review of Materials Science. Vol. 19. 1989. Pp. 121. [ Links ]
Bormann R. Mat. Sc. and Eng. Vol. A226–228. 1997. Pp. 268–273. [ Links ]
Zoz H., Ernst D., Weiss H., Magini M., Powel C., Suryanarayana C., Froes F.H. Fachzeitschrift METALL. Vol. 50. 1996. Pp. 575–579. [ Links ]
Zoz H., Ren H., Reichardt R., Benz H.U. Vol. 1, No. 1. 1999. Pp. 15–19. [ Links ]
Zaluzski L., Zaluska A., Ström–Olsen J.O. Journal of Alloys and Compounds. Vol. 217. 1995. Pp. 245–249. [ Links ]
Liang G., Huot J., Boily S., Van–Neste A., Schulz R. Journal of Alloys and Compounds. Vol. 292. 1999. Pp. 247–252. [ Links ]
Zaluski L., Zaluska A., Tessier P., Ström–Olsen J.O., Schulz R. Journal of Alloys and Compounds.Vol. 217. 1995. Pp. 295–300. [ Links ]
Zaluzski L., Zaluska A., Ström–Olsen J.O. Journal of Alloys and Compounds. Vol. 288. 1999. Pp. 217–225. [ Links ]
Kumar–Singh A., Kumar–Singh A., Srivastava O.N. Journal of Alloys and Compounds. Vol. 227. 1995. Pp. 63–68. [ Links ]
Oelerich W., Klassen T., Bormann R. Journal of Alloys and Compounds. Vol. 315. 2001. Pp. 237–242. [ Links ]
Youp M., Jean–Louis B., Darriet B. Journal of Alloys and Compounds. Vol. 340. 2002. Pp. 256–262. [ Links ]
Inui H., Yamamoto T., Hirota M., Yamaguchi M. Journal of Alloys and Compounds. Vol. 330. 2002. Pp. 117–124. [ Links ]
Martinez–Franco E., Klassen T., Bormann R., Jarmillo D. Materials Science Forum. Vol. 509. 2006. Pp. 141–145. [ Links ]
Schulz R., Boily S., Hout J. [ Links ] Canadian patent. Ser.–Nr.: 2207149. 1999.
Cullity B.D. Elements of X–ray diffraction. 2nd. Ed. Adisson–Wesley Publishing. USA. 1978. [ Links ]
Klassen T., Oelerich W., Zeng K., Bormann R. Magnesium Alloys and their Applications. Ed. by Mordike B.L., Kainer K.U. Werk stoff–Information sgesellschaft GmbH, Frankfurt. 1998. Pp. 307–311. [ Links ]
About the authors
Enrique Martínez–Franco. Bachelor and Graduate Studies on Metalurgical Engineering at ESIQIE–IPN, Mexico. Ph.D. Studies a Sand wich Program at Technical University Hamburg–Harburg, GKSS Research Center both in Germany and ESIQIE–IPN. The topic of the research work during Ph.D was the processing and development of Light Metal Hydrides for Hydrogen Storage. From 2004 up to now is professor at Research Center and Innovative Technological of IPN (CIITEC–IPN). Collaboration with different Research Centers like CINVESTAV Mexico, Zoz GmbH Germany in topics on the field of processing materials for hydrogen storage, metal oxides for electronic applications and use of high energy–ball milling for leaching dust from electric furnaces.
Thomas Klassen. Bachelor and Graduate Studies on Engineering at Goettingen University and Ph.D. studies at Technical University Hamburg–Harburg, Germany. Up to now Prof. Klassen has been participated in more than 60 papers and graduate more than 20 Graduates from different Countries. Leader from more than 10 years in Nanotechnology Department at GKSS Research Center and recently got his Habilitation as Professor and since 2009 is a leader of the Department of Mechanical Engineering at Helmut Schmidt University, Germany.
David Jaramillo–Vigueras. The Professor Jaramillo Vigueras got his Bachelor as Metallurgical Engineering at ESIQIE–IPN. He got his Ph.D at Technology Institute of Nuevo Mexico, USA. He was professor for more than 15 years at ESIQIE–IPN, Director of CIITEC and of the Superior Education in IPN and currently is research–professor at CIITEC. He was pioneer in Mexico with the development of high energy milling concept and has been lead more than 50 theses in Bachelor and Graduate Studies. He joins the group who founded the Ph.D Graduate Studies on Materials at ESIQIE–IPN and all laboratories for the respective research work. Has been participated in more than 100 papers.
Ruediger Bormann. Professor Bormann has been worked more than 20 years as leader in different topics on materials processing. He got more than 10 patents also in different topics all related to the successful research activities. He has Graduate more than 50 Ph.D. students from different Countries. He was professor and leader at Technical University Hamburg–Harburg and Director of Materials Institute at GKSS Research Center. He has participated in more than 150 papers. Currently he is the President of the University of Bayreuth, Germany.














