1. Introduction
The extinction of freshwater snails due to habitat loss is of wide interest and occurs especially among endemic species with restricted distributions, such as those in karst landscapes where lakes, ground-waterfed springs, and other habitats are threatened (e.g., Strong et al. 2008, Régnier et al. 2009). The distribution of extant freshwater gastropods of northern Mexico is poorly known and fresh-water habitats are rapidly being altered by water diversions and dam construction. Only a portion of living species have been studied ecologically, anatomically or using molecular methods. Recent advances have focused mainly on systematics and biogeography of newly collected extant cochliopid and hydrobiid snails or species previously known only from shell morphology (Hershler et al., 2002, 2011a, 2011b, 2014a, 2014b). Without these data our systematic research affiliation of a part of the subfossil material would be much more difficult, or, as in some cases of shell convergences, almost impossible.
The study area is located in the central part of the Laguna District, a small karst region and it comprises the adjoining portions of western Coahuila and eastern Durango states, northern Mexico (Figure 1). The region, a large endorheic basin, was one of the largest paleolakes in Latin America during Late Pleistocene/Early Holocene (Butzer et al., 2008; Czaja et al., 2014b). Although this lake had become significantly smaller during the Holocene, large lakes were still present until the early 1900s (Ortega-Guerrero, 2003; Butzer et al., 2008). In the first half of the 20th century the region became an important center of agriculture and industry in Mexico. This economic process was accompanied by habitat perturbations such as damming and channelization of the two rivers, alteration of springs, and especially by excessive groundwater pumping. This aquifer depletion, described in detail by Wolfe (2013), finally eliminated the habitats provided by the remaining inland lakes and springs in the area of study.
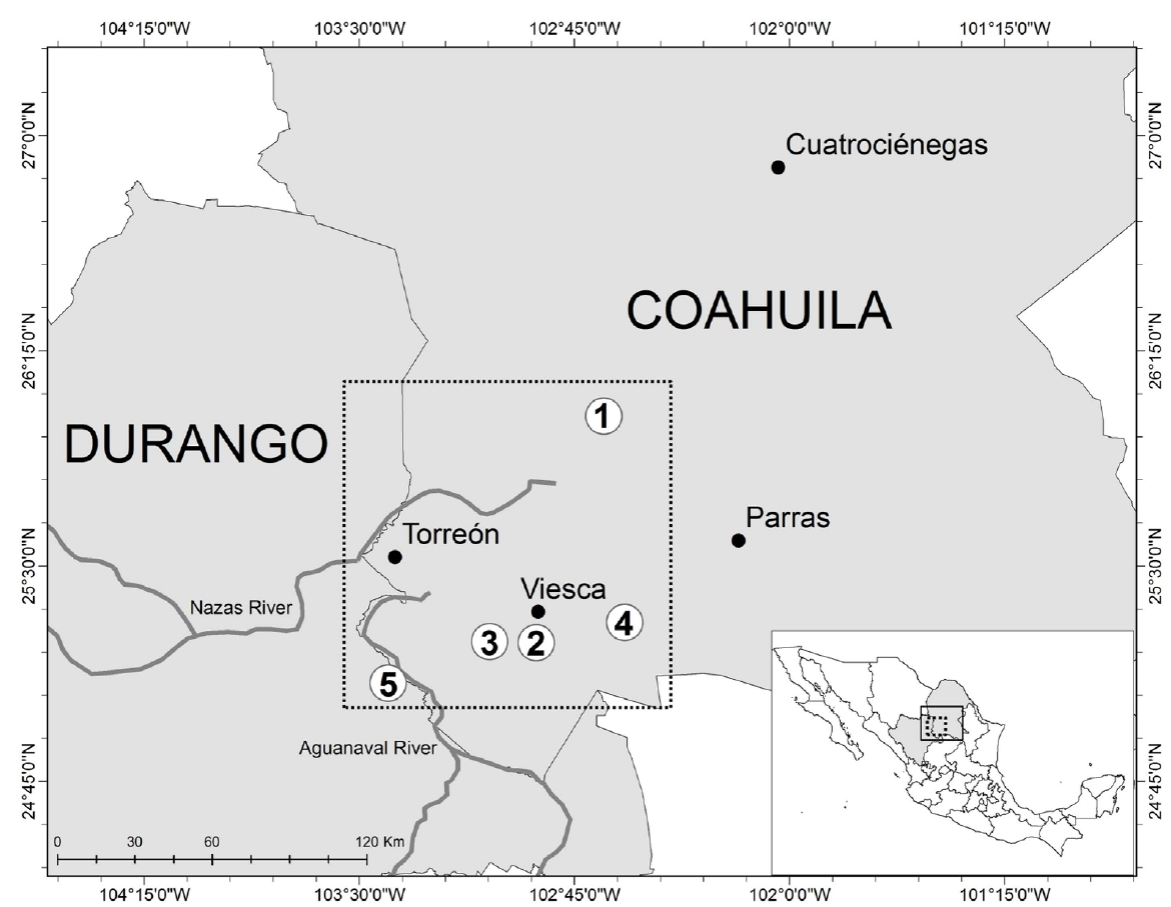
Figure 1 Map of the study area with localization of the sampled sites of the Laguna District of Coahuila and Durango. 1 = Holocene playa deposits of the Paleo-Lake Irritila (Laguna Mayrán). 2 = Springs of Viesca. 3 = Former wetland area of the springs of Viesca. 4 = Lacustrine sub-recent deposits of the Paleo-Lake Viesca. 5 = Sub-recent fluviatile deposits of the Aguanaval River. Dotted squares = rea of study.
From this semi-desert and karst region of south-west Coahuila and northeast Durango, we report subfossil shells belonging to cochliopid, hydrobiid, planorbid and neritid freshwater gastropod genera. The aim of the present study is to describe a new species, report new records and show their original biogeographic distribution prior to the loss of their habitats in recent times. To achieve that, we follow the “near-time” approach sensuDietl and Flessa (2011) which utilizes the fossil record to understand the present-day conditions. In Mexico, this new interdisciplinary approach was applied so far only once (Figueroa-Rangel et al., 2012) but its potential has already been pointed out in detail by Guerrero-Arenas and Jiménez-Hidalgo (2015).
2. Material and methods
Shells of Juturnia gracilis n. sp., J. coahuilae (Taylor, 1966), Pseudotryonia pasajae (Hershler, Liu and Landye, 2011), Promenetus exacuous (Say, 1821) and Tryonia cf. seemani (Frauenfeld, 1863) were collected in present-day superficial sediments of dried-out springs near to the town of Viesca, Coahuila (Figure 1, site 2). All springs were still active in 1959 and dried out in the following years (Blásquez, 1959; Wolfe, 2013). Shells of Biomphalaria hava-nensis (Pfeiffer, 1839) were collected at west of Viesca springs in superficial sediments of a former wetland area of these springs (Figure 1, site 3). Shells of both species of Probythinella Thiele, 1929, Cochliopina riograndensis (Pilsbry and Ferriss, 1906) and Dilatata dilatata (Gould, 1841) were collected in fluviatile deposits of the Aguanaval River and in lacustrine subrecent deposits of the paleo-lake Viesca (Figure 1, sites 5 and 4). A single shell of Vitta cf. usnea (Röding, 1798) was collected in a gray silt horizon with abundant planorbid snails of the former Paleo-Lake Irritila (Figure 1, site 1). These sediments with Vitta were not dated directly but the same horizon with abundant snails from a neighboring site was dated by Butzer et al. (2008) as Holocene playa deposits with radiometric calibrated 14C ages between 3320 ± 100 at the top of the section and not older than 7130 years BP at the bottom.
For a comparison of the subfossil material, empty shells of the extant species Pseudotryonia pasajae were collected in September 2016 from an unnamed spring in San Pedro de Ocuila, Durango, which is the type locality for this species. Empty shells of the extant Juturnia coahuilae from Poza Churince in Cuatrociénegas were collected in summer 2017 directly from sediments of this spring. Only the outflows of the dams of the Nazas and Aguanaval rivers and the small spring La Peña still contain permanent water in the area of study. Table 1 contains the coordinates of the localities that are monitored by the authors since 2009. These three sites were last sampled in summer 2017. The snails were hand collected from stones, sand and aquatic vegetation, 4 kg sediment per site was screened through sieves.
Table 1 The coordinates of three localities that still contain permanent water in the study area (outflows of the dams of the Nazas and Aguanaval rivers and the small spring La Peña).
| Nazas River | Aguanaval River | La Peña spring | |
|---|---|---|---|
| Site 1 | 25°27′56″ N, 103°42′13″ W | 25°07′49″ N, 103°22′22″ W | 25°27′02″ N, 102°35′10″ W |
| Site 2 | 25°27′55″ N, 103°42′12″ W | 25°13′14″ N, 103°26′58″ W | |
| Site 3 | 25°39′30″ N, 103°22′51″ W | 25°01′36″ N, 103°16′09″ W |
To visualize morphological shell differences between subfossil and living populations, a discriminant analysis was carried out. This was done using the software PAST (PAleontogical STatistics) v 3.14 (Hammer et al., 2001). This technique has frequently been used to differentiate closely similar shells of other hydrobiid and cochliopid gastropods (Czaja et al., 2017b). We used following shell morphometric dataset (excluding ratios): Total number of whorls, shell height, shell width, aperture height, aperture width, height of body whorl and width of body whorl. The mean, standard deviation and sample size are given in text (shell measurements).
Sediments of all localities were screened through two sieves with 0.5 mm and 0.3 mm mesh size. Some specimens (especially the apertures of the shells) were cleaned using hydrogen peroxide to remove sediments. All shells were photographed with a Zeiss AxioCam ERc5s-camera attached to a Zeiss Stemi 2000-C microscope. Morphometric variables of the shells were measured using the same microscope. Shell whorls were counted according to the method of Pilsbry (1939). The material is housed in the Malacological Collection of the Faculty of Biological Science of the Juarez State University of Durango (UJED). Paratypes of the new species were deposited in the National Collection of Mollusks, National Autonomous University of Mexico (CNMO).
Abbreviations used in the text: WN, total number of whorls; SH, shell height; SW, shell width; AH, aperture height; AW, aperture width; HBW, height of body whorl; WBW, width of body whorl; sd, standard deviation; UJMC = University Juárez Malacological Collection.
3. Results
3.1. Systematics
We emphasize that in the present paper we apply the morphological species concept widely used for paleobiological and paleontological studies. However, our morphospecies definitions are also based on insight from modern species concepts of living species that includes biogeographic considerations and population genetic aspects. A combination of neo- and paleobiological approach is mutually supportive in these determinations.
Superfamily Neritacea Lamarck, 1809
Family Neritidae Lamarck, 1809
Subfamily Neritininae Poey, 1852
Genus Vitta Mörch, 1852
Type species: Nerita virginea Linnaeus, 1758 Vitta cf. usnea (Röding, 1798) (Figure 2 a to 2e)
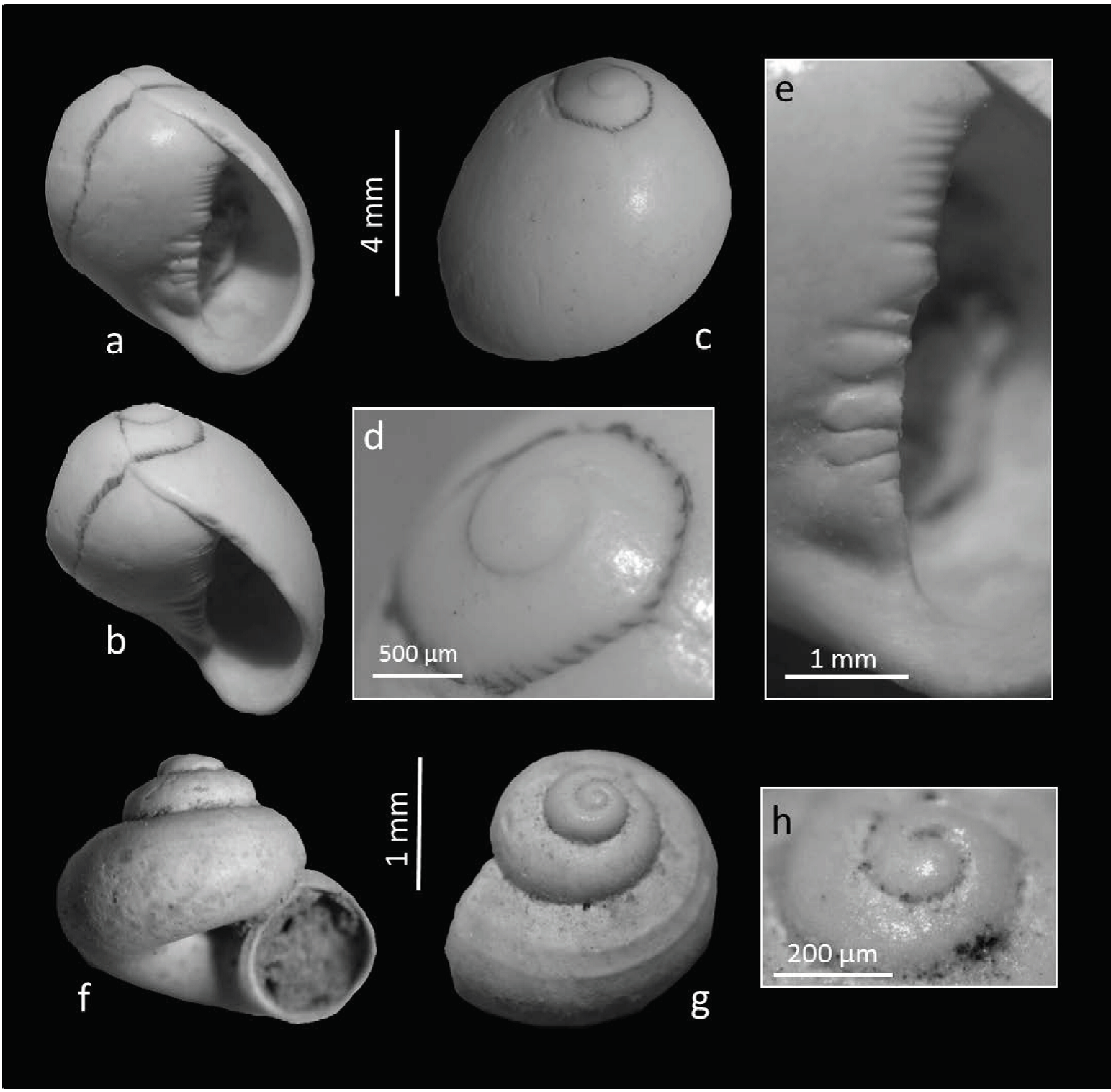
Figure 2 Vitta cf. usnea (Röding, 1798) and Cochliopina riograndensis (Pilsbry & Ferriss, 1906). a-e) V. cf. usnea, UJMC 360. d) Black axial lines as remains of the original color pattering. e) Parietal shelf with irregular teeth. f-h) Cochliopina riograndensis. f) Specimen UJMC361a. g) Specimen UJMC 361b with cords on the bodywhorl. h) Specimen UJMC 361c, protoconch view.
Description: Shell dextral, ovoid, with flat spire, height 7.82 mm, width 6.79 mm, with 3.00 whorls, whorls rapidly increasing in size, bodywhorl constitutes most of shell, surface smooth and porcel-lanous; sutures faintly impressed, with numerous microscopic axial growth lines and no spiral sculpturing, color pattern visible in the area of the sutures consisting of dark, wavy lines, which are closely spaced on a whitish background; aperture ovate, the outer lip is thickened and without teeth, parietal shelf straight with 12 (or 13?) irregular teeth (Figure 2e), teeth absent from the top and the bottom of the inner lip; numerous thin, parallel, brown to black axial lines as remains of the original color pattering (thin, parallel axial lines) visible only near the sutures (Figure 2d).
Shell Measurements: SH = 7.82 mm, SW = 6.79 mm, AH = 5.49 mm, AW = 3.67 mm, WN = 3.00.
Material Examined: 1 specimen (UJMC 360).
Distribution: Florida to Texas, Mexico (gulf coast), West Indies (Eichhorst, 2016).
Remarks: Because it is a single specimen with-out color and pattern on the shell, our material can only be identified tentatively. Further, the specimen shows the teeth on the columellar lip much more defined than on modern Vitta usnea. The outer shell layer on the parietal shelf that leads up to these teeth probably has been broken off, exposing more of the teeth than is normally visible. The species is reported as Neritina reclivata (Say, 1822) in much of the recent literature, but we follow Eichhorst (2016) and placed this species in the genus Vitta because it lacks the characteristics of Neritina. V. usnea, as it occurs in fresh/brackish water, on rocks, plant stems, or other hard surfaces and was found living in freshwater 160 km inland in Florida (Eichhorst, 2016). However, its Late Holocene presence in the study area almost 1000 km from the coast is notable. Although the species has a wide ecological range, occurrences in freshwater at long distances from the coast habitats are not known in its present-day distribution. Last record in the area: Middle/Late Holocene (radiometric calibrated 14C ages between 3320 ± 100 and ̴7130 years BP, Butzeret al., 2008).
Superfamily Truncatelloidea Gray, 1840
Family Cochliopidae Tryon, 1866
Genus Cochliopina Morrison, 1946
Type species: Cochliopa riograndensisPilsbry & Ferriss, 1906 (by original designation)
Cochliopina riograndensis (Pilsbry and Ferriss, 1906) (Figure 2f to 2h)
Description: Shell small, broadly heliciform, height 1.39-2.02 mm, width 1.91-3.01 mm, openly umbilicate; apical whorl with pitted structure; teleoconch nearly smooth or frequently with spiral threads or (two) cords on the bodywhorls, one thread at the shoulder usually more prominent (Figure 2); whorls moderately convex with deep sutures; the aperture is roundly ovate and angled adapically; peristome thin, inner lip partly fused to the penultimate whorl.
Shell Measurements: (mean, with sd in parentheses; N = 10) SH = 1.65 (0.28) mm, SW = 2.41 (0.31) mm, AH = 1.15 (0.08) mm, AW = 1.02 (0.08) mm, WN = 3.40 (0.21).
Material Examined: 10 specimens (UJMC 361a-j).
Distribution: Streams in Val Verde County, Texas and Northwest Mexico (Coahuila, San Luis Potosí, Tamaulipas) (Thompson, 2011).
Remarks: The subfossil shells from Durango and Coahuila correspond morphologically in all details to those from the type locality in Texas. Pilsbry and Ferriss (1906) noticed in the original description of C. riograndensis a great similarity to shells of the genus Valvata (Müller, 1773), but they emphasized that species of the last genus have rounded apertures. According to Riedel (1993), the protoconchs of Valvata exhibit as well typical valvatid sculpture in form of numerous spiral lirae which are radially interconnected. Both shell features are not present in our material so we place the shells into the genus Cochliopina.
Genus JuturniaHershler, Liu and Stockwell, 2002
Type species: Durangonella coahuilaeTaylor, 1966 (by original designation)
Juturnia gracilis Czaja, Covich, Estrada-Rodríguez and Romero-Méndez sp. nov. (Figure 3a to 3g)

Figure 3 Juturnia gracilis new species, Juturnia coahuilae (Taylor, 1966) Hershler, Liu and Stockwell, Probythinella emarginata (Küster) and Probythinella protera Pilsbry. a, b, g) J. gracilis new species, Holotype, UJMC-362. g) Protoconch view. c, d) Paratype 1, UJMC-363. e, f) Paratype 2, UJMC-364. h-j) Juturnia coahuilae (Taylor, 1966) Hershler, Liu and Stockwell from spring Tunel 7, Viesca, Coahuila, Mexico, UJMC-366a. j) Protoconch view. k-m) J. coahuilae from Poza Churince, Cuatrociénegas, Coahuila, Mexico, a present-day specimen. m) Protoconch view. n-p) Probythinella emarginata, UJMC-367a. p) Protoconch view. q-t) Probythinella protera. q, r) Specimen UJMC-368a. s) Specimen UJMC-368b. t) Protoconch view, UJMC-368a.
Diagnosis: Shell medium-sized, turriform, fragile, light tan or transparent, teleoconch of 8.00 to 11½ strongly convex whorls; teleoconch without sculpture, only with very fine and closely spaced growth lines; aperture ovate, inner and outer lip thin; umbilicus rimate to broadly open.
Description: Shell turriform, medium-sized, 4.82-7.32 mm shell length and 1.29-1.78 shell wide, with 8.00 to 11½ strongly convex and smooth whorls; teleoconch sculpture of closely spaced growth lines, from light tan to beige-white or transparent, in some specimens from light tan to brown periostracum preserved; protoconch of 0.7-1.0 whorl, smooth (Figure 3g); aperture ovate, inner lip thin to slightly thickened, broadly adnate to completely separate from parietal wall, outer lip thin or slightly thickened, the peristome is complete; umbilicus rimate to broadly open.
Shell Measurements: (mean, with sd in parentheses; N = 15) SH = 5.75 (0.61) mm, SW = 1.49 (0.13) mm, AH = 1.11 (0.11) mm, AW = 0.95 (0.08) mm, HBW = 2.05 (0.21) mm, WBW = 1.29 (0.12) mm, WN = 10.07 (0.62).
Measurements of Holotype: SH = 5.83 mm, SW = 1.47 mm, HBW = 1.97 mm, WBW = 1.30 mm, AH = 1.07 mm, AW = 1.01 mm, WN = 10¼.
Differential Diagnosis: Of the four extant species of the genus Juturnia, the new species only resembles J. coahuilae but differs clearly from this by having larger shells with considerably more whorls (J. coahuilae is possibly the described freshwater snail with most whorls in North America). Reliable diagnostic distinctions between these two species are as well the whorl height/whorl width ratio that is 0.21-0.29 in J. gracilis n. sp. and 0.30-0.40 in J. coahuilae. As a result of this different ratio, J. gracilis n. sp. has a narrower general form than J. coahuilae (Figure 3a to 3f). Also, the discriminant analysis (Figure 4), based on seven standard shell morphometric data, confirms that the subfossil shells of J. gracilis n. sp. described herein are morphologically clearly distinct to shells of the extant endemic species from the Cuatrociénegas Basin. A combination between the detailed morphological description and morphometric based discriminant analyses were efficient in distinguishing among the recent and subfossil species.
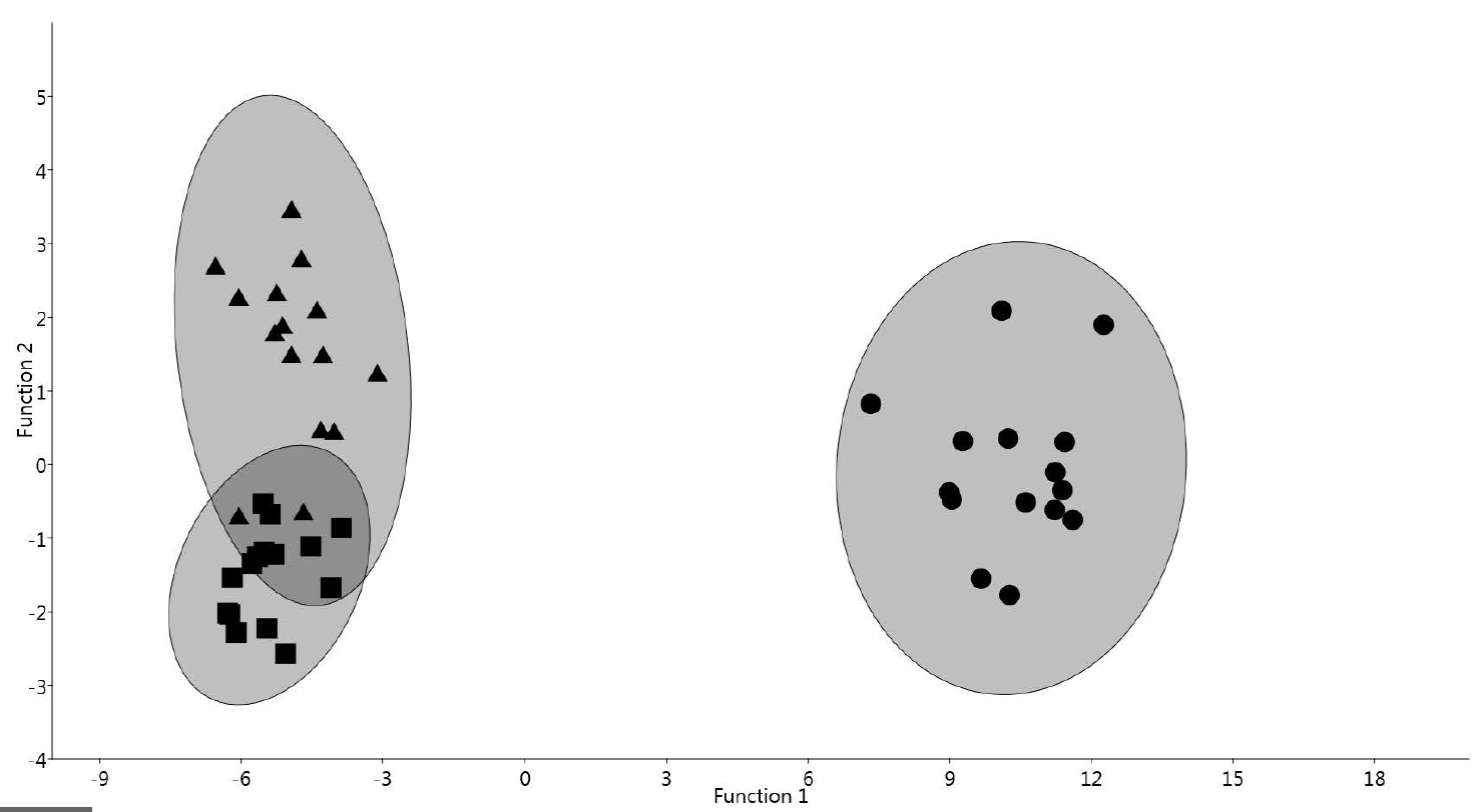
Figure 4 Graphic depiction of the discriminant analysis of morphometric variables of three populations of Juturnia. Circles = Juturnia gracilis new species, subfossil population from Spring Tunel 4, Viesca, Coahuila. Square = J. coahuila (Taylor, 1966), a subfossil population from Spring Tunel 7, Viesca, Coahuila. Triangles = J. coahuila, a present-day population from Poza Churince, Cuatrociénegas, Coahuila.
Type Material: Holotype (Figures 3a, 3b, 3g), UJMC-362. Paratype 1 (Figures 3c, 3d), UJMC-363, Paratype 2 (Figures 3e, 3f), UJMC-364. (leg. Alexander Czaja and José Luis Estrada-Rodríguez, 25/vi/2016). All from type locality.
Type Locality: Mexico, Coahuila state, Viesca, Tunel 4 Spring (25° 20′ 20″ N, 102° 52′ 32″ W, 1099 m a.s.l.)
Stratum Typicum: Spring sediments (layer c. 5 cm below the present-day surface). Latest Holocene, sub-recent.
Other Material Examined: 12 specimens from the same locality (UJMC-365a-l).
Etymology: The species was named after the slender (lat. gracilis) general shape of its shells.
Distribution: J. gracilis n. sp. is endemic to the Túnel 4 spring at Viesca, Coahuila, Mexico.
Remarks: Hershler et al. (2002) mentioned that shells of Juturnia broadly overlap in size, shape, and sculptural features with those of several other epigean cochliopines of southwestern North America such as Eremopyrgus, Littoridinops, Pseudotryonia and Tryonia. However, such narrow and turri-form shells with numerous whorls (more than 7) are reported only from extant members of Juturnia and Tryonia. Both genera are difficult to separate by shells and it seems that conchiologically only the well-developed growth lines on Juturnia shells is the unique useful differentiating feature between both genera. Of the three living species of the genus, J. gracilis n. sp. closely resembles J. coahuilae (Taylor, 1966), an extant endemic species from the Cuatrociénegas Basin. In neighboring springs and spring-outflows in Viesca subfossil shells of J. coahuilae are very common (see below). This geographical proximity and the similarity of the shell structure of J. coahuilae and J. gracilis n. sp. reflect undoubtedly a narrow phylogenic relationship between both species. We suppose that the sub-fossil J. gracilis n. sp. has evolved from J. coahuilae in a separated spring (allopatric evolution) but a confirmation of this hypothesis requires further study of the vertical section of the spring deposits. According to Hershler et al. (2002), all four extant species of Juturnia are associated with saline environments. This can also be assumed for the former springs at Viesca since the groundwater, which once fed the springs with water, had (and has) high levels of salinity (Ortega-Guerrero, 2003).
Juturnia coahuilae (D. W. Taylor, 1966)
Durangonella coahuilaeTaylor, 1966
Durangonella coahuilaeHershler, 1985, 81, fig. 32
Description: Morphologically, the subfossil shells are identical to those described by Taylor (1966) and Hershler (1985) from the type locality of this species.
Shell Measurements: (mean, with sd in parentheses; N = 15) SH = 3.55 (0.34), SW = 1.27 (0.10) mm, AH = 0.95 (0.13) mm, AW = 0.76 (0.04) mm, HBW = 1.80 (0.13) mm, WBW = 1.19 (0.09) mm, WN = 6.12 (0.21).
Material Examined: 15 specimens (UJMC 366a-o).
Distribution: The present-day species is endemic to the spring complex of the Cuatrociénegas basin, Coahuila.
Remarks: The subfossil J. coahuilae from Viesca corresponds in its conchological features in almost all details to the recent species from Cuatrociénegas (Figures 3h to 3m). The unique difference between the subfossil and extant species is the slightly narrower form of the subfossil species, which is also reflected in the discriminant analysis (Figure 4). However, these small morphological differences are, in view of the temporal and spatial distance of both populations, not surprising and we consider them within the variation of the extant species from Cuatrociénegas. The present record extends the (fossil) range of the genus Juturnia c. 300 km to the south.
Genus Probythinella Thiele, 1928
Type species: Paludina emarginata Küster, 1852
Probythinella emarginata (Küster, 1852)
Description: Shell small, sub-globose to narrow-conic or sub-cylindrical, height ranging from 3.24-4.69 mm, width 2.24-3.18 mm; with 4¼-5¼ whorls; whorls inflated, slightly convex to well rounded, sometimes slightly shouldered below suture, suture impressed; apex blunt or depressed, often tilted; body whorl rarely abruptly descending toward end of growth; teleoconch sculptured with strong, regularly spaced collabral striations extending from the suture to the base of whorl; first whorl sometimes planorboid, depressed; aperture ovate, sometimes weakly angled above, peristome usually narrowly adnate, or slightly separated, outer and inner lips thin; shell clear-white, some specimens transparent.
Shell Measurements: (mean, with sd in parentheses; N = 10) SH = 4.11 (0.63) mm, SW = 2.75 (0.41) mm, AH = 1.87 (0.18) mm, AW = 1.45 (0.20) mm, HBW = 3.05 (0.34) mm, WBW = 2.43 (0.30) mm, WN = 4.78 (0.25).
Material Examined: 10 specimens (UJMC 367a-j).
Distribution: Eastern North America in lotic and lentic inland habitats throughout the Mis sissippi River basin; drainages of Great Lakes, Hudson Bay, and Mackenzie River to the north (Hershler, 1996).
Remarks: The genus Probythinella includes only two extant species: P. emarginata and P. protera, both distributed along the eastern part of North America. Both species are well characterized by the extremely blunt apex of their shells (Figures 3p, 3t), a synapomorphy of this small group of North American Hydrobiidae (Hershler, 1996). Our records spread out the (fossil) range of P. emarginata c. 300 km to the south.
Probythinella protera Pilsbry, 1953
Type species: Amnicola emarginata Küster, 1852
Description: Shell medium in size, ovate-conic to pupiform, height ranging from 2.54-2.92 mm, width 1.51-1.82 mm; with 4¼-4½ slightly rounded whorls (accuracy to a ¼ whorl), first whorl almost planorbiform; umbilicus absent; apex blunt, sometimes depressed; aperture small, ovate and inclined approximately 40° away from the coiling axes, inner lip thickened and adnate to the parietal wall, outer lip strongly thickened.
Shell Measurements: (mean, with sd in parentheses; N = 10) SH = 2.69 (0.13) mm, SW = 1.69 (0.10) mm, AH = 1.16 (0.04) mm, AW = 0.99 (0.07) mm, HBW = 2.13 (0.12) mm, WBW = 1.64 (0.10) mm, WN = 5.50 (0.12).
Material Examined: 10 specimens (UJMC 368a-j).
Distribution: Endemic to United States, brackish waters along the eastern Gulf of Mexico coast (Hershler, 1996).
Remarks: Hershler (1996) mentioned that the recent distributions of both Probythinella species appear to be contiguous, but non-overlapping. That does not apply to our area, where both species occurred during the Holocene in lotic and lentic habitats as sympatric species. P. protera occurs in present-day only in coastal regions but our findings confirm that this species was, during the Holocene, also abundant in inland sites.
Genus PseudotryoniaHershler, 2001
Type species: Tryonia alamosae Taylor, 1987 (by original designation)
Pseudotryonia pasajaeHershler, Liu and Landye, 2011
Pseudotryonia pasajaeHershler et al., 2011: 14, 16, figures. 2E-F, I-J, M-O, 3E-H, 4D-E.
Pseudotryonia pasajaeHershler et al., 2014: 59-60.

Figure 5 Pseudotryonia pasajaeHershler, Liu and Landye, 2011 and Tryonia cf. seemani (Frauenfeld, 1863). a-d) P. pasajae, specimen UJMC-369a. c) Male (?) specimen, UJMC-369b. d) Protoconch view, UJMC-369a. e-h) P. pasajae, a present-day specimen from San Pedro de Ocuila, Durango, Mexico. h) Protoconch view. i-m) T. seemani. i-k) T. cf. seemani, subfossil specimen from Viesca, Coahuila, Mexico, UJMC-370. k) Protoconch view. l) T. seemani from Durango, syntype (BMNH 20001099) illustrated by Hershler et al. (2002. Fig. 1b). m) T. seemani, a present-day specimen from an unnamed spring on west side of Nombre de Dios, Durango, Mexico (Hershler et al., 2011a, Fig. 12c).
Description: Morphologically, the shells are identical to those described by Hershler et al. (2011b, p. 16) from the type locality of this species in Durango.
Shell Measurements: (mean, with sd in parentheses; N = 11) SH = 2.21 (0.50) mm, SW = 1.38 (0.22) mm, AH = 0.98 (0.14) mm, AW = 0.77 (0.08) mm, HBW = 1.64 (0.26) mm, WBW = 1.23 (0.19) mm, WN = 4.23 (0.54).
Material Examined: 11 specimens (UJMC 369a-k).
Distribution: P. pasajae occurs living only in two springs northwest of Cuencamé de Ceniceros, Rio Nazas basin, Durango, Mexico (Hershler et al., 2014a).
Remarks: The results of the discriminant analysis did not reveal significant morphological differences among the subfossil shells from Viesca and shells of the endemic extant species P. pasajae from an unnamed spring at San Pedro de Ocuila, Durango, near the type locality (Figure 6). Almost all morphometric characters are within the phenotypic variation of the recent species. Small differences between the subfossil and extant populations remain only in the size of the shells, but these differences are even smaller than differences among the two known populations described by Hershler et al. (2014a) from the type locality and they are also visible in the discriminant analysis (Figure 6). The same author mentioned that P. pasajae shows sexual dimorphism in form of shells size differences, which is also observed in our material. Since all other shell characteristics of both forms are similar, we conclude that they may reflect the sexual dimorphism of the species. The subfossil shells were found in spring El Molino, Viesca, Coahuila, c. 100 km NE from the type locality of this species in Durango.

Figure 6 Graphic depiction of the discriminant analysis of morphometric variables of three populations of Pseudotryonia. Square = P. pasajae, a subfossil population from Spring Tunel 4, Viesca, Coahuila. Circles = P. pasajae, a present-day population from an unnamed spring at San Pedro de Ocuila, Durango. Triangles = P. pasajae, a present-day population from el Tanque, Durango, type locality (data from Hershler et al., 2011b).
Genus Tryonia Stimpson, 1865
Type species: Tryonia clathrata Stimpson, 1865 (by original designation)
Tryonia cf. seemani (Frauenfeld, 1863)
Hydrobia seemaniFrauenfeld, 1863: 1025. Frauen-feld, 1865: 525, pl. 8.
Durangonella seemani (Morrison, 1945): Morrison, 1945, 19, pl. 3, Fig. 1
Description: Shell turriform, medium-sized, height 5.99 mm, with 5½ rounded whorls. Teleo-conch whorls highly convex, evenly rounded with strong impressed sutures. Sculpture of fine growth lines; aperture pyriform, parietal lip complete; umbilicus absent; periostracum tan.
Shell Measurements: SH = 5.99 mm, SW = 2.54 mm, AH = 2.09 mm, AW = 1.56 mm, HBW=3.64 mm, WBW = 2.25 mm, WN = 5½.
Material Examined: 1 specimen (UJMC-370).
Distribution: Endemic to a few springs near Durango City, Durango State (Hershler et al., 2011a).
Remarks: Our shell closely resembles shells from Durango described originally as Hydrobia seemani by Frauenfeld (1863). This species was later redescribed and placed as type species in the new erected genus Durangonella by Morrison (1945). Hershler et al. (2002) finally examined the rehydrated material and transferred this species to the genus Tryonia (Figure 5m). Our single high spired and strongly convex-whorled specimen is morphologically near identical to the syntypes of the species from Durango illustrated by Hershler et al. (2002) (Figure 5l) and can therefore be considered as conspecific. A convergence is unlikely, however, since it is a single specimen we identify the subfossil shell from Viesca only tentatively. The single specimen was found in superficial sediments of the Juan Guerra Spring, Viesca.
Family Planorbidae H. and A. Adams, 1855
Genus Biomphalaria Preston, 1910
Type species: Biomphalaria smithii Preston, 1910
Biomphalaria havanensis (Pfeiffer, 1839)
Biomphalaria obstructa (Morelet 1849): Test. Noviss. I: 16.
Biomphalaria “temascalensis” Rangel-Ruiz 1987: 25-34; pl. 1.
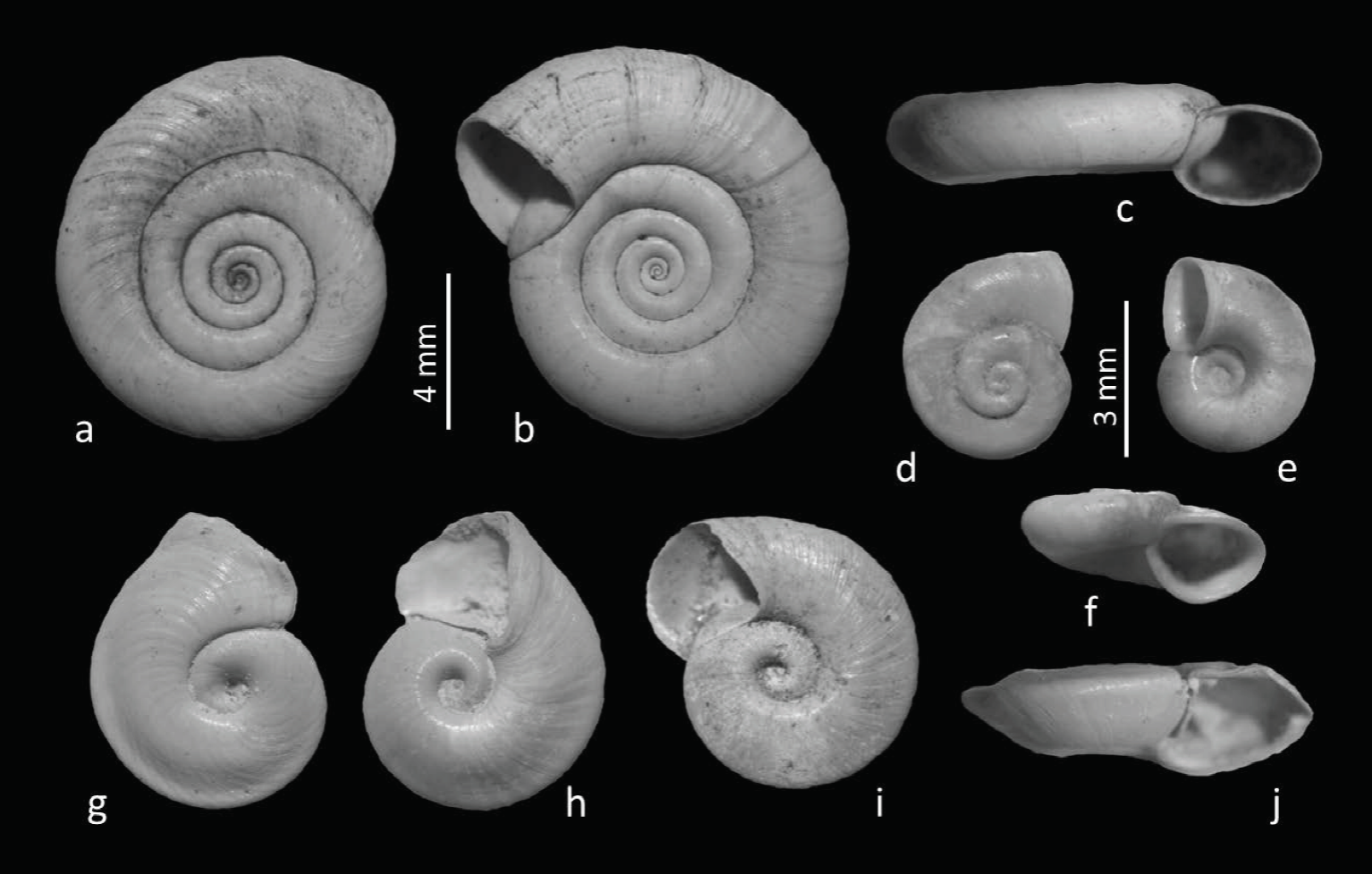
Figure 7 Biomphalaria havanensis (Pfeiffer, 1839), Dilatata dilatata (Gould, 1841) and Promenetus exacuous (Say, 1821). a-c) B. havanensis, fossil specimen UJMC-371a from a former wetland area of Viesca, Coahuila, Mexico. d-f) D. dilatata, subfossil specimen UJMC-372a from fluviatile deposits of the Aguanaval River, Durango, Mexico. g-j) P. exacuous subfossil specimens from spring el Molino, Viesca, Coahuila, Mexico. g, h, j) Specimen UJMC-373a. i) Specimen UJMC-373b.
Description: Shells medium to large (maximum diameter 13.25 mm), flattened, discoidal; with 4 – ‒ 5 – rounded whorls that increase moderately in diameter; spire flattened; sculpture of fine lines of growth pronounced on the bodywhorl, whorls separated by deep sutures; aperture ovate, the peristome is thin, without apertural lamellae.
Shell Measurements: (mean, with sd in parentheses; N = 8) SH = 3.41 (0.20) mm, SW (= shell diameter) = 12.10 (0.83) mm, WN = 5.19 (0.35).
Material Examined: 8 specimens (UJMC 371a-h).
Distribution: The species occurs in southeastern USA, West Indies, Mexico and Central America. South American distribution remains to be determined (Thompson, 2011). According to Naranjo-García (2003), B. havanensis is the most common species of the genus in Mexico and inhabits mainly in areas along the Atlantic and Pacific coasts.
Remarks: The taxonomic history and systematics of the recent members of the genus Biomphalaria are long and confusing (DeJong et al. 2001). The subfossil shells from Viesca resemble shells of two extant species reported frequently from United States and Mexico: B. havanensis and B. obstructa. Both species were considered for a long time as different species (DeJong et al., 2001; Thompson, 2011) but recent molecular studies show that B. obstructa (as well as B. tamascalensis Rangel-Ruiz from Oaxaca, Mexico) is a junior synonym of B. havanensis (Aguiar-Silva et al., 2014; Rosser et al., 2016).
Genus Dilatata Clessin, 1885
Type species: Planorbis dilatatus Gould, 1841
Dilatata dilatata (Gould, 1841)
Description: Shell small, ultradextral, flattened, discoidal (flat spiral), with few rapidly enlarging whorls; body whorl with less well-developed carina (keel), placed just above the center of the body; with 3 whorls (accuracy to a ¼ whorl), whorls rapidly increasing in size, sculpture of fine lines of growth; umbilicus deep; aperture large, expanded; lips slightly thickened.
Shell Measurements: Specimen 1: SH = 1.63 mm, SW (= shell diameter) = 3.23 mm, AH =1.28 mm, AW = 1.59 mm, WN = 3. Specimen 2: SH = 1.22 mm, SW (= shell diameter) = 2.68 mm, AH = 1.11 mm, AW = 1.31 mm, WN = 2.75.
Material Examined: 2 specimens (UJMC 372a-b).
Distribution: Dilatata dilatata has a broad present-day distribution across United States from Florida and Texas to Canada (Baker, 1945; Burch, 1989). In Mexico in Zacatecas and Puebla (Albrecht et al., 2007; Thompson, 2011 as Micromenetus dilatatus).
Remarks: The two subfossil specimens correspond in their shell morphology in all details to the recent material of this species. It is the first fossil record of this genus from Mexico.
Genus Promenetus Baker, 1935
Type species: Planorbis exacuous Say, 1821
Promenetus exacuous (Say, 1821)
Menetus exacuous (Say, 1821).
Baker, 1928, Wisconsin Geol. Nat. History Survey Bull. 70, pt. 1, p. 361, pi. 23, fig. 1-5.
Promenetus exacuous exacuous (Say, 1821): Baker, 1945,
Molluscan family Planorbidae, p. 182.
Promenetus exacuous megas (W.H. Dall, 1905): Baker,
Molluscan family Planorbidae, p. 188.
Description: Shells medium (maximum diameter 8.43 mm), flattened, discoidal (flat spiral), ultradextral, with carinate periphery (sharply keeled); keel close to or above the center of the body whorl; with 2¼‒3½ whorls, whorls rapidly increasing in size; spire flattened; sculpture of fine lines of growth and fine spiral lines; umbilicus deep; aperture triangular or ovate, outer and inner lip thin to slightly thickened: some specimens with shell repair scars.
Shell Measurements: (mean, with sd in parentheses; N = 10) SH = 1.91 (0.07) mm, SW (= shell diameter) = 6.96 (0.59) mm, WN = 2.60 (0.36).
Material Examined: 10 specimens (UJMC 373a-j).
Distribution: P. exacuous has a broad present-day distribution across Canada and United States (including Alaska) but does not reach Mexico.
Remarks: The prominent keel on the body whorl is the main characteristic of the shell of this species. Although there are no anatomical or radular differences, some authors such as Clarke (1981) and Prescott and Curteanu (2004) distinguish two sub-species of the Keeled Promenetus: P. e. exacuous and P. e. megas. However, we follow Burch (1989) and Turgeon et al. (1998) and do not recognize these subspecies because we considered that small differences in shell size alone are insufficient for a distinction. The southern limit of occurrence of living P. exacuous is Tarrant County, Texas (Pratt, 1983), and our present record spread out the (fossil) range of the genus c. 1000 km to the south. It is the first fossil record of this genus from Mexico.
4. Discussion
There are few data about extinctions of freshwater gastropods in Mexico but Johnson et al. (2013) suggested that similar or greater extinctions likely occurred in Mexico and North America. The largest single modern extinction event of freshwater snails in North America took place in Coosa River, Alabama, United States, where within 50 years 33 species went extinct (Johnson et al., 2013). Our results from northern Mexico show a similar rapid loss of local freshwater snail species as in United States. This general decline of mollusks diversity began in the early to middle Holocene (Czaja et al., 2014a, 2014b), but then increased rapidly in the 20th century. The estimated times of local gastro-pod extirpation in the area (Table 2) are based on species described in the present study as well several other subfossil species reported recently from the same area (Czaja et al., 2014a, 2014b, 2015, 2017a, 2017b, 2017c). Of the 32 species present through the Holocene in Laguna District only four (12.5%) are still living here. A quarter (25%) is totally extinct, half of them (50%) still live in Mexico and three species (9%) occur only in United States and Canada. This means the loss of 88% of the number of freshwater gastropod species in the area of study (Figure 8). The area is now a very dry region and belongs to the most populated regions in Mexico. Only the outflows of the dams of the Nazas and Aguanaval rivers and the small spring La Peña contain permanent waters. These sites are monitored by the authors since 2009 and contain only four gastropod species (Table 2).
Table 2 List of gastropod species of the study area and estimated time prior of the local extirpation or extinction based on species described in the present study as well subfossil species reported previously from the same area. * = Endemic species, bold = Species described in present paper.
| Family | Species | Holocene Records | 20th Century Records | Present-day Occurrence in are |
|---|---|---|---|---|
| Assimineidae | Assiminea cienegensis* | X | X | |
| Cochliopidae | Coahuilix hubbsi* | X | ||
| Cochliopidae | Coahuilix landyei* | X | ||
| Cochliopidae | Coahuilix parrasense* | X | X | |
| Cochliopidae | Cochliopina riograndensis | X | X | |
| Cochliopidae | Juturnia coahuilae* | X | X | |
| Cochliopidae | Juturnia gracilis* | X | X | |
| Cochliopidae | Mexipyrgus viescaensis* | X | X | |
| Cochliopidae | Paludiscala thompsoni* | X | X | |
| Cochliopidae | Phreatoceras taylori | X | ? | |
| Cochliopidae | Tryonia cf. seemani | X | X | |
| Cochliopidae | Tryonia hershleri* | X | ||
| Cochliopidae | Tryonia paleocircumstriata* | X | X | |
| Hydrobiidae | Cincinnatia integra | X | X | |
| Hydrobiidae | Probythinella emarginata | X | X | |
| Hydrobiidae | Probythinella protera | X | X | |
| Hydrobiidae | Pseudotryonia pasajae* | X | X | |
| Hydrobiidae | Pyrgulopsis manantiali* | X | ||
| Hydrobiidae | Pyrgulopsis paleoacarinatus* | X | X | |
| Hydrobiidae | Pyrgulopsis paleominckleyi* | X | X | |
| Lymnaeidae | Galba obrussa | X | X | |
| Neritidae | Vitta cf. usnea | X | ||
| Physidae | Physella acuta | X | X | X |
| Physidae | Physella pomilia | X | ||
| Planorbidae | Biomphalaria havenensis | X | X | |
| Planorbidae | Ferrissia californica | X | X | X |
| Planorbidae | Gyraulus parvus | X | X | X |
| Planorbidae | Hebetancylus excentricus | X | X | |
| Planorbidae | Helisoma anceps | X | X | X |
| Planorbidae | Dilatata dilatata | X | X | |
| Planorbidae | Planorbella duryi | X | ||
| Planorbidae | Promenetus exacuous | X | X |
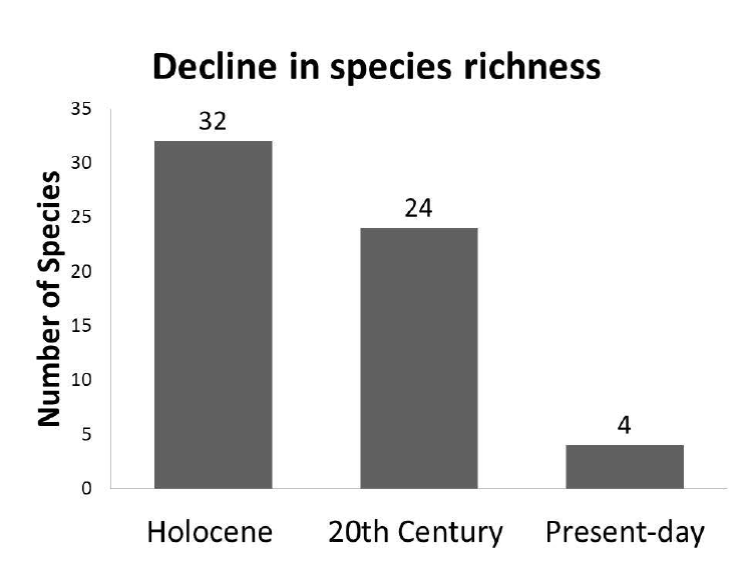
Figure 8 Decline in species richness of freshwater gastropods in the area of study during the Holocene.
Snails with small geographic ranges were more vulnerable to such extinction events than species with a wider distribution. Of the 28 species that disappeared from the area during the Holocene, 14 (43%) were endemics (Table 2). Extirpation of all endemic species in this area apparently depended on, among others factors, their limited dispersal ability, which is especially true for the phreatic species of Coahuilix, Paludiscala and Phreatoceras. Similar patterns of extinctions of endemics were also reported from other sites (Hershler et al., 2014b). However, also taxa with relatively wide recent distribution such as Promenetus, Dilatata, Cincinnatia, Biomphalaria and both species of Probythinella did not survive in this area. The genera Promenetus and Probythinella apparently disappeared completely from Mexico. Only Physella acuta (Draparnaud, 1805), Ferrissia californica (Rowell, 1863), Helisoma anceps (Menke, 1830) and Gyraulus parvus (Say, 1817) survived. Not surprisingly, all of them are known as very tolerant species with wide geographic ranges. It seems that of the 28 disappeared species only Planorbella duryi (Wetherby, 1879), Tryonia hershleriCzaja and Estrada-Rodriguez, 2015, Physella pomilia (Conrad, 1834) and Vitta usnea (Röding, 1798) disappeared from the area of study before human activities impacted the region.
The families most affected by the local extirpation were the Cochliopidae (12) and Hydrobiidae (7 species) and none of both currently have members in the area. These families belong to the most vulnerable freshwater gastropod families in North America, and they depend on groundwater inflows. Almost all endemic species of Cuatro-ciénegas also belong to these hydrobiid families.
The environmental impact was especially severe in the complex of 15 springs of Viesca in the central part of the Laguna District that dried up completely in the second half of the last century. The exact time of the drying is difficult to determine but in 1959 all 15 springs were still active (Blásquez, 1959). In 1961, some freshwater habitat must have existed around Viesca because type specimens of the Viesca Mud Turtle (Kinosternon hirtipes megacephalum Iverson) were collected (Legler and Vogt, 2013). Rhodin et al. (2011) reported 1970 as the approximate extinction date of the Viesca Mud Turtle and this could also be time of extirpation of the freshwater snails. That most snails from Viesca were still living in the 20th century is established by their common occurrence with Melanoides tuberculata (Müller, 1774) in the superficial sediments of the springs. This exotic species was introduced to North America in the 1930’s (Rader et al., 2003).
The loss of aquatic habitats in Viesca occurred after decades of extensive overexploitation of groundwater pumping principally for irrigated agricultural production. In addition, the construction of two large dams further altered the ground-water hydrology. Wolfe (2013) has described how groundwater resources in Laguna District (especially at Viesca) were depleted from the 1920s to the 1960s. In this period, the number of water pumps increased from 12 to almost 3000 and, as a result, the groundwater level dropped by almost 100 meters. A few years later the lakes disappeared and also all 15 springs at Viesca had dried out (Figure 9). Similar local extinction events documented by Hershler et al. (2014b) from various sites in arid western North America coincide in time with the environment change in Laguna District. Also, there were especially hydrobid snails affected in these arid regions.

Figure 9 Spring Tunel 1 (Bilbao), Viesca, Coahuila, Mexico, that dried up in the second half of the last century (May 2018).
Assiminea cienegensis, J. coahuilae, T. seemani, P. pasajae and Pyrgulopsis manantiali (Hershler, 1985) are still living near the area of study but all five species are now endemic to very restricted areas, including single springs. The subfossil records show that they had a wider distribution; hence, they are currently not only endemics but also relicts.
Although Promenetus exacuous and Probythinella emarginata are extirpated in Mexico, they have a wide-spread distribution in eastern United States and Canada and their populations are currently stable (Johnson et al., 2013). However, both species occur in semi-arid region of United States in only a very few sites. P. protera, a species common in late Holocene Rivers and lakes in the area of study, shows currently a very limited distribution in United States (Hershler, 1996). However, contrary to the more widespread P. emarginata, this species is not included in the IUCN Red List and does also not appear in the list of endangered freshwater species of Canada and the United States published by Johnson et al. (2013).
It cannot be ruled out that invasive species such as Melanoides tuberculata are at least partly responsible for the loss of gastropod diversity in the Laguna District. This species has long been suspected of displacing native populations (Thompson, 2004; Almeida et al., 2018). Although extirpation and invasion coincide temporally and spatially in the area of study, a causal relationship such as interspecific competition or increased parasitism is unclear. The current sampling demonstrates that the superficial sediments contain only a few specimens of Melanoides and its relative abundance never exceeds 0.1%. Today, however, Melanoides is now by far the most abundant species in the localities that still contain water in the study area.
5. Conclusions
The local extirpation event described here from the Laguna District happened silently and unrecognized only some two hundred kilometers away from one of the currently greatest hotspots of gastropods diversity in North America, the Cuatro-ciénegas Basin. Unfortunately, the same human activities, especially groundwater pumping, that caused loss of aquatic habitat in the study área continue to endanger the ecosystems of Cuatro-ciénegas. One of the mottos of the Conservation Paleobiology concept created by Dietl and Flessa (2011) was “Using the Past to Manage for the Future”. We hope that the large number of extirpated species reported here will strengthen the efforts to conserve threatened freshwater habitats in Mexico, especially in Cuatrociénegas basin.











 text new page (beta)
text new page (beta)


