Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Agrociencia
On-line version ISSN 2521-9766Print version ISSN 1405-3195
Agrociencia vol.51 n.7 Texcoco Oct./Nov. 2017
Animal Science
Implications of urban and rural agricultural practices on the transmission of leptospirosis
1Departamento de Ciencias Básicas. Programa de Biología. Universidad de la Salle.
2Facultad de Ciencias Agropecuarias. Programa Medicina Veterinaria. Universidad de la Salle.
3Grupo Biomigen (Biología Molecular e Inmunogenética). Universidad de la Salle.
4Grupo de Investigación Epidemiología y Salud Pública. Universidad de la Salle. Colombia. (phernandez@unisalle.edu.co).
Changes in agricultural and livestock practices and climate and geographic conditions in the tropics, associated to great biological diversity, favor the propagation of diseases, the appearance of new pathogens, or the reappearance of some that have already been controlled. Therefore, leptospirosis is an emerging zoonosis associated to flooding in urban and rural zones, affecting at least 160 animal species, and because water is an important transmission vehicle, it is necessary to perform inter-disciplinary work to minimize its occurrence and its impact on human and animal health, and especially on the ecosystem (“One Health”). Therefore, the objective of this essay was to analyze the implications of urban and rural agricultural and livestock practices on the transmission of leptospirosis. For this purpose a thematic review was carried out with databases from 2000 to 2015, which allowed analyzing the health restrictions in tropical agriculture, and contextualizing the implications of agricultural and livestock practices, urban and rural, on leptospirosis. The analyses performed showed the need for interdisciplinary studies to address the epidemiology of this zoonosis in an integral manner, with the participation of sciences such as microbiology, molecular biology, veterinary medicine, and bioeconomy, among other areas.
Key words: urban and rural agriculture; health restrictions; leptospirosis; One Health
Las modificaciones en las prácticas agropecuarias y las condiciones climáticas y geográficas del trópico, asociadas con una amplia diversidad biológica, favorecen la propagación de enfermedades, la aparición de nuevos patógenos, o la reaparición de algunos ya controlados. Por lo tanto, y debido a que la leptospirosis es una zoonosis emergente asociada con inundaciones en zonas urbanas y rurales, que afecta mínimo 160 especies animales, y que el agua es un vehículo importante de transmisión, es necesario un trabajo interdisciplinar que minimice su presentación, y el impacto sobre la salud humana, animal y sobre el ecosistema (“Una Salud”). Por consiguiente, el objetivo de este ensayo fue analizar las implicaciones de las prácticas agropecuarias urbanas y rurales, sobre la transmisión de la leptospirosis. Para esto se realizó una revisión temática en bases de datos, desde el 2000 al 2015, la cual permitió analizar las limitantes de la salud en la agricultura tropical, y una contextualización de las implicaciones de las prácticas agropecuarias, urbanas y rurales sobre la leptospirosis. Los análisis realizados evidenciaron la necesidad de estudios interdisciplinares para abordar la epidemiología de esta zoonosis de forma integral, con la participación de ciencias como microbiología, biología molecular, medicina veterinaria, y bioeconomía entre otras áreas.
Palabras clave: agricultura urbana y rural; limitantes de salud; leptospirosis; una salud
Introduction
nadequate agricultural and livestock practices, the wrong use of water, intensive agriculture, an increase in emerging and reemerging diseases, and environmental changes have local and global consequences related to an increase in pathogen transmission (McMichael et al., 2004; Jones et al., 2013; Liverani et al., 2013). Thus, emerging and reemerging zoonoses, such as leptospirosis, make evident the interaction between health and agriculture since the factors associated with the presence of this zoonosis show points in common for the development of sustainable agriculture and adequate health.
Climate change is included among the factors associated based on which reports have been made about alterations in ecosystems since 1998, fostering variations in the transmission of zoonotic infections, as well as in the distribution and in the types of environmental contamination (OMS, 1998; OMS, 2010; Vanasco et al., 2000). In this sense, the World Health Organization (WHO, 2010), established the Leptospirosis Burden Epidemiology Reference Group (LERG), which, through indicators of mortality and disability, such as the Disability Adjusted Life Years (DALY), quantifies and describes the burden of the disease on diverse populations, something that can be used to formulate policies and implement prevention and control measures. Therefore, it is important to carry out joint activities between the health, agriculture, environment and education sectors, to develop innovating solutions, which contribute to the connection between human, animal and ecosystem health, because leptospirosis affects wild and domestic animals that become reservoirs and sources of infection for the people who reside primarily in rural and urban zones with sanitary deficiencies (Meites et al., 2004; Agudelo-Flórez et al., 2006; Escamilla et al., 2007). Inter-sectorial work will be promoted in this way, allowing a transformation of the policies for primary prevention of health and agricultural/livestock problems (Lilenbaum and Martins, 2014).
Therefore, the purpose of this essay was to analyze the implications of urban and rural agricultural and livestock practices on the transmission of leptospirosis. To reach this objective a thematic revision of original research articles and reviews from 2000 to 2015 in PubMed, Scopus, Science Direct, SciELO, Redalyc, and Latindex was performed. The descriptors in Spanish and English were: “leptospirosis” “urban and rural agriculture” “urban and rural livestock production” “One Health”. After reading the abstracts, the articles that allowed analyzing the health restrictions in tropical agriculture were identified, as well as the effect on agricultural and livestock practices, urban and rural, on leptospirosis. Articles related to the impact of leptospirosis on human, animal and ecosystem health were also analyzed.
Health restrictions in tropical agriculture
Productive agriculture and health systems that protect the greater part of the population are essential elements to reduce poverty. However, social and political conflicts, as well as inequality, cause for agriculture and health systems to have unresolved challenges. In agriculture the challenges are: limitation of natural resources, extreme climatic conditions, agricultural plagues, globalization, environmental deterioration, and lack of strategies to sustain agricultural production in situations of conflict; these constitute serious problems in the national, regional and continental scope, without effective solution strategies (Hawkes and Ruel, 2006).
According to Hawkes and Ruel (2006), there are still old challenges for health systems such as malaria, tuberculosis, diarrheic diseases, respiratory infections, and malnutrition, which in the 21st century claim many victims. On the contrary, fever, chronic, transmissible diseases, and resistance to medicines and insecticides are new challenges in health; this, associated to interventions of low effectiveness, increase agricultural and health problems which are reflected from the local to the global, so it is necessary to find solutions at different scales (Grace, 2011).
This is evidenced by the appearance of epidemics and outbreaks due to climatic, cultural and social factors. From 1999 to 2010 there were outbreaks and epidemics of endemic leptospirosis in tropical countries, and from 1991 to 2010 the annual average impact in China was 0.70 cases for every 100 000 inhabitants. During those two decades, three large leptospirosis outbreaks were produced due to rains and flooding (McMichael et al., 2004; Rodríguez et al., 2000; Zhang et al., 2012). In Central and South America there is an increase in leptospirosis epidemics after natural disasters (Zaki et al., 1996; Trevejo et al., 1998; Schneider et al., 2013). Therefore, since 1995 this disease is considered to be emerging infectious (Farmer, 2001). This zoonosis has global importance, particularly in humid tropical and subtropical countries, where the environmental conditions favor the survival and growth of the bacteria (Escamilla et al., 2007). In rural and urban zones, sanitary deficiencies, exposure to contact with rodents, and flooding, are factors associated to the disease; also, in people whose work implies frequent contact with animals or their products, hikers, and those who practice aquatic sports (Meites et al., 2004; Escamilla et al., 2007).
Despite the broad distribution, in most countries the epidemiologic vigilance programs for leptospirosis present limitations and few have laboratories for effective diagnosis (Vanasco et al., 2000). In addition, an alteration of the public health measures is produced in face of natural catastrophes because they become inefficient and hard to operate, making the human population more vulnerable to the infection (King, 2011).
Urban and rural agriculture: Its implications for the transmission of leptospirosis
Urban agriculture arises as a process of territorial expansion that serves as a mechanism to harbor rural families that reach the cities looking for better opportunities or fleeing from precarious security conditions in the country. This migration promotes the agricultural and livestock practices in urban and metropolitan spaces, and generates modifications in the ecosystems (Méndez et al., 2005; Franco et al., 2008). The highest levels of urban and metropolitan vegetables are found in China, where 79 % of the fruits come from metropolitan areas (Lee-Smith and Prain, 2006). In Beijing and Shanghai, vegetables are produced at less than 10 kilometers from the sales points, in 85 % and in 76 %, respectively (Lee-Smith and Prain, 2006). The production of fruits and vegetables is 31 % for urban zones, and 64 % for metropolitan zones of Beijing (Lee-Smith and Prain, 2006). Of the land in the metropolitan zone of Manila, 6 % is destined to agriculture and 2 % to fish farming, which covers two thirds of the fish demand (Hawkes and Ruel, 2006; FAO, 2012).
In the urban zones of Latin America, 12 % of the land is used for agriculture and provides jobs for 117 000 people. In Lima (Peru), 70 % of some species of the vegetables consumed are contributed by this type of agriculture, and between 15 % and 20 % of the households participate in urban and metropolitan agriculture. In addition, these families have fowl and small livestock (Hawkes and Ruel, 2006).
Manizales, Armenia and Pereira are urban centers in the Colombian coffee axis, and the urban practices of agriculture and livestock production in these are an expression of change in the dichotomous concept between rurality and urbanism, because rural municipalities around it concentrate the social infrastructure that supplies foods to the region, and are the point of residence and work of the population displaced from the country to the city (Méndez et al., 2005). Bogotá depends on nearby municipalities for the supply of vegetables harvested in 27 548 ha, and Cundinamarca is the department with greatest influence on vegetable production, with a national participation of 28 %. This department has 220 000 ha of land apt for cultivation with sufficient water resources, and 30 000 ha of land in different thermal layers (Cámara de Comercio Bogotá, 2011). In addition, in the productive chain of the vegetable sector, from Sabana de Bogotá, small-scale and medium-scale producers are connected as suppliers of provision centers for the whole country (Miranda et al., 2008).
These agricultural strategies, which connect the country with the peripheral surroundings of the large metropolis, result from the new rurality, which implies a change in the concept of what is rural and what is urban. Rural is related to the agricultural sphere, low development, and poverty; and urban with the opposite; however, in the context of new rurality these differences have lost and continue to lose clarity, and the urban scope is interpreted as a fundamental component of rurality and vice versa (Méndez et al., 2005; Ramírez et al., 2008).
The phenomenon of urban agriculture integrates the rural activity to the city’s environment, characterized by the transformation, industrialization and unproductive use of the soil; therefore, the implementation in cities or peripheries of primary agricultural and livestock production models makes the division between rurality and urbanism become increasingly more limited (Rodríguez et al., 2000). This is explained, first, by the direct implementation of rural practices in the city and peripheries; and then, because city residents participate in agricultural and livestock activities indirectly through the sale of inputs, transport, as intermediaries, in implementation of processes, and commerce at a lower and higher scale (Mikkelsen, 2013).
The analysis of agricultural and livestock practices in the city and in the country shows that the territorial physical support for their establishment determines their main difference. Thus, cultivating plants and breeding animals in rural areas, based on traditional processes, require a large and adequate land extension. However, this practice in the urban context takes advantage of any available space, whether covered or not. Thus, in the city plant production is done directly on the soil or in different containers, and livestock production is adapted, according to the practice that will be implemented, in terraces, courtyards, backyards, parks, public zones or fallow lands (Franco et al., 2008; Teubal et al., 2001).
In rural agriculture, the main elements are open and wide spaces, adequate for agriculture and livestock production, and urban agriculture is related to rather closed and built spaces where the area available for production is restricted. In addition, the urban agricultural and livestock practices, compared to the rural ones, are close to large human concentrations and promote opportunities and risks (Teubal et al., 2001; Méndez et al., 2005).
According to FAO (2008), the opportunities are related to access to consumption markets because this decreases the packaging, storage and transport of foods. There is also an increase in the possibilities for employment and agricultural income, and a higher attainment of foods by consumers of scarce resources. However, risks are present such as inadequate agricultural and aquaculture practices, and reduction in the ability of the environment to limit the contamination because animals and humans share resources like soil, air and water. This competition limits the resources, affects human, animal and environmental health, and generates greater possibilities for the transmission of diseases that increase the prevalence and incidence of pathologies, primarily zoonotic. These environmental difficulties and of competition over the use of resources derived from agriculture, also takes place in rural zones, but the space limitation and the human settlements increase the possibilities and risks in urban zones (Aguilar, 2014).
Agricultural and livestock practices in urban centers foster changes in the distribution of pathogens, and risk factors are established for a zoonosis like leptospirosis that can strengthen its presentation in tropical and humid regions (Escamilla et al., 2007). Knowing about the prevalence in a locality, defining its hosts for maintenance, and monitoring the emergence of new serovars of Leptospira spp., are essential steps to understand the epidemiology of the disease in a region and to focus the control strategies. In addition, Leptospira spp., etiologic agent of leptospirosis, can survive in different environments, which is why the impact of the disease on public health is evident. The survival of the bacteria in diverse environments is explained because its reproduction and survival is favored in the soil or in the water with a neutral or slightly alkaline pH, and in zones with a high degree of humidity; also, it can resist temperatures between 7° and 36 °C (OMS, 2008). This is associated to the diversity in reservoirs for the bacteria, such as rats, cattle, dogs, pigs, horses, goats, rabbits and bats; the first two are the most important ones and characteristic in the rural and urban context (Meites et al., 2004; Agudelo-Flórez et al., 2010). In the reservoirs, the pathogenic lepstospires are established in renal tubules; they are excreted with urine and reach the environment, foods and water. This mechanism is essential in the transmission of the pathogen, in the human-animal-environment interphase. The conditions of humidity, the presence of puddles, lagoons, and stagnant waters that are easily contaminated, become permanent focuses of dissemination (Hernández-Rodríguez et al., 2011; Brihuega, 2008; Evangelista and Coburn, 2010; Hartskeerl et al., 2011).
The prevalence of infections from the different serovars of Leptospira spp. in livestock farms of tropical and subtropical countries is not known. According to studies related with leptospirosis, in the USA’s livestock industry the prevalence is 35 to 50 % and most of these are probably due to the Hardjo serovar (Grooms, 2006). In addition, it is difficult to determine the economic losses, primarily from difficulties related to the diagnosis of the disease.
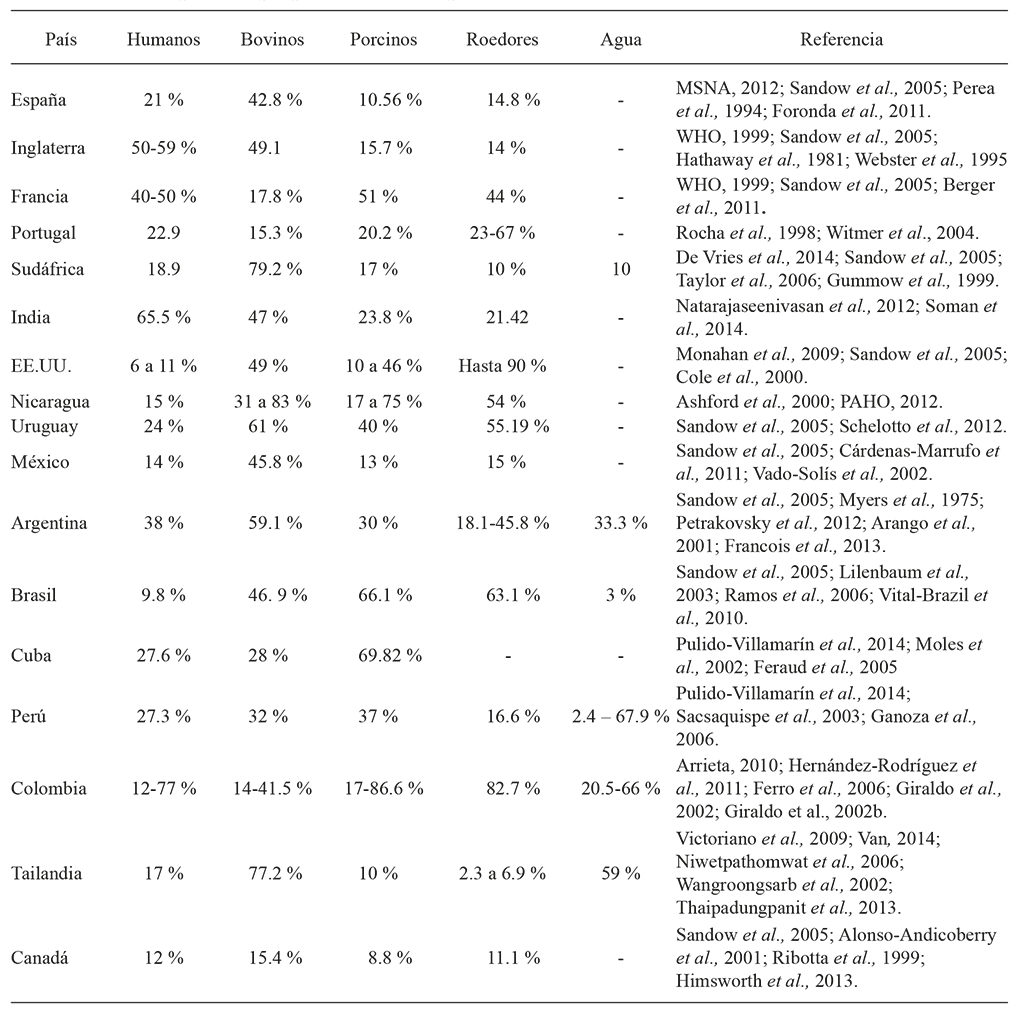
The prevalence of leptospirosis varied between continents, countries, regions, and between animal species in the world. The frequency of this zoonosis increases from flooding caused by natural factors, and from the inadequate use of the land that generated leptospirosis outbreaks between 1995 and 2009. Some examples: in Nicaragua (1995), Malaysia (2000), Ecuador and Peru (1998), Orissia (1999), Bombay (2000, 2005), Jakarta (2002), and the Philippines (2009). According to the HealthMap database, between 2007 and 2013 there were 787 global alerts of this zoonosis: 63 % in America, especially in Brazil, Nicaragua and Argentina, 15 % in the Western Pacific; 14 % in Southeast Asia; Europa 8 %; Africa 1 % and Western Mediterranean 0.5% (Schneider et al., 2013). These natural factors and the changes associated with the urban and rural agricultural and livestock practices have determined prevalence of infection in different species of importance in Public Health and Livestock Production (Table 1).
In the region of the Colombian Caribbean, a positivity of 38.2 % was reported for bovines, in Pie de Monte Llanero 24.8 % and in the Andes Region 14.4 %, and for the country the average is 21.7 % (Monsalve et al., 2009). In the Department of Córdoba a study with a sample of 600 pigs showed 43.0 % of seropositivity for Leptospira spp., and is one of the highest in the country, if compared to seropositivities of 26.0 % for the Andes Region, 23 % for the coffee-producing axis, and 24.0 % for Valle del Cauca. In Villavicencio a seropositivity of 16.0 % was reported, with a sample of 358 pigs (Monsalve et al., 2009).
According to epidemiological studies, with information in the Animal Health bulletins from the Colombian Agricultural and Livestock Institute (Instituto Colombiano Agropecuario, ICA), and the deficit in strategies for prevention and control, bovine leptospirosis is a common disease in the country and affects livestock production (Orjuela et al., 2002). The economic impact of the disease in bovines is evidenced by reproductive problems, inefficient treatments and vaccination, as well as by 5.0 % to 10 % of abortions in cows infected and by embryo mortality (Orjuela et al., 2002). These events cause economic losses, from morbidity and a decrease in production, and show the economic impact of the disease; therefore, leptospirosis is a disease that must be controlled (Faine et al., 1982; Adler and Moctezuma, 2010).
With regards to human leptospirosis, information from 2013, up to epidemiological week 52, shows notification to the Sivigila of 2 263 cases in Colombia, and the increase is 13.94 % in the event’s notification compared to 2012. The incidence is 1.7 cases for 100 000 inhabitants (INS, 2014). In addition, according to statistics from week 1 to 14 of 2015, 715 cases of leptospirosis were reported in Sivigila, representing an increase of 1.51 % in the notification, compared to the year 2014, and 25 probable causes of death from leptospirosis are reported in the country (INS, 2015).
According to data from the National Health Institute, during 2010 and 2011 the number of cases of leptospirosis increased, which is related to the rain season and flooding. This explains the relevance of water as vehicle in the transmission of Leptospira spp. to human beings, and other species, and reflects the importance of its study, as well as its impact on public health (Giraldo et al., 2002). However, the adaptability of the bacteria to survive in fresh water and adjacent soils where there is a lack of nutrients stands out and the hypothesis of interaction with other microorganisms in the environment is suggested, and even with germs of its species, for growth. A study carried out in Quito (Ecuador) showed the viability of Leptospira interrogans serovar Canicola, during 98 d of incubation in distilled water (UNESCO, 2003).
Concerning the bacteria’s maintenance cycle, a defining factor in the transmission is that different serovars of Leptospira spp. can be present in the same species and several animal species can carry the same serovar (Ramadass et al., 1990; Levett et al., 2010). Some animals are adapted to particular serovars of Leptospira spp., serve as reservoirs and eliminate the bacteria from the environment during years, without manifesting clinical signs of the disease, favoring the human-animal-environment transmission; the soil, and mainly water, are important factors in the transmission (Calderón et al., 2014). The animal species adapted to Leptospira serovars are: equines to the serovar Bratislava (Bernard, 1993), canines to the serovar Canicola (Ortega-Pacheco et al., 2008), porcines to the serovar Pomona (Ellis et al., 1986), and bovines to the serovar Hardjo (Grooms, 2006). Thus, L. borgpetersenii serovar Hardjo (hardjo-bovis), is the most common source of bovine leptospirosis in the world, and L. interrogans serovar Hardjo (hardjoprajitno) has been isolated in specific regions, such as in isolates from bovines in the United Kingdom (Grooms, 2006). Infections caused by L. interrogans serovar Pomona and by L. interrogans serovar Grippotyphosa are associated to reproductive failure. This causes notable economic losses (Kingscote and Wilson, 1986; Barr and Anderson, 1993).
The implementation of biosafety norms in the primary chain of the livestock industry has an important function in the prevention and control of leptospirosis, particularly the programs designed to control plagues such as rodents and stagnated waters (Sanderson and Gnad, 2002). Various research groups center their efforts in developing vaccines that confer a greater protection to animals, and the following vaccines stand out: recombinant (of external membrane proteins, lipoproteins, and virulence factors), liposaccharides (LPS), inactive, attenuated, and DNA. However, results from clinical assays to quantify the effectivity of these biological agents are unsatisfactory (Wang et al., 2007; Murray et al., 2010). Therefore, more studies are required to characterize the disease and understand the molecular mechanisms of the physiopathology of this zoonosis.
Based on what has been exposed, it is important to improve the diagnosis and evaluate human, animal and environmental health to optimize a true epidemiological vigilance. In this sense, it is necessary to address leptospirosis from the approach of a single health, because it is defined as “a strategy that seeks the interdisciplinary collaboration, and communication, in every aspect of healthcare for people, animals and the environment” (Gibbs, 2014). From this perspective, generating strategies for prevention and control is essential, for which it is priority to strengthen the diagnosis systems that allow identifying this pathogen in animal species, in the environment and in infected humans.
Zoonosis within the context of one health: Leptospirosis as a model
In humans there are 1 461 diseases, of which 60 % correspond to pathogens that interact with different species, and 75 % of the emerging infectious diseases are zoonotic. Therefore, the increase in the interaction with animals and their products, and the relationship of the origin of zoonoses with agriculture, are critical factors for health in the human-animal-environment interphase (Taylor et al., 2001; Grace and Jones, 2011). A problem faced by public health is the lack of an integrated control of diseases, especially of zoonoses, from the articulation and the joint work between sectors of human health, animal health and the environment. Thus, in mid-1999 the interaction mechanisms between human, animal and ecosystem health began to be explored, to generate work with joint benefits. This is how the One Health initiative emerged, supported by FAO, the World Health Organization (WHO), the World Organisation for Animal Health (OIE), the UNICEF, and the World Bank, allowing to structure a strategy in 2008, in order to respond to the increase in the risk of emerging and reemerging diseases (Gibbs, 2014).
In Colombia, the limited inter-sectorial communication and the scarce availability of precise diagnostic tests in the health and livestock sector has limited the leptospirosis vigilance programs. Dechner (2014) reported that 85 % of the leptospirosis studies carried out in Colombia is based on the MAT test that presents difficulties in management and interpretation, and concluded that the prevalence and incidence of the disease is unknown. The implementation of control measures for this zoonosis is difficult for the prolonged survival of leptospires in the soil and the water, because of the abundance in animal reservoirs, and due to the more than 300 serovars (Bourhy et al., 2013; Picardeau, 2013). In addition, the vaccines are serovar-specific, the protection conferred is of short duration, and it does not provide crossed immunity against heterologous serovars of Leptospira (Picardeau, 2013; Rajapakse et al., 2015).
According to Guerra (2013), leptospirosis is recognized as an occupational disease in humans that affects specific at-risk groups, such as workers in rice fields and other agricultural crops, as well as miners and maintenance workers in infected environments like sewage systems, and soldiers stationed in rain forest zones. Leptospirosis affects workers in farms, whole sale centers or butchers, animal product processing plants, and veterinaries. In addition, the increase in human population and the invasion of habitats of wild animals increase the opportunities to interact and, therefore, the transmission of this pathogen in the human-animal-environment interphase. Therefore, it is important to address this disease from the concept of One Health and in an interdisciplinary manner, to understand it and intensify the control measures. Due to the environmental, social and economic conditions that characterize leptospirosis, the importance of this zoonosis in the world is evident, and particularly in tropical zones. Thus, it is necessary to carry out an approach which, from an inter-sectorial viewpoint, allows strengthening the current diagnosis systems, making them more efficient and accessible, to achieve in the medium range the decrease in under-reporting, improving the epidemiological vigilance and knowledge of the actual rates of prevalence and incidence of this zoonosis in many tropical regions.
Thus, the importance of determining the true impact of the disease is emphasized, as well as improving the vigilance and diagnosis systems in the world to decrease under-reporting, and to understand the geographic distribution, the presence of new reservoirs, and primarily, the actual incidence of the disease. In addition, the interventions and strategies for control must be evaluated, as well as calculating in humans the economic losses from incapacity of individuals in productive age, and the treatment costs. Insofar as the livestock part, the losses in the productive sector from alterations in reproduction and production must be established, attributable to the infection from Leptospira (WHO, 2010).
Conclusions
The implementation of new forms in agriculture and livestock production in urban and metropolitan spaces, as well as the family dietary demand, has contributed to the division between the urban and the rural spheres to be increasingly less evident. These conditions modify the distribution of zoonotic pathogens, and foster a change in paradigm in the control and vigilance of zoonoses, such as leptospirosis, which with global distribution require an inter-sectorial approach that integrates the actions of the public officers responsible for health, environment and agriculture policies.
The health limitations in tropical agriculture are strategic points that may be addressed from different stances: human and veterinary medicine, agricultural and livestock production, epidemiology, microbiology and molecular biology, as an integration strategy that leads to productive agriculture and to a health system that protects and covers most of the population, whose consolidation will be reflected in a reduction of poverty, and in benefits for the human and animal population, and also for the ecosystems in which these populations reside
Literatura Citada
Adler, B., y A. Moctezuma. 2010. Leptospira and leptospirosis. Vet. Microbiol. 140: 287-296. [ Links ]
Agudelo-Flórez, P., J. C. Arango, E. Merizalde, A. F. Londoño, V. H. Quiroz, y J. D. Rodas. 2010. Serological evidence of Leptospira spp circulation in naturally-exposed rats (Rattusnorvegicus) in a Colombian urban area. Rev. Salud Públ. (Bogotá) 12: 990-999. [ Links ]
Agudelo-Flórez P., M Restrepo, y M. A. Lotero. 2006. Evaluation of indirect immunofluorescence assay for diagnosis of human leptospirosis. Biomedica 26: 216-223. [ Links ]
Restrepo, B. N., M. Arboleda, y P. Agudelo-Flórez. 2007. Situación de la leptospirosis en el Urabá antioqueño colombiano: Estudio seroepidemiológico y factores de riesgo en población general urbana. Cad. Saude Public. 23: 2094-2102. [ Links ]
Aguilar, E. 2014. Los nuevos escenarios rurales: de la agricultura a la mutifuncionalidad. Endoxa 33: 73-98. [ Links ]
Alonso-Andicoberry, C., F. J. García, y L. M. Ortega-Mora. 2001. Epidemiología, diagnóstico y control de la leptospirosis bovina (Revisión). Invest. Agr.: Prod. Sanid. Anim. 16: 205-225. [ Links ]
Arango J., E Cittadino, A. Agostini, G. Dorta, C. Álvarez, M. Colusi, A. Koval, A. Cabrera, y F. Kravetz. 2001. Prevalencia de leptospiras en Rattus rattus y Rattus norvegicus en el Gran Buenos Aires, Argentina. Ecol. Austral 11: 25-30. [ Links ]
Arrieta, G., V. Rodríguez, y A. Calderón. 2010. Seroepidemiología de Leptospira spp., en porcinos de algunos municipios del Sinú medio, departamento de Córdoba-Colombia. Rev. MVZ Córdoba 15: 2023-2024. [ Links ]
Ashford, D. A., R. M. Kaiser, R. A. Spiegel, B. A. Perkins, R. S. Weyant, S. L. Bragg, B. Plikaytis, C. Jarquin, J. O.De Los Reyes, and J. J. Amador. 2000. Asymptomatic infection and Risk Factors for Leptospirosis in Nicaragua. Am. J. Trop. Med. Hyg. 63: 249-254. [ Links ]
Barr, B. C., and B. L. Anderson. 1993. Infectious diseases causing bovine abortion and fetal loss. Vet. Clin. North Am. Food Anim. Pract. 9: 343-368. [ Links ]
Berger, S. A. 2011. Infectious Diseases of France. Gideon e-books. France. 687 p. [ Links ]
Bernard, W. 1993. Leptospirosis. Vet. Clin. North Am. Equine Pract. 9: 435-444. [ Links ]
Brihuega, B. 2008. Leptospirosis: Diagnóstico y tipificaciónde leptospiras. In: Cacchione, R., R. Durlach, y P. Martino (eds). Temas de Zoonosis IV. Asociación Argentina de Zoonosis, Buenos Aires, Argentina. pp: 221-227. [ Links ]
Bourhy, P., C. Herrmann-Storck, R. Theodose, C. Olive, M. Nicolas, and P. Hochedez. 2013. Serovar diversity of pathogenic Leptospira circulating in the French West Indies. PLoS Negl Trop Dis, 7: e2114. [ Links ]
Calderón, A., V. Rodríguez , S. Máttar, and G. Arrieta. 2014. Leptospirosis in pigs, dogs, rodents, humans, and water in an area of the Colombian tropics. Trop. Anim. Health Prod. 46: 427-32. [ Links ]
Cámara de Comercio Bogotá. 2011. Invest in Bogotá. Recuperado el 140215, de Invest in Bogotá & Cundinamarca: Recuperado el 140215, de Invest in Bogotá & Cundinamarca: http://es.investinbogota.org (Consulta: marzo 2016). [ Links ]
Cárdenas-Marrufo, M. F., I. Vado-Solís, C. Pérez-Osorio, and J. Segura-Correa. 2011. Seropositivity to leptospirosis in domestic reservoirs and detection of Leptospira spp. from water sources, in farms of Yucatan, México. Trop. Subtrop. Agroecos. 14: 185-189. [ Links ]
Cole D., L. Todd, and S. Wing. 2000. Concentrated swine feeding operations and public health: A review of occupational and community health effects. Environ. Health Perspect. 108: 685-699. [ Links ]
Dechner, A. 2014. A retrospective analysis of the leptospirosis research in Colombia. J. Infect. Dev. Ctries. 8: 258-264. [ Links ]
De Vries, S. G., B. J. Visser, I. M. Nagel, M. G. Goris, R. A. Hartskeerl, and M. P. Grobusch. 2014. Leptospirosis in Sub-Saharan Africa: A systematic review. Int. J. Infec. Dis. 28: 47-64. [ Links ]
Ellis, W. A., P. J. McParland, D. G. Bryson, and J.A. Cassells. 1986. Prevalence of Leptospira infection in aborted pigs in Northern Ireland. Vet. Rec. 118: 63-65. [ Links ]
Escamilla, H., J. Martínez, M. Medina, and E. Morales. 2007. Frequency and causes of infectious abortion in a dairy herd in Queretaro, Mexico. Can. J. Vet. Res. 71: 314-317. [ Links ]
Evangelista, K. V., and J. Coburn J. 2010. Leptospira as an emerging pathogen: A review of its biology, pathogenesis and host immune responses. Future Microbiol. 5: 1413-1425. [ Links ]
Faine, S., and N. D. Stallman. 1982. Amended descriptions of the genus Leptospira Noguchi 1917 and the species L. interrogans (Stimson 1907) Wenyon 1926 and L. biflexa (Wolbach and Binger 1914) Noguchi 1918. Int. J. Syst. Bacteriol. 32: 461-463. [ Links ]
FAO. 2008. Water and the rural poor interventions for improving livelihoods in sub-Saharan Africa. Rome. pp: 6-10. [ Links ]
FAO. 2012. The State of Food Insecurity in the World. Economic growth is necessary but not sufficient to accelerate reduction of hunger and malnutrition. Rome. pp: 15-28. [ Links ]
Feraud, D., y M. Abeledo. 2005. Primer reporte en Cuba de Leptospira interrogans serovar Tarassovi y caracterización clínica epizootiologica en focos de Leptospirosis porcina. REDVET 6: 1-35. [ Links ]
Ferro B. E., A. L. Rodriguez, M. Perez, and B. L. Travi. 2006. Seroprevalence of Leptospira infection in habitants of peripheral neighborhoods in Cali, Colombia. Biomedica 26: 250-257. [ Links ]
Foronda, P., A. Martin-Alonso, B. Castillo-Figueruelo, C. Feliu, H. Gil, and B. Valladares. 2011. Pathogenic Leptospira spp. in wild rodents, Canary Islands, Spain. Emerg. Infect. Dis. 17: 1781-1782. [ Links ]
Franco, S., S. Mattar, M. Urrea, y V. Tique. 2008. Seroprevalencia de Leptospira sp., Rickettsia sp. Ehrlichia sp. en trabajadores rurales del departamento de Sucre, Colombia. Infectio 12: 90-95. [ Links ]
Francois, S., B. Brihuega, S. Grune, V. Gattarello, C. Correa, J. Petrakovsky, C. Gualtieri, y M. Arestegui. 2013. Aislamiento de Leptospira borgpetersenii de fuentes de agua en Argentina. Rev. Cubana Med. Trop. 65: 177-184. [ Links ]
Farmer, P. 2001. Desigualdades sociales y enfermedades infecciosas emergentes / Social inequities and emerging infectious diseases. Rev. Fac. Nac. Salud Pública 19: 111-126. [ Links ]
Ganoza, C., M. Matthias, D. Collins-Richards, K. Brouwer, C. Cunningham, E. Segura, R. Gilman, E. Gotuzzo, and J. Vinetz. 2006. Determining risk for severe Leptospirosis by molecular analysis of environmental surface waters for pathogenic Leptospira. PLoS Medicine, 3: 1329-1340. [ Links ]
Gibbs, E. P. 2014. The evolution of One Health: a decade of progress and challenges for the future. Vet. Rec . 174: 85-91. [ Links ]
Giraldo, G., A. Orrego, y A. Betancurth. 2002. Los roedores como reservorios de Leptospiras en planteles porcinos de la zona central cafetera de Colombia. Arch. Med. Vet. 34: 69-78. [ Links ]
Giraldo, G., A. Orrego, y M. Santacruz. 2002b. Leptospirosis. Las aguas de la explotación porcina como vehículo de la Leptospira, en la zona central cafetera de Colombia. Arch. Med. Vet . 34: 79-87. [ Links ]
Grace, D., and B. Jones. 2011. Zoonoses: Wildlife domestic animal interactions. A report to DFID. Nairobi and London: International Livestock Research Institute and Royal Veterinary College. pp: 51-68. [ Links ]
Grooms, D. L. 2006. Reproductive looses caused by bovine viral diarrhea virus and leptospirosis. Theriogenology 66: 624-628. [ Links ]
Guerra, M. 2013. Leptospirosis: Public health perspectives. Biologicals 41: 295-297. [ Links ]
Gummow,B., J.Myburgh, P.Thompson, J.Van der Lugt, and B.Spencer. 1999. Three case studies involving Leptospira interrogans serovar Pomona infection in mixed farming units. J. S. Afr. Vet. Assoc. 70: 29-34. [ Links ]
Hartskeerl, R. A., M. Collares-Pereira, and W. A. Ellis. 2011. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin. Microbiol. Infect. 17: 494-501. [ Links ]
Hathaway, S. C., T. W. Little, and A. E. Stevens. 1981. Serological and bacteriological survey of leptospiral infection in pigs in southern England. Res. Vet. Sci. 31: 169-173. [ Links ]
Hawkes, C., and M. Ruel. 2006. Agriculture and nutrition linkages: Old lessons and new paradigms. In: Hawkes, C . and M. T. Ruel (eds). Understanding the Links Between Agriculture and Health. 2020 Focus 13. Washington, DC: International Food Policy Research Institute. 78 p. [ Links ]
Hernández-Rodríguez, P., C. Díaz, E. Dalmau, and G. Quintero. 2011. A comparison between Polymerase Chain Reaction (PCR) and traditional techniques for the diagnosis of leptospirosis in bovines. J. Microbiol. Methods 84: 1-7. [ Links ]
Himsworth, C.G., J.Bidulka, K. L.Parsons, A.Y. Feng, P. Tang, C.M. Jardine, T. Kerr, S. Mak, J. Robinson, and D.M. Patrick. 2013. Ecology of Leptospira interrogans in Norway rats (Rattus norvegicus) in an inner-city neighborhood of Vancouver, Canada. PLoS Negl Trop. Dis. 7: 1-9. [ Links ]
INS-Instituto Nacional de Salud. Bogotá. Colombia. 2013. Sivigila Semana Epidemiológica 15 de 2013. pp: 4-6. [ Links ]
INS-Instituto Nacional de Salud. Bogotá. Colombia. 2014. Sivigila Semana Epidemiológica 2 de 2014. pp: 7-10. [ Links ]
INS, Dirección de Vigilancia y Análisis del Riesgo en Salud Pública. Semana 1 del 2015. Boletín epidemiológico semanal: Semana epidemiológica número 1 de 2015. Edición Rodríguez A. Bogotá, Colombia. 27 p. [ Links ]
INS, Dirección de Vigilancia y Análisis del Riesgo en Salud Pública. Semana 4 del 2015. Boletín epidemiológico semanal: Semana epidemiológica número 4 de 2015. Edición Rodríguez A. Bogotá, Colombia. pp: 33-34. [ Links ]
Jones, B., D. Grace, R. Kock, S. Alonso, J. Rushton, M.Y. Said, D. McKeever, F. Mutua, J. Young, J. McDermott, and D.U. Pfeiffer. 2013. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. USA 110: 8399-8404. [ Links ]
King, L. 2011. 79 Sesión General, Comité Internacional OIE. París 2004 SAPUVENET: Red de Salud Pública Veterinaria. pp: 189-190. [ Links ]
Kingscote, B. F., and D. Wilson. 1986. Leptospira pomona abortion storm in cattle herd in Saskatchewan. Can. Vet. J. 27: 440-442. [ Links ]
Lee-Smith, D., and G. Prain. 2006. Urban agriculture and health. In: Hawkes, C. and M.T.Ruel(eds). Understanding the Links Between Agriculture and Health. 2020 Focus 13. Washington, DC: International Food Policy Research Institute . pp: 26-28. [ Links ]
Levett, P., and D. Haake. 2010. Leptospira species (leptospirosis). In: Madell, G. L., J. E. Bennett, and R. Dolin. Principles and Practice of Infectious Diseases. Philadelphia: Churchill Livingstone Elsevier Editions. pp: 3059-3667. [ Links ]
Lilenbaum, W., and G. N. Souza. 2003. Factors associated with bovine leptospirosis in Rio de Janeiro, Brazil. Res. Vet. Sci . 75: 249-251. [ Links ]
Lilenbaum, W ., and G. Martins. 2014. Leptospirosis in cattle: a challenging scenario for the understanding of the epidemiology. Transbound Emerg. Dis. 61 Suppl. 1: 63-8. doi: 10.1111/tbed.12233 [ Links ]
Liverani, M. et al. 2013. Understanding and managing zoonotic risk in the new livestock industries. Environ. Health Perspect . 121: 873-877. [ Links ]
McMichael, A. J. et al. 2004. Global climate change. In: Ezzati, M., A. D. Lopez, A. Rodgers, and C. J. L. Murray (eds). Comparative Quantification of Health Risks. Geneva, Switzerland: World Health Organization. 2: 1543-1649. [ Links ]
Meites, E., M. T. Jay, S. Deresinski, W. J. Shieh, S. R. Zaki, and L. Tompkins. 2004. Reemerging leptospirosis, California. Emerg. Dis. 10: 406-412. [ Links ]
Méndez, M., L. Ramírez, y A. Alzate. 2005. La práctica de la agricultura urbana como expresión de emergencia de nuevas ruralidades: reflexiones en torno a la evidencia empírica. Cuaderno de Desarrollo Rural, 55: 51-70. [ Links ]
Mikkelsen, C. A. 2013. Debatiendo lo rural y la ruralidad: un aporte desde el sudeste de la provincia de Buenos Aires; el caso del partido de Tres Arroyos. Cuad. Geogra. Rev. Colomb. Geogr. 22: 235-256. [ Links ]
Miranda, D., C. Carranza, C. Rojas, C. Jerez, G. Fischer, y J. Zurita. 2008. Acumulación de metales pesados en el suelo y plantas de cuatro cultivos hortícolas, regados con agua del río Bogotá. Rev. Colomb. Cienc. Hortic, 2: 180-191. [ Links ]
Moles, L. P., M. A. Cisneros, D. Gavaldón, N. Rojas, y J. I. Torres. 2002. Estudio serológico de leptospirosis bovina en México. Rev. Cubana Trop. 54: 24-27. [ Links ]
Monahan, A., I. Miller, and J. Nally. 2009. Leptospirosis: risks during recreational activities. J. Appl. Microbiol. 107: 707-716. [ Links ]
Monsalve, S., S. Mattar , y M. González. 2009. Zoonosis transmitidas por animales silvestres y su impacto en las enfermedades emergentes y reemergentes. Rev. MVZ Córdoba 14: 1762-1773. [ Links ]
MSNA(Ministerio de Salud de la Nación Argentina). 2012. Leptospirosis. Situación Mundial. Provincia de Santafé, Argentina. pp: 1-6. [ Links ]
Myers, D. M., and F. Jelambi. 1975. Isolation and identification of leptospira hardjo from cattle in Argentina. Trop. Geogr. Med. 27: 63-70. [ Links ]
Murray, G. L. et al. 2010. Mutations affecting Leptospira interrogans lipopolysaccharide attenuate virulence. Infect. Immun. 78: 701-709. [ Links ]
Natarajaseenivasan, K., V. Raja, and R. Narayanan. 2012. Rapid diagnosis of leptospirosis in patients with different clinical manifestations by 16S rRNA gene based nested PCR. Saudi J. Biol. Sci. 19: 151-155. [ Links ]
Niwetpathomwat, A., S. Luengyosluechakul, and S. Geawduanglek. 2006. Investigation of leptospirosis in sows from Central Thailand. South. Asian J. Trop. Med. Public. Health. 37: 716-719. [ Links ]
OMS - Organización Mundial de la Salud. 2010. Global school-based student health survey Ginebra. http://www.who.int/ncds/surveillance/gshs/en/ . (Consulta: agosto 2016). [ Links ]
OMS - Organización Mundial de la Salud. 2008. Leptospirosis humana. Guía para el diagnóstico, vigilancia y control Rio de Janeiro: Guía para el diagnóstico, vigilancia y control Rio de Janeiro: http://www.med.monash.edu.au/microbiology/staff/adler/guia-esp.pdf (Consulta: julio 2016). [ Links ]
OMS - Organización Mundial de la Salud. 1998. Enfermedades y daños a la Salud. Salud en América 1: 143-7. [ Links ]
Orjuela, J., M. Navarrete, y L. Betancourt. 2002. Salud y productividad en bovinos de la Costa Norte de Colombia. Instituto Colombiano Agropecuario ICA. http://www.fao.org/docrep/u5700t/u5700T07.htm (Consulta: octubre 2015). [ Links ]
Ortega-Pacheco, A., Colin-Flores, R.F., Gutiérrez-Blanco, E., and Jiménez-Coello, M. 2008. Frequency and type of renal lesions in dogs naturally infected with Leptospira species. Ann. N. Y. Acad. Sci. 1149: 270-274. [ Links ]
PAHO-Pan American Health Organization. 2012. National Forum of Leptospirosis of Nicaragua and International Meeting of Countries that are Facing Outbreaks of Leptospirosis in the Americas. Ministry of Health of Nicaragua. Ministry of Agriculture of Nicaragua. University of León, Nicaragua. 2012; Report of the Meetings. Managua, Nicaragua. pp: 1-38. [ Links ]
Perea, A., R. García, A. Maldonado, M. C. Tarradas, I. Luque, R. Astorga, and A. Arenas. 1994. Prevalence of antibodies to different Leptospira interrogans serovars in pigs on large farms. J. Vet. Med. 41: 512-516. [ Links ]
Petrakovsky, J., J. Tinao, y J. Esteves. 2012. Leptospirosis porcina: prevalencia serológica en establecimientos productores de la República Argentina. Rev. MVZ Córdoba 18: 3282-3287. [ Links ]
Picardeau, M. 2013. Diagnosis and epidemiology of leptospirosis. Med. Mal. Infect. 43: 1-9. [ Links ]
Pulido-Villamarín, A., G. Carreño-Beltrán, M. Mercado-Reyes, y P. Ramírez-Bulla. 2014. Situación epidemiológica de la leptospirosis humana en Centroamérica, Suramérica y el Caribe. Univ. Sci. 19: 247-264. [ Links ]
Rajapakse, S., R. Chaturaka, S. M. Handunnetti, and S. Sumadhya. 2015. Current immunological and molecular tools for leptospirosis: diagnostics, vaccine design, and biomarkers for predicting severity. Ann. Clin. Microbiol. Antimicrob. 14: 1-8. [ Links ]
Ramadass, P., and R. B. Marshall. 1990. Species differentiation of Leptospira interrogans serovars hardjo strain Hardjo bovis from strain Hardjoprajitno by DNA slot blot hybridisation. Res. Vet. Sci . 49: 194-197. [ Links ]
Ramírez-Hernández, L., C. M. Sánchez, y C. Gómez-Caldas. 2008. Agricultura urbana en Bogotá: situación, perspectivas y retos. Coordinación Programa Ciudades Cultivando para el Futuro. Universidad del Rosario-Jardín Botánico Bogotá. Alcaldía Mayor de Bogotá. pp: 8-14. [ Links ]
Ramos, A., G. Souza, and W. Lilenbaum. 2006. Influence of leptospirosis on reproductive performance of sows in Brazil. Theriogenology 66: 1021-1025. [ Links ]
Ribotta, M., and R. Higgins. 1999. Swine leptospirosis: Low risk of exposure for humans? Can. Vet. J . 40: 809-810. [ Links ]
Rocha, T. 1998. A review of leptospirosis in farm animals in Portugal. Revue scientifique et technique. Bull. Off. Int. Epizoot. 17: 699-712. [ Links ]
Rodríguez, A., H. Gómez, y R. Cruz de Paz. 2000. Leptospirosis humana: ¿Un problema de Salud? Rev. Cub. Salud Pública 26: 27-34. [ Links ]
Sacsaquispe, R., M. Glenny, y M. Céspedes. 2003. Estudio preliminar de leptospirosis en roedores y canes en Salitral, Piura-1999. Rev. Peru. Med. Exp. Salud Pública 20: 39-40. [ Links ]
Sanderson, M. W., and D. P. Gnad. 2002. Biosecurity for reproductive diseases. Vet. Clin. North Am. Food Anim. Pract . 18: 79-98. [ Links ]
Sandow, K., y W. Ramírez. 2005. Leptospirosis. REDVET 6: 1-61. [ Links ]
Schelotto, F., E. Hernández, S. González, A. Del Monte, S. Ifran, K. Flores, L. Pardo, D. Parada, M. Filippini, V. Balseiro, J. P. Geymonat, and G. Varela. 2012. A ten-year follow-up of human leptospirosis in Uruguay: An unresolved health problem. Rev. Inst. Med. Trop. Sao Paulo 54: 69-75. [ Links ]
Schreier, S., G. Doungchawee, and D. Triampo. 2012. Development of a magnetic bead fluorescence microscopy immunoassay to detect and quantify Leptospira in environmental water samples. Acta Trop. 122: 119-25. [ Links ]
Schneider, M., M. Jancloes, D. Buss, S. Aldighieri, E. Bertherat, P. Najera, D. Galan, K. Durski, and M. Espinal. 2013. Leptospirosis: A silent epidemic disease. Int. J. Environ. Res. Public Health 10: 7229-7234. [ Links ]
Soman, M., V. Jayaprakasan, and M. Mini. 2014. Epidemiological study on human and canine leptospirosis in Central and North Kerala. Vet. World 7(10):759-764, ISSN: 2231-0916. [ Links ]
Taylor, L. H., S. M. Latham, and M. E. Woolhouse. 2001. Risk factors for human disease emergence. Philos. Trans. Royal Soc. Lond. B Biol. Sci. 356: 983-9. [ Links ]
Taylor, P. 2006. Managing urban rats and rodent-borne diseases in a squatter camp - the Cato Crest Model? Palmnut Pos 9: 18-20. [ Links ]
Teubal, M. 2001. Globalización y nueva ruralidad en América Latina. In: Giarracca, N. (coord). ¿Una nueva ruralidad en América Latina? Buenos Aires, Argentina: Agencia Sueca de Desarrollo Internacional, Consejo Latinoamericano de Ciencias Sociales (Clacso). pp: 45-65. [ Links ]
Thaipadungpanit, J. et al. 2013. Short report: Leptospira species in floodwater during the 2011 floods in the Bangkok Metropolitan Region, Thailand. Am. J. Trop. Med. Hyg . 89: 794-796. [ Links ]
Trevejo, R. T., Y. Rigan-Perez D., and A. Ashford. 1998. Epidemic Leptospirosis associated with pulmonary hemorrhage, Nicaragua 1995. Infect. Dis. 178: 1457-1463. [ Links ]
UNESCO-United Nations Educational, Scientific and Cultural Organization. 2003. Water for People, Water for Life. Executive Summary of the UN World Water Development Report. Mundi-Prensa Libros. Paris, France. pp: 134-137. [ Links ]
Vado-Solís, I., M. F. Cárdenas-Marrufo, B. Jiménez-Delgadillo, A. Alzina-López, H. Laviada-Molina, V. Suarez-Solís, and J. E. Zavala-Velázquez. 2002. Clinical-epidemiological study of Leptospirosis in humans and reservoirs in Yucatan, Mexico. Rev. Inst. Med. Trop. Sao Paulo 44: 335-340. [ Links ]
Van, N. Encyclopedia of Agriculture and Food Systems. 1. 2014. Segunda Edición. UK: Elsevier. 464 p. [ Links ]
Vanasco, N., G. Sequeiro, M. Fontana, S. Fusco, M. Sequeiro, y D. Enría. 2000. Descripción de un brote de leptospirosis en la ciudad de Santa Fe, Argentina, marzo-abril 1998. Rev. Panam. Salud Publica 7: 27-32. [ Links ]
Victoriano, A., L. Smythe, N. Gloriani-Barzaga, L. Cavinta, T. Kasai, K. Limpakarnjanarat, B. Ong, G. Gongal, J. Hall, C. Coulombe, Y. Yanagihara, S. Yoshida, and B. Adler. 2009. Leptospirosis in the Asia Pacific Region. BMC Infect. Dis. 9:147. [ Links ]
Vital-Brazil, J. M., I. T. Balassiano, F. S. De Oliveira, A. D. De Souza, L. Hillen, and M. M. Pereira. 2010. Multiplex PCR-based detection of leptospira in environmental water samples obtained from a slum settlement. Mem. Inst. Oswaldo Cruz 105: 353-355. [ Links ]
Wang, Z., L. Jin, and A. Wegrzyn. 2007. Leptospirosis vaccine. Microb. Cell Fact. 6: 39. https://microbialcellfactories.biomedcentral.com/articles/10.1186/1475-2859-6-39. [ Links ]
Wangroongsarb, P., W. Petkanchanapong, S. Yasaeng, A. Imvithaya, and P. Naigowit. 2002. Survey of Leptospirosis among rodents in epidemic areas of Thailand. J. Trop. Med. Parasitol. 25: 55-58. [ Links ]
Webster, J. P., W. A. Ellis , and D. W. Macdonald. 1995. Prevalence of Leptospira spp. in wild brown rats (Rattus norvegicus) on UK farms. Epidemiol. Infect. 114: 195-201. [ Links ]
Weiss, R., and A. McMichael. 2004. Social and environmental risk factors in the emergence of infectious diseases. Nat. Med. 10: S70-6. [ Links ]
WHO-World Health Organization. 2010. Report of the First Meeting of the Leptospirosis Burden Epidemiology Reference Group, WHO Library Cataloguing-in-Publication Data, Geneva, Switzerland. pp: 1-34. [ Links ]
WHO-World Health Organization. 1999. Weekly Epidemiological Record. 74. Geneva, Switzerland: 237-244. [ Links ]
Witmer, G. W., H. Martins, and L. Flor. 2004. Leptospirosis in the Azores: The rodent connection. USDA National Wildlife Research Center - Staff Publications, Paper 401: 217-220. [ Links ]
Zhang, C., H. Wang, and J. Yan. 2012. Leptospirosis prevalence in Chinese populations in the last two decades. Microbes Infect. 14: 317-23. [ Links ]
Zaki, S. R., W. V. Shieh, and Epidemic Working Group. 1996. Leptospirosis associated with outbreak of acute febrile illness and pulmonary hemorrhage, Nicaragua. 1995. Lancet 347: 553 [ Links ]
Received: August 2016; Accepted: June 2017











 text in
text in