Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Agrociencia
On-line version ISSN 2521-9766Print version ISSN 1405-3195
Agrociencia vol.51 n.6 Texcoco Aug./Sep. 2017
Plant Protection
CONTROLLING FUSARIUM WILT OF CHICKPEA (Cicer arietinum L.) WITH NATIVE MICROORGANISMS OF SINALOA, MEXICO
1Universidad Autónoma de Sinaloa. Facultad de Agronomía. Carretera Culiacán-Eldorado km. 17.5. 80398. Culiacán de Rosales, Sinaloa, México.
2Centro de Ciencias de Sinaloa. Avenida de las Américas 2771 Norte. Culiacán de Rosales, Sinaloa, México.
3Universidad Tecnológica de Culiacán, carretera Culiacán-Imala km 2, en la Ciudad Educadora del Saber. 80014. Culiacán de Rosales, Sinaloa, México.
Fusarium wilt in chickpea (Cicer arietinum L.) has spread, due to the difficulty to control it. Chemical control diminishes the biodiversity and pollutes the environment, so sustainable measures must be developed to limit the disease. Therefore, the objective of this study was to determine the potential of Sinaloa’s native bacterial and fungal strains, as biological control agents of this disease in chickpea plants grown in greenhouses. Because native bacterial and fungal strains inhibit Fusarium oxysporum f. sp. ciceris, the hypothesis was that those strains are an alternative to control fusarium wilt in chickpea. The radial growth of Fusarium oxysporum f. sp. ciceris, race 5 -the principal agent that causes this disease in Sinaloa- was evaluated to study the inhibition of biological control strains, using the dual culture technique in a PDA growing medium. The experiments were carried out in a greenhouse. The experimental design was completely random, with five repetitions per treatment; each repetition had ten pots, and each pot had two plants. Plant vigor, foliage wilt, and root canker were evaluated using a subjective scale; plant height (cm), chlorophyll (SPAD 502 units), wet and dry biomass (g), stem diameter (mm), and yield (g plant-1) were quantified. Trichoderma sp. HRG-060 native strain showed the highest in vitro mycelial inhibition (PIRC: 80.07%), due to its ability to colonize 100% of the surface of the mycelium that grew out of the pathogen in 3 d. The highest grain yield was obtained in plants with the Trichoderma sp. HRG-060 strain (6.13g plant-1); meanwhile, the Bacillus sp. T442 strain created a favorable atmosphere for the highest chlorophyll production (55.45 SPAD units), as well as the greatest wet and dry biomass weight (41.73 g plant-1 and 30.03 g plant-1, respectively).
Key words: biological control; native strains; Bacillus; Trichoderma; Pseudomonas; Fusarium oxysporum f. sp. ciceris.
La fusariosis vascular del garbanzo (Cicer arietinum L.) aumenta debido a la dificultad para controlarla. El control químico disminuye la biodiversidad y contamina el ambiente, por lo cual se deben desarrollar medidas sustentables para limitar la enfermedad. Por lo tanto, el objetivo de este estudio fue determinar el potencial de cepas bacterianas y fúngicas, nativas de Sinaloa, como agentes biocontrol de esta enfermedad en plantas de garbanzo cultivadas en invernadero. La hipótesis fue que las cepas bacterianas y fúngicas nativas son una alternativa para controlar de la fusariosis vascular del garbanzo porque inhiben Fusarium oxysporum f. sp. ciceris. La inhibición de las cepas de control biológico se evaluó sobre el crecimiento radial de Fusarium oxysporum f. sp. ciceris raza 5, el principal agente causal de la enfermedad en Sinaloa, mediante la técnica de cultivo dual en medio de cultivo PDA. Los experimentos se realizaron en invernadero, el diseño experimental fue completamente al azar con cinco repeticiones por tratamiento, cada repetición tuvo 10 macetas, y cada maceta contenía dos plantas. Vigor de planta, marchitez en follaje y cáncer de raíz se evaluaron con una escala subjetiva; se cuantificaron altura de planta (cm), clorofila (unidades SPAD 502), biomasa húmeda y seca (g), diámetro de tallo (mm) y rendimiento (g planta-1). La cepa nativa de Trichoderma sp. HRG-060 destacó por la mayor inhibición micelial in vitro (PIRC: 80.07 %) y por su capacidad de colonizar el 100 % de superficie del micelio crecido del patógeno en 3 d. El mayor rendimiento de grano fue en las plantas con la cepa Trichoderma sp. HRG-060 (6.13 g planta-1), y la cepa de Bacillus sp. T442 propició la mayor producción de clorofila (55.45 unidades SPAD) y peso de biomasa húmeda (41.73 g planta-1) y seca (30.03 g planta-1).
Palabras clave: control biológico; cepas nativas; Bacillus; Trichoderma; Pseudomonas; Fusarium oxysporum f. sp. ciceris
Chickpea (Cicer arietinum L.) is the second most important nourishing leguminous plant in the world, after beans (Phaseolus vulgaris L.). Almost all the production of chickpea crops in Sinaloa, Mexico, is exported, and this generates significant revenues. Sinaloa is the country’s main producer and exporter of white chickpea (Guerrero et al., 2015). In 2013, 61 600 ha of white chickpea were sowed in Sinaloa, and the production reached 103 645 Mg (SIAP, 2014). All over the world, one of the main factors that limits the success of this crop production is fusarium wilt, which is caused by the Fusarium oxysporum f. sp. ciceris pathogen (Jiménez and Jiménez, 2001). Losses caused by this fungi range from 10 to 40 % of the annual harvest, and can completely devastate crops, if there are favorable conditions for the development of the resulting disease (Guerrero et al., 2015).
This pathogen is difficult to control, as a consequence of its resistance to major fungicides and fumigants; besides, fungi can lie dormant in the soil, through chlamydospores, structures that represent initial inoculums for the epidemics during subsequent crop cycles. Fusarium wilt is the most important disease of chickpea in Sinaloa (Velarde et al., 2013), and the main control method is to apply fungicides and fumigants on the soil. However, some of these products can harm the crops due to their phytotoxic effect; they can also eradicate benefic organisms, because they are not specific. This situation -along with safety and public health problems associated with the manufacture and use of agrochemicals, as well as the potential diffuse pollution of ground water bodies- leads to the pursuit and establishment of environmentally-friendly management alternatives. Therefore, identifying and studying native antagonistic microorganisms strains are essential to optimize their success as biological control agents of the fusarium wilt of chickpea, due to their proven adaptation to the source environment.
Trichoderma spp. and Bacillus spp. are proposed as biological control agents, particularly against soilborne phytopathogens (Verma et al., 2007). Trichoderma spp. uses antagonistic action mechanisms, such as mycoparasitism, lyses, antibiosis, nutrient and space competition, or resistance induction, or both, in the host (De la Garza, 1996). Bacillus spp. is a plant-growth promoting bacteria; additionally, it has a protecting effect against soilborne pathogens (Butt et al., 1999). Antibiotic and enzyme production, solubilization of phosphates (Chen et al., 2006), and biological nitrogen fixation (Ooi et al., 2008) have shown the potential of the Bacillus species genus.
The hypothesis of this study was that native bacterial and fungal strains represent an alternative to control fusarium wilt in chickpeas, because their activities inhibit F. oxysporum f. sp. ciceris, and promote the growth and vigor of chickpeas, which will result in better grain yield. The objectives were as follows: 1) to determine the potential of Sinaloa’s native bacterial and fungal strains as biological control agents against fusarium wilt in chickpea, by means of in vitro and greenhouse tests; 2) to identify the fungal strains’ control mechanisms; 3) to evaluate the effect that bacterial and fungal strains have on the height, chlorophyll content, and wet and dry plant biomass weight, as well as grain yield.
Materials and Methods
Isolates’ origin
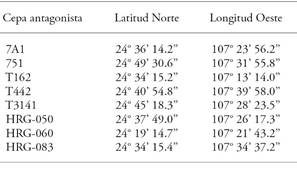
Isolate 303CS of F. oxysporum f. sp. ciceris, race 5 -GenBank access number KJ000584- used on this study was provided by the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pesqueras. As biological control agents, the following strains -taken from the Centro de Ciencias de Sinaloa (CCS) collection- were used: bacteria of the genus Bacillus sp., T442, T3141 strains; bacteria of the genus Pseudomonas sp., T162, 7A1, and 751 strains; fungi of the genus Trichoderma sp., HRG-050, HRG-060, and HRG-083 strains. These strains were previously isolated in chickpea crops, infected with the disease (Table 1).
Isolate activation
Bacteria were cultured using an inoculated loop sampling method in a Petri dish. Pseudomonas strains were cultured in a medium based on Agar FLO (20 g peptones mix, 14 g agar-agar, 15 g K2HPO4, 15 g MgSO4, and 1.0 L distilled H2O); Bacillus was cultured in a nourishing agar medium (5 g peptone gelatine, 3 g meat extract, 15 g agar-agar, and 1.0 L distilled H2O); and, fungal strains and Foc race 5 were grown using a portion inclusion method (Leslie and Summerell, 2006). To carry out the experiments, 1 cm diameter dishes of each strain were placed on the center of the Petri plates, with a PDA culture (4.0 g potato infusion, 20 g dextrose, 15 g agar, and 5.6±0.2 final pH). The plates were incubated at 28 °C, for 24 h and 5 d, for bacteria and fungi, respectively.
In vitro antagonism tests against Foc, race 5
Strain antagonistic activity was studied using the dual culture technique (Ezziyyani et al., 2004), in Petri plates with a PDA culture medium. Confrontations between bacteria strains versus Foc race 5 were arranged as follows: first, a 1 cm wide phytopathogen disk grown in PDA was placed on one side of the plate; 3 d later, a bacterial strain sample was put in the center of the plate, using an inoculating loop. The portion method was used to culture antagonistic fungal strains (Howell, 2003): a 1 cm wide disk containing Foc race 5 was placed on one side, and, 5 d later, an antagonist disk of the same size was placed at the other end of the plate. The inoculated plates were incubated 7 d at 28 °C. The strains’ antagonistic capacity was evaluated using the percentage inhibition of radial growth (PICR), using the following formula:
PICR=[(R1-R2)/100] (Suárez et al., 2008)
where
PICR is the inhibition percentage of phytopathogen mycelium growth.
R1 is the highest radial growth (pathogen-control mycelium radius).
R2 is the lowest ratio (pathogen mycelium radius on dual culture).
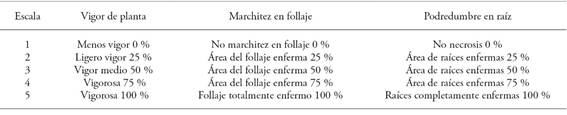
The mycoparasitism degree exercised by Trichoderma strains was determined based on the surface of the Petri dish that it colonized when the phytopathogen was present, according to the scale of Bell et al. (1982) (Table 2).
The confrontations were observed using an optical microscope, in order to determine the antagonistic mechanism used by Trichoderma on the pathogen fungi. Additionally, the total amount of haustoria generated by Trichoderma strains during their confrontations with Foc were quantified 5 d after they were cultured. For this purpose, five 1-cm2 samples were randomly taken from the Petri dish where the confrontation between the antagonist and Foc had taken place, and they were observed under the microscope.
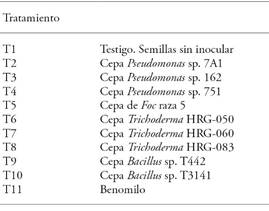
The experimental design was completely random, with four repetitions per treatment (Table 3); each repetition contained a confrontation (experimental unit). The data were subject to an ANOVA, and to a mean comparison Tukey test (p≤0.05), using SAS 9.0 (SAS Institute, 2002).
Inoculum production
Microorganism inoculum production was carried out using 500 mL flasks, using a inoculating loop to sample the biological material, under aseptic conditions, inside a laminar flow bell, and using as culture medium for Pseudonomas: 300 mL of tryptic soy broth (17 g casein peptone, 3 g soy peptone, 5 g NaCl, 2.5 g K2HPO4, 2.5 g dextrose, and final pH of 7.3±0.2); for Bacillus: 300mL of nurturing broth was use (5 g peptone, 3 g meat extract, and final pH 6.9±0.2); 300 mL of dextrose potato agar (PDA) were used to produce Trichoderma. Once the flasks were inoculated with their respective strains, they were incubated during 5 d at 28 °C, stirring them at 150 rpm. After this, their content was put on 50 mL test tubes (Falcon) and they were centrifuged at 3800 rpm for 20 minutes, at room temperature; the biomass was later harvested. Then, Sinaloa 92 white chickpea seeds were treated with a 1×108 ufc mL-1 concentration with the bacterial strains, using the Mc Farland 139 method (Ortigoza and Ruiloba, 1998). The turbidity method was used to standardize bacterial suspensions. The seeds with the fungal strains were treated with a concentration of 1×108 conidium mL-1, using the Neubauer chamber method (Ferron, 1981).
Greenhouse experiments
Inoculated seeds were sown on pots containing 12 L of substrate (Peat Moss). Before the sowing, the substrate (sterilized in autoclave) was inoculated with F. oxysporum f. sp. ciceris (Foc) race 5, using a 1×108 conidium mL-1 concentration. The experimental design was completely random, with five repetitions per treatment (Table 3); each repetition had 10 pots, and each pot had two plants (20 plants per repetition). The seeds were sown on December 2013; they were watered 15 d after the sowing, and Murashige-Skoog (1962) nourishing solution was used.
The following response variables were evaluated: plant vigor (development and sagging percentage of the plant); wilt (chlorophyll percentage); and root rot (necrosis percentages with subjective visual assessment scales) (Table 4). In addition, the following elements were evaluated: greenness (chlorophyll content on the leaves), using a SPAD 502 (Spectrum Technologies, Inc.); plant height (cm), from the stem base to the top of the plant; wet biomass (g); and dry biomass weight (g) of plants dehydrated in an oven at 80 °C, for 24 hours. Additionally, the stem diameter (mm) was evaluated using a digital Vernier caliper, while yield (g plant-1) was calculated using a digital scale.
Data analysis
A normality and homogeneity of variance test was carried out using the inhibition percentage data; the means were compared with the Tukey test (p≤0.05), using SAS 9.0 (SAS Institute, 2002). The data obtained from the greenhouse treatments of the non-parametric variables (plant vigor, foliage wilt, and root rot), along with their replication per treatment, were analyzed using the Kruskal-Wallis test. Parametric variable data (height, stem diameter, chlorophyll content, fresh weight, dry weight, and yield) were subject to an ANOVA test and a Tukey (p≤0.05) mean difference test, using SAS 9.0 (SAS Institute, 2002).
Results and Discussion
Antagonistic activities of bacterial and fungal strains
Statistical analysis showed that in vitro radial growth of F. oxyspourm f. sp. ciceris (Foc) race 5 diminished 80.07 % to 38.89 % (p≤0.05) when it was evaluated in relation to biological control microorganisms (Table 5). The highest Foc PICR (80.07 %) was observed in its confrontation with the HRG-060 strain and it was different (p≤0.05) to the PICR of other antagonist strains. On that matter, Michel-Aceves et al. (2005) report 16.40 % to 77.80 % inhibitions, when in vitro isolated native Trichoderma are evaluated against F. oxysporum f. sp. lycopersici and 13.10 % and 94.40 % inhibitions when are evaluated against S. rolfsii. With regard to bacterial strains, the highest PICR was observed in T442 strain, which had a 59.26 % inhibition of Foc.
Table 5 In vitro mycelial growth inhibition of Foc race 5 against native microorganisms.
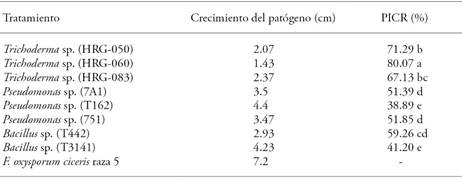
Different letters indicate statistically significant differences (Tukey; p≤0.05).
Three days after inoculation, the HRG-060 Trichoderma strain had colonized 100 % of the surface of the medium where Foc had grown; HRG-050 and HRG-083 colonized the surface on the fourth day, as a result of which they held the 1st degree of antagonism in the scale of Bell et al. (1982). According to Guédez et al. (2012). This indicates that these strains are highly aggressive, even more aggressive than Trichoderma spp. strains (Sánchez García et al., 2017), which colonized 100 % of the medium where the phytopathogen of F. solani, F. oxysporum, and F. verticillioides grew, 10 d after inoculation.
During its confrontation with Foc, the HRG-060 strain formed 123±15 haustoria cm-2 per confrontation, while the HRG-050 and HRG-083 strains formed 49±12 and 58±9 haustoria per confrontation, respectively. The highest amount of haustoria formed by the Trichoderma HRG-060 strain highlighted its higher efficiency against F. oxysporum f. sp. ciceris, since it facilitated the entrance of hyphae and the mycoparasitism of Foc. This proves that Trichoderma strains have a high mycelial growth inhibition capacity, particularly strain HRG-060 which formed more haustoria than the other fungal strains.
The control mechanism exercised by Trichoderma strains over Foc was the result of mycoparasitism: Trichoderma’s hyphae stuck to Foc’s hyphae and phialides, formed haustoria, and penetrated the hypha. These results are similar to those observed by Kexiang et al. (2002) who reported that mycoparasitism eliminated the pathogen’s mycelial growth.
There are different types of hypha interaction for Trichoderma (such as parasitism), and they are considered to be potential bioregulators of soil fungi (Infante, 2009): Trichoderma hyphae coil up and penetrate F. oxysporum f. sp. cubense and penetrate Phythium sp. and R. solani hyphae. Rivero (2008) evaluated four Trichoderma isolates against Alternaria padwickii, Bipolaris oryzae, Curvularia lunata, and Phoma sp., and found out that it had a high competitive capacity, with two or more kinds of hypha interaction, except in the case of Phoma sp.
Protective effect of biological control microorganisms
The F. oxysporum f. sp. ciceris isolate used in our study is highly aggressive: it causes a 93.27 % rot in the positive control plant roots (Table 6).
The vigor of T7 (Trichoderma sp. HRG-060) treatment plants was greater than with other treatments (Table 6); meanwhile T3 (Pseudomonas sp. T162) plants showed the lowest vigor (p≤0.05). In the case of other treatments, the plants showed higher values than control and negative control. Treatment plants whose seeds were inoculated with Trichoderma (T6, T7, and T8) showed lower wilt, but they were less wilted (p≤0.05) that T5 (positive control) and T11 (Benomyl) plants. The lower root rot percentage occurred in T7 (Trichoderma sp. HRG-060) plants, and it was 41 % and 19 % lower than the symptomatology showed by T5 (positive control) and T11 (Benomyl) plants, respectively (Table 6). Additionally, the presence of the disease in T2, T6, T8, T9, and T10 plants was similar to its presence in T11 plants, and lower than in T5 plants (Table 6).
Table 6 Protective effect on the fusarium wilt of chickpeas, under greenhouse conditions.
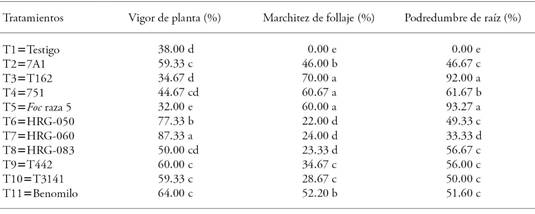
Means with different letters in each variable indicate statistically significant differences (Tukey; p≤0.05).
The greatest protective effect against fusarium wilt in the greenhouse bioassay was provided by the Trichoderma HRG-060 strain treatment (Table 5), which also had the best PICR (80.07 %). The PICRs of the HRG-050 and HRG-083 strains were 71.29 and 67.13 %, respectively, but there was no beneficial protective effect in chickpea plants inoculated with Foc. This information does not match the recommendations made by Sánchez-García et al. (2017) who mention that strains with good in vitro control also have that effect in vivo in inoculated plants, and recommend in vivo tests as an adequate tool to estimate biological control in plants.
The good results obtained from PICR means, from Trichoderma native strains, can be the result of the antagonists’ coadaptation with pathogen fungi, because they originated in similar climates and habitats (Andrews, 1992). Therefore, there might not have been enough recognition between Foc and Trichoderma HRG-050 and HRG-083 strains. This recognition would have occurred through the lectin-carbohydrate interaction which agglutinates cells that interact with pathogen’s cell surface and created a favorable atmosphere for mycoparasitism (Infante et al., 2009); therefore, the level of mycoparasitism was not the same as in the in vitro tests. This information confirms that coadaptation between the antagonist fungus and the pathogen fungus is required for control (Baker and Cook, 1974). Therefore, carrying out plant tests is necessary to prove the true potential of the biological control agents. In all treatments, the pathogen was once more isolated from the necrotic tissue of all inoculated plants and it was morphologically confirmed to be the same as the inoculated strain. No symptoms were observed in the non-inoculated controls.
Plant growth promotion by bacterial and fungal antagonists
Plants in T7 (Trichoderma sp. HRG-060) were the highest: 26 and 57 % higher than control plants (T1) and positive control (T5), respectively (Table 7); the greatest stem diameter was shown by T4, T7, and T10. The chlorophyll content in T9 (Bacillus sp. T442) and T4 (Pseudomonas sp. 751) plants was greater than in other treatments (p≤0.05). The wet biomass weight was higher (p≤0.05) in T9 plants (Bacillus sp. T442) that in T1 (control), T5 (positive control), and T11 (Benomyl). In other treatments -except T2 (Pseudmonas sp. 7AI) and T3 (Pseudomonas sp. 162)-, the wet biomass weight was greater, in relation to T1 (control) and T11 (Benomyl) plants, when T9 (Bacillus sp. T442) plants had a greater wet biomass weight. Likewise, dry biomass weight showed a significant increase in T9 (Bacillus sp. T442) plants, in relation to plants in the remaining treatments.
Plants in T9 (Bacillus sp. T442) had the greatest chlorophyll content, wet biomass weight, and dry biomass weight values, but T7 (Trichoderma sp. HRG-060) plants showed a significant increase (p≤0.05) in grain yield, in relation to other treatments (Table 7); there was a significant relation (R2=0.91) between disease control in the plant and grain production yield. In our study, a greater (wet and dry) biomass production and a greater quantity of chlorophyll does not entail a greater grain yield. On that matter, Howell (2003) and Mohiddin et al. (2010) report that Trichoderma species promote an increase in plant yield and contribute to disease resistance or tolerance, when they interact with plants. Mohiddin et al. (2010) point out that Trichoderma is located inside and outside the rhizosphere, where it can colonize and protect the roots. Trichoderma does not only protect the plant, but it also helps its development; according to Stefanova (2007), treating tobacco seeds with Trichoderma reduces external pollutants (such as Rhizopus stolonifer).
Table 7 Effect of inoculating seeds with bacterial and fungal microorganisms with the chickpea plant growth parameters.
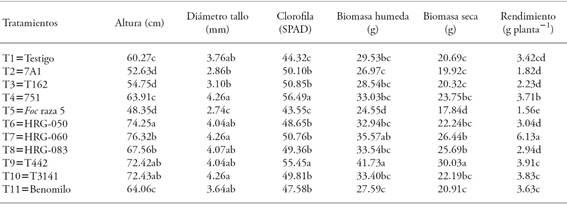
Means with different letters in each variable are statistically significant (Tukey; p≤0.05).
Various substances that promote plant growth are produced by certain rhizosphere microorganisms -including bacteria from the Bacillus genus, and fungi from the Trichoderma genus- and they can have a direct or indirect influence on the metabolism and physiology of the plant (Bhattacharyya and Jha, 2012), because they synthesize and excrete phytostimulant substances, such as plant growth regulators and volatile organic compounds that reinforce plant immunity (Ahmad et al., 2008; Ambreen and Shahida, 2010). Therefore, studying the interaction between the Trichoderma HRG-060 strain and the chickpea plant and subsequently influencing greater grain yield are essential.
Conclusions
The Trichoderma sp. HRG-060 native strain stood out owing to its greater in vitro mycelial inhibition and to its capacity to fully (100 %) colonize the surface of the mycelium that grew from the pathogen over the course of three days. The strain’s high aggressiveness is the result of the formation of a greater number of haustoria, which facilitated mycoparasitism over the pathogen’s hyphae. This strain induced the greater increase yield-wise, but Bacillus sp. T442 favored the highest production of chlorophyll and greater wet and dry biomass weight. The Trichoderma sp. HRG-060 strain represents a biological control agent against Fusarium oxysporum f. sp. ciceris.
Likewise, our study shows that in vitro and plant tests are necessary to identify microorganisms with biological control potential
Literatura Citada
Ahmad, F., I. Ahmad, and M. S. Khan. 2008. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 163: 173-81. [ Links ]
Ambreen, A., and H. Shahida. 2010. Auxin-producing Bacillus sp.: Auxin quantification and effect on the growth of Solanum tuberosum. Pure Appl. Chem. 2: 313-319. [ Links ]
Andrews, J. H. 1992. Biological control in the phyllosphere. Ann. Rev. Phytopathol. 30: 603-635. [ Links ]
Baker, K. F., and J. R Cook. 1974. Biological Control of Plants Pathogens. Firth edition. CRS Press. Freeman, San Francisco, CA. USA. 157 p. [ Links ]
Bell D.K., H.D. Wells, and C.R. Markham. 1982. In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology 72: 379-382. [ Links ]
Bhattacharyya, N., and K.Jha D. 2012. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J. Microbiol. Biotechnol. 28: 1327-1350. [ Links ]
Butt TM, Harris J, Powell K. 1999. The European scene opportunities for biopesticides. In: Hall, Franklin R., Menn, Julius J. (Eds.) Bio-Pesticides: Use and Delivery. Volume 5. Humana Press, Totowa, N. J. pp: 23-44. [ Links ]
Chen, Y.P., P.D.Rekha, A.B.Arun, F.T.Shen, W.Lai, and C.C.Young. 2006. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 34: 33-41. [ Links ]
De la Garza, J.L. 1996. Fitopatología General. Universidad Autónoma Nuevo León. Facultad de Agronomía. Marín, Nuevo León. 515 p. [ Links ]
Ezziyyani, M., C.Pérez-Sánchez, A.Sid, E. Requena M., y E. Candela M. 2004. Trichoderma harzianum como fungicidas para el control de Phytophtora capsici en plantas de pimiento (Capsicum annuum L.). Anales Biol. 26: 35-45. [ Links ]
Ferron, P. 1981. Pest control by the fungi Beauveria and Metarhizium. In: Burge, H. D. (ed). Microbial Control of Pests and Plant Diseases 1970-1980. Academic. Press, London and New York. pp: 465-481. [ Links ]
Guédez, C., L.Cañizales, C.Castillo, y R.Olivar. 2012. Evaluación in vitro de aislamientos de Trichoderma harzianum para el control de Rhizoctonia solani, Sclerotium rolfsii y Fusarium oxysporum en plantas de tomate. RSVM 32: 44-49. [ Links ]
Guerrero, Z., J.A. Acosta G., B.M. Sánchez G., P.F. Ortega M., y M.M. González C. 2015. Razas patogénicas de Fusarium oxysporum f. sp. ciceris en garbanzo cultivado en Guanajuato, México. Rev. Fitotec. Mex. 38: 183-190. [ Links ]
Howell, C. R. 2003. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 87: 4-10. [ Links ]
Infante, D., B.Martínez, N. González, y Y. Reyes. 2009. Mecanismos de acción de Trichoderma frente a hongos fitopatógenos. Rev. Protec. Veg. 24: 14-21. [ Links ]
Jiménez, M., and M.M. Jiménez G. 2011. Integrated management of Fusarium wilt diseases. In: Alves S., F.M., and J. Díez (eds). Control of Fusarium Diseases. Research Signpost, Kerala, India. pp: 177-215. [ Links ]
Kexiang, G., L. Xiaoguang, L. Yonghong, Z. Tianbo, and W. Shuliang. 2002. Potential of Trichoderma harzianum and T. atroviride to control Botryosphaeria berengeriana f. sp. piricola, the cause of apple ring rot. J. Phytopathol. 150: 271-276. [ Links ]
Leslie J., F., and A. Summerel B. 2006. The Fusarium Laboratory Manual. Blackwell Publishing. Ames, Iowa, USA. 388 p. [ Links ]
Michel-Aceves, A. C. et al. 2005. Producción y efecto antagónico de quitinasas y glucanasas por Trichoderma spp., en la inhibición de Fusarium subglutinans y Fusarium oxysporum in vitro. Rev. Chapingo Serie Hortic. 11: 273-278. [ Links ]
Mohiddin, F.A., M.R. Khan, S.M. Khan, and B.H. Bhat. 2010. Why Trichoderma is considered super hero (super fungus) against the evil parasites?. Plant Pathol. J. 9: 92-102. [ Links ]
Murashige, T., and F. Skoog. 1962. A revised medium for rapid growht and bio-assays with tobacco cultures. Phy. Plantarum 15: 473-497. [ Links ]
Ooi, T.C., A.B. Ariff, M.S. Halimi, and Z.H. Shamsuddin. 2008. Growth kinetics of diazotrophics Bacillus sphaericus UPMB cultured using different types and concentrations of carbon and nitrogen sources. Malay. J. Microbiol. 4: 15-25. [ Links ]
Ortigoza F., J., y S. L. Ruiloba L. 1998. Microbiología Práctica. Departamento de Microbiología. 2a Ed. ENCB-IPN. México. 205 p. [ Links ]
Rivero, D. 2008. Identificación y control in vitro con quitosana y Trichoderma spp. de hongos que causan el manchado del grano en arroz (Oryza sativa L.). Rev Protec. Veg. 23: 67. [ Links ]
Sánchez-García, B. M., E. Espinosa-Huerta, E. Villordo-Pineda, R. Rodríguez-Guerra, M. A. Mora-Avilés. 2017. Identificación molecular y evaluación antagónica in vitro de cepas nativas de Trichoderma spp. sobre hongos fitopatógenos de raíz en frijol (Phaseolus vulgaris L.) cv. Montcalm. Agrociencia 61: 63-79. [ Links ]
SAS (Statistical Analysis System Institute). 2002. SAS/STAT User’s Guide, Software version 9.0. SAS Institute Inc. Cary, N.C. 27513. USA. [ Links ]
SIAP. SAGARPA. 2014. http://www.sagarpa.gob.mx/Delegaciones/sinaloa . (Consulta: junio 2014). [ Links ]
Stefanova, N. M. 2007. Introducción y eficacia del biocontrol de fitopatógenos con Trichoderma spp. en Cuba. Fitosanidad 11:75-79. [ Links ]
Velarde F., S. et al. 2013. Distribución y presencia de la fusariosis del garbanzo (Cicer arietinum L.) en el Noroeste de México y búsqueda de resistencia en condiciones controladas. In: Simposio Nacional de Garbanzo. SAGARPA/INIFAP. 2013. Memoria pp: 54-64. [ Links ]
Verma, M., S.K. Brar, R.D. Tyag, R.Y. Surampalli, and J.R. Valero. 2007. Review: Antagonistic fungi, Trichoderma spp.: Panoply of biological control. Biochem. Eng. J. 37: 1-20. [ Links ]
Received: November 2016; Accepted: May 2017











 text in
text in