Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Agrociencia
On-line version ISSN 2521-9766Print version ISSN 1405-3195
Agrociencia vol.51 n.4 Texcoco May./Jun. 2017
Crop science
Monitoring protein quality of o2 maize (Zea mays L.) in inbred lines and their F1 and F2 progeny
1Programa de Maíz, Campo Experimental Valle de México, Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. km 13.5 Carretera Los Reyes-Texcoco. 56250. Coatlinchán, Texcoco, México. arevajolu@yahoo.com.mx.
2Facultad de Química, Universidad Nacional Autónoma de México. Avenida Universidad y Copilco. 04510. Ciudad de México, México jorman@unam.mx, elpidio@unam.mx.
3Departamento de Bioprocesos, Unidad Profesional Interdisciplinaria de Biotecnología, Instituto Politécnico Nacional. Avenida Acueducto s/n. 07340. La Laguna Ticomán. Ciudad de México, México. ygomezipn@hotmail.com, enredipn@yahoo.com.mx.
Because of its high contents of essential amino acids lysine and tryptophan, opaque-2 (o2) maize (Zea mays L.) is an option for improving human nutrition in social sectors whose diet is based on maize. For this reason, its genetic improvement is relevant. This study analyzed the o2 maize hybrids from the parent lines up to the generation of grain for consumption (F2). The objectives were to determine the contents of tryptophan, lysine and total protein and the quality protein index of the six o2 maize lines, of the 15 single crosses formed with these lines and the F2 generation of these crosses, as well as to observe whether these variables decreased between F1 and F2. The o2 maize lines (M1 to M6) and the 15 single direct crosses formed with these lines were sown in 2011, whereas generation F2 of the crosses and H70 (regional control) were sown the following year. The experimental design was completely randomized with two replications. Contents of lysine, tryptophan and total protein and the quality protein index were determined in harvested grain of each genotype, and differences in these values between F1 and F2 were estimated. Lines M2 and M3 and the crosses M2 x M4, M2 x M6 and M3 x M6 had higher contents of lysine, tryptophan and total protein and a higher quality protein index. Protein quality was not stable in F2, with the exception of M3 x M6, which maintained the ICP in this generation and was the most consistent and stable cross. Inbreeding depression did not affect the crosses with intermediate protein quality (M2 x M5 and M5 x M6) nor did it affect lysine content, which was the experimental variable with the best response in all of the breeding stages.
Key words: essential amino acids; quality protein; inbreeding depression; lysine; opaque2; QPM; tryptophan
El maíz opaco 2 (o2) (Zea mays L.) es una opción para mejorar la nutrición humana en sectores sociales que basan su alimentación en este cereal, por sus elevados contenidos de los aminoácidos esenciales lisina y triptófano, por lo cual su mejoramiento genético es relevante. En este estudio se analizó la obtención de híbridos de maíz o2, desde las líneas parentales hasta la generación de consumo de grano (la F2), Los objetivos fueron determinar el contenido de triptófano, lisina, proteína total e índice de calidad de proteína de seis líneas de maíz o2, de las 15 cruzas simples formadas con ellas y de la generación F2 de esas cruzas, y además observar si esas variables disminuyen al pasar de la F1 a la F2. En el año 2011 se sembraron las líneas de maíz o2 (M1 a M6) y las 15 cruzas simples directas formadas con las líneas anteriores, mientras que la generación F2 de las cruzas anteriores y el H70 (testigo regional) se sembraron al año siguiente. El diseño experimental fue completamente al azar con dos repeticiones. En el grano cosechado de cada genotipo se determinaron los contenidos de lisina, triptófano, proteína total e índice de calidad de proteína y se estimaron las diferencias de estos valores entre F1 y F2. Las líneas M2 y M3 y las cruzas M2 x M4, M2 x M6 y M3 x M6 presentaron los contenidos más altos de lisina, triptófano, proteína total e índice de calidad de proteína. La calidad de proteína no fue estable en la F2, a excepción de M3 x M6 que conservó el índice de calidad de proteína en esta generación y fue la cruza más consistente y estable. La depresión endogámica no incidió sobre las cruzas con calidad intermedia de proteína (M2 x M5 y M5 x M6) y tampoco sobre la lisina, que fue la variable experimental de mejor respuesta en todas las etapas genotécnicas.
Palabras clave: aminoácidos esenciales; calidad de proteína; depresión endogámica; lisina; opaco2; QPM; triptófano
Introduction
Maize grain is the major component of human diets in Latin America and Africa (OCDE/FAO, 2011). In Mexico, it is the main crop, occupying 33 % of the country’s cultivated area (7.5 million ha). In 2013, 22.7 million to of grain was produced, accounting for 18 % of the value of agricultural production (78 billion pesos) (FAOSTAT, 2014); the national mean yield is 3.2 t ha-1. Apparent annual per capita consumption of the grain is estimated at 210 kg (Morris and López, 2000).
Maize is the food of highest consumption and the main source of proteins (39 %) and energy (59 %) (Fernández et al., 2013), particularly for rural and marginalized urban populations (Nuss and Tanumihardjo, 2011). But, its proteins are deficient in lysine and tryptophan, which are essential amino acids for cell metabolism and, because humans cannot synthesize them, they must by supplied by food from animal sources.
Maize endosperm contains 75 % starch and 15 % protein (Manicacci et al., 2009). The latter is stored mainly as zein, which is deficient in lysine and tryptophan (Serna et al., 2008). For this reason, only 65 % of total proteins in consumed maize grain is used (Gerdes et al., 2012).
To contribute to reducing malnutrition and to optimize the food situation, the Centro Internacional de Mejoramiento de Maíz y Trigo (CIMMYT) and the HarvestPlus program promote biofortified crops (Nuss and Tanumihardjo, 2011) and work hand in hand with national programs. Among their objectives is to increase the nutritional value of maize grain with the gene opaque2 (o2).
The mutant gene o2 is expressed in recessive homozygous conditions and determines a higher content of lysine and tryptophan (Mertz et al., 1964; Krivanek et al., 2007). Initially, the high nutritional value of these maize varieties, thus called opaque2, was linked to undesirable characteristics of the kernels, such as soft endosperm, low weight and low resistance to diseases and storage pests (Bjarnason and Vasal, 1992). These characteristics limited their use until, with traditional breeding techniques, genes that modify opaque2 maize endosperm texture were incorporated (Vasal, 1994; Larkins et al., 1994), and maize with characteristics very similar to unmodified genotypes were obtained (Mendoza-E. et al., 2006) and denominated quality protein maize (QPM).
Breeding o2 maize is complex because there are numerous genes that regulate the development of the endosperm (Holding et al., 2008), and only some of them have been identified. Moreover, expression of quality protein is affected by the environment (Landry and Delhaye, 2007), especially by the dosage of fertilization (Gutiérrez et al., 2014). For this reason the denomination QPM implies a genotypic and phenotypic response of the cultivar to production technology (Vázquez et al., 2012). Success of the QPM technology lies in the preservation of its nutritional advantages up to the F2 generation, which is destined to become human and animal food and could be used for seed in the following crop cycle, given the high cost of hybrid seed.
Growers sow F2 seed to establish the crop, and the harvested grain is generation F2, which is for food. For this reason, it is relevant to assure that the attributes of high quality protein is coded in the genome of the parent lines, preserved in F1 and expressed in F2.
There is little information on monitoring protein quality through the different stages of o2 maize hybrid formation, from parent lines to food grain harvest. Therefore, the objectives of this study were 1) to determine the tryptophan, lysine, and total protein contents and quality protein index of six o2 maize lines, of 15 single crosses formed by these lines and of generation F2 of these crosses; and 2) to observe whether these variables decrease from F1 to F2.
Materials and Methods
Field planting
Field planting was carried out in the Campo Experimental Valle de México of the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (CEVAMEX, INIFAP) located in Chapingo, México (19° 29’ N and 98° 53’ W), altitude 2240 m. The climate is temperate with 643 mm of rainfall in summer and average annual temperature of 15.1 °C, and the soil is sand clay.
Lines and F1 crosses were sown on April 27, 2012, and both crops werere managed as described below.
The experimental design was complete randomized blocks with three replications. The genetic material was planted in plots comprising two furrows measuring 5.0x0.80 m, with 26 plants in each row 0.20 m apart. Plant density was 65 thousand plants ha-1 and the dosage of fertilizer (kg ha-1) was N (120), P (60), K (30). Auxiliary irrigation was applied 40, 90 and 120 d after planting (DAS).
Around physiological maturity of the crop (150 DAS), ten plants with complete competition per replication were selected at random. Ears were harvested and shelled manually; the kernels of the replications were mixed and two 100 g samples were taken to be used in the chemical determinations. The kernels, with 10 % moisture, were stored until analysis.
Genetic material
The genetic material was of the following types.
1) Six inbred lines of opaque2 (o2) maize, with white grain and normal textured endosperm (M1 to M6), derived from crossing lines CML 244 (S4), CML 352 (S5), CML 242 (S4), CML 246 (S3), CML 349 (S5), and CML 354 (S3) with the CIMMYT line CML 176 [(P63-12-2-1/P67-5-1-1)-1-2-B-B], which was the donor of the o2 gene. They were then backcrossed toward normal material and self-pollinated. Massive increases were then done to recombine and stabilize them (Table 1).
Table 1 Quality protein maize lines used in the study (CEVAMEX, 2011).
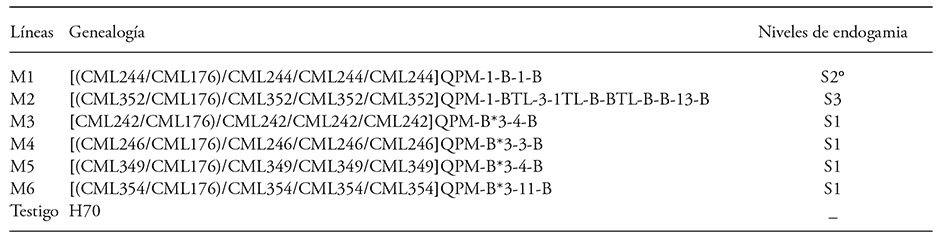
° = Self-pollinations after backcrosses.
2) The 15 single direct crosses formed with the above lines (S1, S2 or S3 plants, depending on their self-pollinations after backcrossing, with F1 embryos in the crop field).
3) The F2 generation of the above crosses (F2 plants with F2 embryos, in the crop field). H70, a commercial hybrid with normal protein and endosperm, was included as the regional control.
The genotypes studied are adapted to prosper in the Central Highland Plateau of Mexico (with altitudes of 2200 to 2600 m), and H70 predominates in this region (Arellano et al., 2011), the reason it was chosen as the regional control.
Determination of tryptophan, lysine and total protein contents and quality protein index
Chemical determinations were done during 2013 in the Molecular Biotechnology Laboratory of the Biotechnology Interdisciplinary Professional Unit of the Instituto Politécnico Nacional (UPIBI, IPN) in Mexico City. The lysine (Lys), tryptophan (Trp) and total protein (TP) contents and quality protein index (ICP) were quantified for each genotype (six lines, 15 F1 crosses and their respective F2), and the two replications of 100 grains mentioned above were used.
The grains were ground and defatted with hexane for 6 h. One hundred milligrams of this flour was used for spectrophotometric determination (Zuzi 4201/20) of the amino acids (Galicia et al. 2009). For Lys, the protocol was based on the reaction of 2-chloro, 3, 5-dinitropyridine (390 nm) and for Trp it was based on that of glyoxylic acid (560 nm).
Total protein was calculated by multiplying the content of total nitrogen, determined following Galicia et al. (2009), by the factor 6.25 (Salinas and Vázquez, 2006). The quality protein index (ICP) was calculated with the formula ICP = [tryptophan (%) x 100] [protein (%)]-1 (Galicia et al. 2009). The results were expressed as percentages, dry weight base, and were averages of the two replications.
Variation in quality protein between generations F 1 and F 2
To estimate the stability of quality protein variables between generations F1 and F2, the differences (%) in the values between the two generations were calculated: F1-F2 = (F1-F2) (100) (F1)-1; where F1 = value of the single cross and F2 = value of the corresponding segregating cross multiplied by (-1) to indicate reduction of the variable (adapted from Hernández-Leal et al., 2013).
Statistical analysis
With the data, an ANOVA was performed. Lysine, Trp and protein contents, and the ICP, between and within genotypes, were compared using the Tukey test (p≤0.05). To evaluate quality protein stability of each cross between their F1 and F2 generations (inbreeding depression), the t test for paired data was used (p≤0.05). All the statistical analyses were carried out with SAS software (SAS Institute, ver. 9.2).
Results and Discussion
Chemical quantifications
Differences (p≤0.01) were found in Trp, Lys, and ICP for F1 and F2 lines and crosses and the regional control (H70) (Table 2). Thus, the genotypes expressed significant variation in the quantified characteristics of protein quality and, therefore, the means obtained in each case were compared (Table 3).
Table 2 Mean squares and statistical significance of the chemical traits evaluated in maize inbred lines, F1 and F2 crosses, and regional control.

**p ≤ 0.01; CV = Coefficient of variation.
Table 3 Averages (% dry base) of tryptophan, lysine and protein contents, and quality protein index in maize inbred lines.

Means with different letters in a column are statistically different (Tukey; p ≤0.05).
Diversity in expression of the evaluated characteristics may be because the genealogy of the six parent lines was different, although all of them shared line CML 176, the donor of the o2 gene. That is, the lines conserved their individuality and high level of inbreeding since only the o2 gene was incorporated and later backcrossed toward their respective unmodified, or normal, material (Table 1).
The lines (Table 3) and the F1 crosses (Table 4) significantly surpassed the regional control (H70) in all of the measurements of protein quality evaluated, especially in essential amino acids and ICP, which are priority chemical traits for quality protein. Therefore, it may be pointed out that the evaluated materials showed large potential for producing hybrids with high protein quality.
Table 4 Averages (% dry base) of tryptophan, lysine and protein contents, and quality protein index in F1 and F2 generations of maize crosses.

Means with different letters in a column are statistically different (Tukey; p≤0.05).
Lines M2 and M3 had the highest contents (p≤0.05) of Trp and PT and the highest ICP, followed by line M6, while M1, M4 and M5 had the lowest performance (Table 3). That is, there was genetic variation among the lines for Trp, PT and ICP, but not for LIS since the content of this amino acid did not vary among lines nor between F1 and F2 crosses. It was thus the experimental variable with the best response in all the study.
The F1 crosses with the highest, most consistent values over measurements were obtained by crossing the lines situated in the first two significant levels (M2, M3, M4 and M6) (Table 3), but the crosses between less prominent lines generated hybrids with fewer quality protein attributes. In both cases, with hybridization, the intrinsic protein quality of the lines was conserved.
The crosses M2 x M5 and M5 x M6 had intermediate, but consistent, performance in generations F1 and F2 (Table 4). For this reason, they are a breeding option for formation of QPM, that is, hybrids with fewer, but more stable, quality protein attributes in F2, which is the generation used for food and that in which expression of the beneficial maize quality protein is sought. The lines described above as superior (M2, M3 and M6) were parents of seven outstanding crosses for PT (p≤0.05), of which only three stood out also in Trp, Lys and ICP. Thus, the crosses consistently superior in all of the variables of the study were M2 x M4, M2 x M6 and M3 x M6 (Table 4).
The crosses mentioned as more favorable for PT than for preponderant factors of high quality protein, such as the essential amino acids and ICP, does not suggest that the proteins of the experimental materials had a good balance of amino acids. This argument coincides with the absence of correlation between protein content and quality protein index, which was reported by Gutiérrez et al. (2014).
Variation in protein quality between generations F 1 and F 2
Examination of quality protein of the crosses passing from generation F1 (controlled pollination) to F2 (random pollination) showed that inbreeding depression was not significant in 70 % of the cases (Table 5). This indicated that, despite random recombination, most of the crosses, in generations F1 and F2, maintained similar contents of the studied variables, mainly Lys and ICP, followed by Trp and finally PT (Table 5). Thus, PT was the chemical component that the crosses maintained with greatest difficulty in the food grain generation (F2).
Table 5 Inbreeding depression (%) of the chemical traits evaluated between the F1 and F2 generations.

°=Student test of paired data. In columns: *p ≤ 0.05; *p ≤ 0.01; ns=Not significant.
The stability of the crosses between the F1 and F2 generations could also be explained by the high level of inbreeding of the parent lines with respect to o2 (as mentioned above), so that recombination of F2 occurred among alleles of the same gene and, consequently, in most of the crosses there was no inbreeding depression in the variables that are determined by the o2 gene, which are Lys, Trp and ICP. For ICP, determination is indirect since it is the proportion of the quantity of Trp present in the grain proteins.
Another outstanding aspect of Lys is that its F1 - F2 differences were positive in 73 % of the crosses (Table 5), meaning that its content increased in the F2 generation and thus there was no inbreeding depression, which did occur with Trp, PT and ICP.
The increases in Lys in F2 would be attributable to the recombination between homozygous lines for o2, with high amino acid content, with which the F2 crosses were obtained. They were also outstanding in the same variable and random pollination favored their Lys accumulation.
The crosses M2 x M4, M2 x M6 and M3 x M6 mentioned above as outstanding for their high significant levels of Trp, Lys, TP and ICP had reduced responses because of inbreeding depression. The exception was M3 x M6 which maintained its ICP with no changes in F2. This cross was the best of the study because it showed high significant values in quality protein in F1 and did not exhibit significant inbreeding depression.
The crosses M2 x M5 and M5 x M6, with intermediate response, were highly stable in F2. They did not exhibit inbreeding depression (p≤0.05) in primordial aspects of quality protein (essential amino acids and ICP). For this reason, they are also valuable hybrids.
Some crosses maintained the level of quality of their lines and others did not, possibly because of their genetic constitution since expression of o2 is regulated by the genotype of the inbred line into which it was introduced (Gutiérrez-Rojas et al., 2008). Moreover, there are genes that modify development of the endosperm (Holding et al., 2008) and should vary with genotype.
Thus, there were different genotypic responses for inbreeding depression. Crosses with superior quality protein were more susceptible than allele combinations with lower quality attributes. Random genetic recombination had a favorable repercussion on the Lys variable and diminished amino acid content and ICP.
Obtaining o2 hybrids is a complex, but viable, process for which it is desirable to evaluate and characterize experimental germplasm based on the amount of essential amino acids and especially on ICP and stability of the manifestation of the virtues of o2 into generation F2. In addition, the environment and the production technology level it requires should be defined. It is also possible to integrate the above elements using mathematical models that contribute to knowledge of the process.
Conclusions
Lines M2 and M3 and the crosses M2 x M4, M2 x M6 and M3 x M6 had the highest contents of lysine, tryptophan, and total protein, as well as the highest quality protein index. The quality of the protein of these crosses was not stable in F2, with the exception of M3 x M6, which was the most consistent and stable cross.
Inbreeding depression did not affect crosses of intermediate quality protein or the lysine content, which was the experimental variable with the best response in all of the breeding stages.
Acknowledgements
We would like to express our gratitude to the participating institutions of the Interdisciplinary Maize Research Group (GIIM): INIFAP, CIMMYT, UNAM and IPN
REFERENCES
Arellano V., J. L., J. Virgen V., M. Á. Ávila P., e I. Rojas M. 2011. H-70 Híbrido de maíz de alto rendimiento para áreas de temporal y riego del altiplano central de México. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). Campo Experimental Valle de México (CEVAMEX). México, D.F. Ficha Téc. 13. 34 p. [ Links ]
Bjarnason M., S, and K. Vasal. 1992. Breeding of quality protein maize (QPM). In: Jules. J. (ed). Plant Breeding Reviews Vol. 9. Purdue University. John Wiley and Sons. pp: 181-210. [ Links ]
Fernández S., R., L. A. Morales C., y A. Gálvez M. 2013. Importancia de los maíces nativos de México en la dieta nacional. Una revisión indispensable. Rev. Fitotec. Mex. 36 Supl. 3-A: 275-283. [ Links ]
Galicia, L., E. Nurit , A. Rosales, and N.Palacios R. 2009. Laboratory protocols: Maize nutrition quality and plant tissue analysis laboratory. CIMMYT. El Batán, Texcoco, México. 42 p. [ Links ]
Gerdes, S. et al. 2012. Plant B vitamin pathways and their compartmentation: A guide for the perplexed. J. Exp. Bot. 63: 5379-5395. [ Links ]
Gutiérrez H., G. F. et al. 2014. Formación de híbridos de maíz con calidad proteica: lisina, triptófano e índice de calidad. Rev. Fac. Agron. 31: 171-189. [ Links ]
Gutiérrez-Rojas A., M. Paul-Scott, O. R. Leyva, M. Menz, and J. Beltrán. 2008. Phenotypic characterization of quality protein maize endosperm modification and amino acid contents in a segregating recombinant inbred population. Crop Sci. 48:1714-1722. [ Links ]
Hernández-Leal, E., R. Lobato-Ortiz, J. J. García-Zavala, D. Reyes-López, A. Méndez-López, O. Bonilla-Barrientos, y A. Hernández-Bautista. 2013. Comportamiento agronómico de poblaciones F2 de híbridos de tomate (Solanum lycopersicum L.). Rev. Fitotec. Mex . 36: 209-215. [ Links ]
Holding D. R., B. G. Hunter, T. Chung, B. C. Gibbon, C. F. Ford, A. K. Bharti, J. Messing, B. R. Hamaker, and B. A. Larkins. 2008. Genetic analysis of opaque-2 modifier loci in quality protein maize. Theor. Appl. Genet. 117: 157-170. [ Links ]
KrivanekA.F., H.De Groote, N.S. Gunaratna, A.O. Diallo, and D.Friesen. 2007. Breeding and disseminating quality protein maize (QPM) for Africa. Afr. J. Biotechnol.: 6: 312-324. [ Links ]
Landry J, and S. Delhaye 2007. Influence of genotype and texture on zein content in endosperm of maize grains. Ann. Appl. Biol. 151: 349-356. [ Links ]
Manicacci, D. et al. 2009. Epistatic interactions between O2 transcriptional activator and its target gene CyPzPDK1 control kernel trait variation in maize. Plant Physiol. 150: 506-520. [ Links ]
Mendoza-E., M. et al. 2006. Contenido de lisina y triptófano en genotipos de maíz de alta calidad proteica y normal. Universidad y Ciencia 22: 153-161. [ Links ]
Mertz E. T., L. S. Bates, and O. E. Nelson. 1964. Mutant gene that changes protein composition and increases lysine content of maize endosperm. Science 145: 279-280. [ Links ]
Morris M. L., y M. A. López P. 2000. Impactos del mejoramiento de maíz en América Latina 1996 - 1997. CIMMYT. El Batán, Texcoco, México. 45 p. [ Links ]
Nuss, E. T., and S. A. Tanumihardjo. 2011. Quality protein for Africa: Closing the protein inadequacy gap in vulnerable populations. Am. Soc. Nutr. 2: 217-224. [ Links ]
Organización de las Naciones Unidas para la Agricultura y la Alimentación. Div. Estadísticas (FAOSTAT). 2014. http://faostat3.fao.org (Consulta: abril 2016). [ Links ]
Organización para la Cooperación y el Desarrollo Económico y Organización de las Naciones Unidas para la Agricultura y la Alimentación (OCDE/FAO). 2011. Perspectivas Agrícolas 2011-2020. OECD Publishing/FAO. 227 p. [ Links ]
Salinas M., Y., y G. Vázquez C. 2006. Metodologías de análisis de la calidad nixtamalera-tortillera en maíz. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). Campo Experimental Valle de México (CEVAMEX). México D. F. Ficha Téc. 23. 91 p. [ Links ]
Serna S., S. O., C. A. Amaya G., P. Herrera M., J. L. Melesio C., R. E. Preciado O., A. D. Terrón I., and G. Vázquez C. 2008. Evaluation of the time-cooking and tortilla making properties of quality protein maize hybrids grown in Mexico. P. Food Human Nutr. 63: 119-125. [ Links ]
Statistical Analysis System (SAS). 2002. SAS/STAT Ver. 9. SAS Inst. Inc. Cary NC, USA. [ Links ]
Vázquez C., G. M., H. Mejía A., C. Tout C., y N. Gómez M. 2012. Características de granos y tortillas de maíces de alta calidad proteínica desarrollados para los valles altos centrales de México. Rev. Fitotec. Mex . 35: 23-31. [ Links ]
Received: January 2016; Accepted: July 2016











 text in
text in 


