Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Agrociencia
On-line version ISSN 2521-9766Print version ISSN 1405-3195
Agrociencia vol.50 n.6 Texcoco Aug./Sep. 2016
Animal Science
Biomass of Urochloa brizantha cv. Toledo as raw material for bioethanol production
1Postgrado en Recursos Genéticos y Productividad-Ganadería, Colegio de Postgraduados, Campus Montecillo. Km. 36.5 Carretera Federal México-Texcoco. 56230. Montecillo, Texcoco, Estado de México. México. (hernan@colpos.mx).
2Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, Campo Experimental, San Martinito. Km 56.5 Carretera Federal México-Puebla, San Martinito, Tlahuapan, 74100 Puebla, México. (honorato.amador@inifap.gob.mx).
3Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Campo Experimental, La Posta. Km. 22.5 Carretera Veracruz-Córdoba, Col. Paso del Toro, Medellín de Bravo, 94277, Veracruz, México.
Evaluation of fast-growing and high biomass-yieldinggrasses has increased worldwide because they are potential energy crops for the production of second-generation bioethanol. In Mexico, studies on high biomass-producing grasses have begun with the aim of using them for biofuel production. The objective of this study was to evaluate the biomass of Urochloa brizantha cv. Toledo as raw material for bioethanol production. Yearly biomass production, calorific power, chemical composition, immediate analysis and theoretical bioethanol yield were determined for six cutting frequencies (30, 60, 90, 120, 150 and 180 d after regrowth). The data were analyzed with GLM (SAS) and treatments means were compared with the Tukey test (p≤0.05). The highest content of hemicellulose and cellulose (p≤0.05) was 30.1 and 44.1 % at the frequencies of 60 and 150 d. The lowest content of lignin (p≤0.05) was 16.2 % at 60 d. The highest contents of ash and protein (p≤0.05) was 9 and 8 % at 30-d. Calorific power did not differ (p≤0.05) between cuttings. With 180 days cutting frequency, the highest quantity (p≤0.05) of biomass (27.95 Mg ha-1 year-1), the largest percentage of holocellulose (73.6 %) and the highest yield of bioethanol (281.3 L Mg dry matter) accumulated. The chemical characteristics and yield of biomass underline the potential of U. brizantha for production of bioethanol in tropical areas.
Key words: Urochloa brizantha; bioethanol; chemical composition; immediate analysis; calorific power
La evaluación de pastos de crecimiento rápido y rendimiento alto de biomasa ha aumentado en el mundo porque son cultivos energéticos potenciales para producir bioetanol de segunda generación. En México se inician estudios en pastos con producción alta de biomasa para usarlos en la producción de biocombustibles. El objetivo de este estudio fue evaluar la biomasa de Urochloa brizantha cv. Toledo, como materia prima para la producción de bioetanol. La producción anual de biomasa, el poder calorífico, la composición química, el análisis inmediato y el rendimiento teórico de bioetanol se determinaron en seis frecuencias de corte (30, 60, 90, 120, 150 y 180 d después del rebrote). Los datos se analizaron mediante el procedimiento GLM (SAS) y las medias de los tratamientos se compararon con la prueba de Tukey (p≤0.05). El contenido mayor de hemicelulosas y celulosa (p≤0.05) fue 30.1 y 44.1 % a los 60 y 150 d del corte. El contenido menor de lignina (p≤0.05) fue 16.2 % a los 60 d del corte. El contenido mayor de cenizas y proteína (p≤0.05) fue 9 y 8%,y a los 30 d del corte. El poder calorífico no difirió entre frecuencias de corte (p≤0.05). Con 180 d de frecuencia de corte se acumuló cantidad mayor (p≤0.05) de biomasa (27.95 Mg ha-1 año-1), el porcentaje mayor de holocelulosa (73.6 %) y el rendimiento mayor de bioetanol (281.3 L Mg materia seca). Las características químicas y el rendimiento de biomasa destacan el potencial de U. brizantha para producir bioetanol en áreas tropicales.
Palabras clave: Urochloa brizantha; bioetanol; composición química; análisis inmediato; poder calorífico
Introduction
In recent decades, different sources of alternative energy have been researched aiming to gradually substitute fossil fuels. Today, interest in biofuels, especially for use in the transportation sector, is due to four factors: 1) safety in supplying energy, 2) decrease in hydrocarbon reserves, 3) volatility in oil prices, and 4) mitigation of the effects of climate change.
First-generation biofuels produced from maize and sugarcane drove food prices up in 2007 and 2008. This caused economic instability and debate over the role of biofuels in the food crisis. Thus, biofuels from lignocellulosic biomass emerged as an alternative using forest and farm residues, such as sugarcane bagasse, maize cobs and stalks, wheat and rice straw; industrial waste such as pulp and paper processing waste; solid urban residues; besides, pasture crops and fast-growing trees.
Lignocellulosic biomass is a dense matrix constituted mainly of cellulose, hemicellulose and lignin (Demirbas, 2004). Cellulose is a linear chain of glucose units linked by β(1"4) bonds. Hemicelluloses are mainly five sugars: glucose, galactose, mannose, xylose, and arabinose. Lignin is made up of phenolic compounds that act as inhibitors of sugar fermentation (Robinson et al., 2002).
In Mexico, there is potential for the production of bioethanol from lignocellulosic material. However, there are still technical and economic limitations that impede efficient bioconversion of biomass into bioethanol. Ethanol yields from lignocellulosic biomass can be between 0.11 and 0.27 m3 Mg DM-1 (ORNL, 2006).
Ethanol is used as the chemical raw material or as an octane promotor of gasolines. Mixtures of ethanol:gasoline (v:v) were produced in proportions of 8:92 (Hernández-Salas et al., 2009). Bioethanol is combined with 10 % lead-free gasoline (E10) and is used in unmodified internal combustion engines. With the E10 mixture, the maximum permitted amount of ethanol is defined for unmodified motors, because of automobile manufacturer warranties (Yacobucci, 2010). In Mexico, a law on bioenergetics was enacted in 2008. This law foments the use of bioethanol in domestic gasoline to decrease the use of oxygenating agents such as methyl tertbutyl ether, which is harmful because it is a stable, soluble compound that pollutes ground water (Nava et al., 2007). Currently, biofuels are considered a complementary energy source in the supply of energy for the transportation and electricity sectors.
Some fast-growing grasses were evaluated as raw material for second-generation bioethanol production. The three main species are Panicum virgatum (Sarath et al., 2008; Schmer et al., 2008), Pennisetum purpureum (Anderson et al., 2008) and Miscanthus giganteus (Murnen et al., 2007).
In Mexico, evaluation of fast-growing grasses is initiating in order to determine their potential as sources of raw material for bioethanol production. The objective of this study was to evaluate the potential of Urochloa brizantha (Sin. Brachiaria brizantha) cv. Toledo at six cutting frequencies for bioethanol production in tropical areas.
Materials and methods
Study area
Biomass yield was evaluated in the Papaloapan Experimental Site of INIFAP (18° 06’ N, 95° 31’ W and 65 m altitude), in Ciudad Isla, Veracruz, Mexico. Climate is Awo and mean temperature 25.7 °C (García, 1988). Soil is orthic acrisol, sandy loam, pH 4 to 4.7, poor in organic matter, nitrogen, calcium, and potassium, and medium to high contents of phosphorus and magnesium (Enríquez and Romero, 1999). Physical-chemical determinations of forage dry matter (DM) were done in the soil fertility and environmental chemistry laboratory of the Colegio de Postgraduados, Campus Montecillo. The variables evaluated were biomass yield, extractives, holocellulose, cellulose, lignin, N and calorific power. In addition, immediate analysis was carried out for moisture, fixed carbon, volatile matter and ash.
Plot setup
Grass was sown on July 22, 2011, in rows 0.50 m apart in experimental plots 5 m wide by 15 m long, and replicated three times. The fertilizer formula 120-80-00 kg ha-1 N and P2O5 was applied twice, 43 and 112 d after sowing.
Biomass yield (DM ha -1 )
Biomass accumulation was determined per unit of area for each cutting frequency: every 30, 60, 90, 120, 150 and 180 d after the uniformity cut (DAUC) in destructive samples per year. On five occasions, a square metal frame (7 m2) was tossed at random into each plot and all of the forage (intact plant) was cut; the remainder was left 20 cm tall for the plant to recover. The harvested biomass was weighed on a precision balance (Ohaus, Mod. GT-4000; 6.200 kg 0.1 g). The weight of a subsample was recorded. The subsample was then dehydrated in a forced air oven (Felisa, Mod. FE-243A), at 55 °C, until constant weight and DM was calculated.
Sample preparation for analyses
The dried samples were pulverized in a mill (Wiley; PA, USA) and sifted through No. 40 (0.42-1.00 mm) and No. 60 (0.25-0.42 mm) meshes. After this, chemical determinations, immediate analysis and calorific power calculations were performed.
Chemical composition
TAPPI T-204 (TAPPI, 2007) norm was used for release of the extractives. Holocellulose content was determined by the acid chlorination method (Rowell et al., 2005) and cellulose with the procedure of the ASTM D1103 norm (ASTM, 1977). Hemicelluloses were obtained after extracting the cellulose from holocellulose. The lignin was determined according to the TAPPI T-222 os-74 (TAPPI, 2007) norm, and nitrogen content by the semi-micro Kjeldahl method (AOAC-984.13; 1990). Six determinations were performed for each cutting frequency in two replications per sample.
Immediate analysis
To determine humidity, the method included in the ASTM E871 norm was used; volatile material was determined following ASTM E872 norm, and ash according to ASTM D 1102-84 (ASTM, 2012). Fixed carbon was calculated with the formula CF 100 (moisture ash volatile material). Three replications were evaluated per sample and nine per cutting frequency.
Calorific power
Calorific power was determined in a calorimeter adiabatic bomb (Isoperibol, Parr 1266), following the standard ASTM (E711), at 30 0.5 °C, with tablets of maximum 1 g. Moisture content was determined in a thermal balance Ohaus MB45. Five determinations per sample were analyzed and 15 replications per cutting frequency.
Theoretical bioethanol yield
Theoretical bioethanol yield was determined based on reactions of hydrolysis and of transformation of sugars into ethanol, with their respective chemical equations and reaction stoichiometry of cellulose and hemicelluloses, and the following formulas (Badger, 2002; Dien, 2010):
For cellulose
For hemicelluloses
Total
where: RET: L Mg-1 DM; C: kg cellulose Mg-1 biomass, H: kg hemicelluloses Mg-1 biomass, C g/c : concentration of glucose (1.111 kg glucose kg-1 cellulose), H x/h : concentration of xylose (1.136 kg xylose kg-1 hemicelluloses), Ecc: efficiency of cellulose conversion (0.76), Ech: efficiency of hemicellulose conversion (0.90), Ret: stoichiometric yield of ethanol (0.511 kg ethanol kg-1 glucose; 0.511 kg ethanol kg-1 xylose), Efg: efficiency of glucose fermentation (0.75), Efx: efficiency of xylose fermentation (0.50), Det: ethanol density (0.78 Mg m3).
Moreover, the theoretical yearly amount of bioethanol (L) that 1 ha of Toledo produces was calculated for each cutting frequency. The procedure consisted of multiplying theoretical yield of bioethanol per unit of biomass by annual biomass yield.
Statistical analysis
The experimental design was randomized blocks with split plots: whole plot was the genotype and subplot was the cutting frequency (30, 60, 90, 120, 150 and 180 d), with three replications. An ANOVA was carried out using the GLM procedure and means were compared with the Tukey test (p≤0.05). The data were analyzed to estimate the effect of cutting frequency using SAS for Windows version 9 (SAS Institute, 2002).
Results and discussion
Biomass yield
Differences (p≤0.05) were observed in annual biomass yield and cutting frequency. Production of biomass increased as cutting interval increased. The exception was with cutting every 150 d, with which the amount of biomass decreased 21.9 %, relative to the cutting frequency of 120 d. Production of biomass was 28 Mg ha-1 year-1 with the frequency of 180 d (Table 1). With cutting every 30 d, biomass production was lower (11.12 Mg ha-1 year-1). Cab et al. (2007) obtained 16.8 Mg ha-1 with Toledo grass cut every 32 d. Switchgrass (Panicum virgatum) is studied with energy goals and is suitable raw material because of its high biomass yields: 17.3 Mg ha-1 (Parrish and Fike, 2005) to 37.6 Mg ha-1 (Thomason et al., 2004). Miscanthus sinensis hybrids are also considered suitable for energy production. In northern Europe, it yields 25 Mg ha-1 and, in central and southern Europe, can yield 38 Mg ha-1 (Lewandowski et al., 2003). The increase in the proportion of cell wall with age of the grass may be the main cause of increased DM, and this is affected by factors such as availability of water and fertilization, root development, time of year, pests and diseases.
Table 1 Biomass yield and theoretical production of bioethanol from Urochloa brizantha cv. Toledo grass at six harvest frequencies.
| Frecuencias de cosecha (días) | Rendimiento (Mg ha -1 año -1 ) | Poder calorífico (MJ kg -1 ) | Producción de energía (GJ ha -1 año -1 ) | Humedad (%) |
|---|---|---|---|---|
| 30 | 11.1f | 16.37a | 182.2f | 8.40c |
| 60 | 13.7e | 16.33a | 224.2e | 7.40c |
| 90 | 18.4c | 16.64a | 307.2c | 8.20a |
| 120 | 20.7b | 16.37a | 340.1b | 7.90c |
| 150 | 16.2d* | 16.72a | 271.5d | 6.80bc |
| 180 | 28.0a | 16.69a | 466.6a | 7.70ab |
| Media | 18.40 | 16.52 | 298.7 | 7.79 |
| EE | 2.40 | 0.07 | 40.70 | 0.19 |
EE: Standard error. Different letters indicate differences between cuttings (Tukey, p≤0.05). *Two cuttings 150 d apart.
Biomass yield of the variety Toledo is lower than that of Miscanthus and Switchgrass, but production conditions and growing site affect biomass yield. For this reason, the information from different studies is useful even when results are modified by environment.
Calorific power and moisture content
High moisture reduces combustion efficiency because large part of the heat released is used to evaporate water, instead of chemically reducing the material (Hakkila, 1989). Moisture content was different (p≤0.05) among cutting frequencies (Table 1).
Calorific power per unit of mass is the variable that determines the energy available in the biomass (Godin et al., 2013), and there were no differences among cutting frequencies (p>0.05) (Table 1). Its interval was 16.3 MJ kg-1 to 16.7 MJ kg-1. In our study, calorific power did not exhibit a direct relationship with cutting frequency, the opposite of what is observed in woody species. Firewood, the most used material for generating energy, has a calorific power of approximately 20 MJ kg-1 . Values found for Switchgrass are 18.1 MJ kg-1 (Jenkins et al., 1998) and 17.4 MJ kg-1 (Mckendry, 2002). The value of pinewoods is 20.5 MJ kg-1 and that of wood from broadleaf trees is 20.2 MJ kg-1 (CEN, 2005). The average calorific power of Toledo is 16.52 MJ kg-1, making it inferior to wood and other grasses.
The highest annual production of energy (466.56 gigajoules; GJ) per hectare of Toledo grass was achieved by harvesting every 180 d (two cuts per year) (Table 1). Annual yields with eucalyptus (Eucalyptus spp.) in Brazil (24 Mg ha-1) and annual energy per hectare (448.52 GJ) (Roger et al., 2011) were lower than those obtained with Toledo grass.
Chemical composition
Total extractives
Chemical composition enables definition of pretreatment, hydrolysis and fermentation methodology. The extractives function as metabolic intermediaries, energy reserves, or form part of mechanisms of protection; they are responsible for color, odor and resistance to plant wilting (Olanders and Steenari, 1995). Differences (p≤0.05) in frequencies of these components were observed (7.7 to 11.8 % in 90 and 180 d). Frequency in Maralfalfa (Pennisetum glaucum Pennisetum purpureum), Bermuda (Cynodon dactylon) and King (Pennisetum hybrids) grass was 10.7, 5.5 and 16.9 % (Lee et al., 2009; Mateus et al., 2012; Cardona, 2013; Table 2). Rowell et al. (2005) report 8 and 9 % in pinewoods and broadleaf wood. The quantity of extractives depends on the physiology and morphology of the plant and environmental conditions. Extractives cannot be transformed into ethanol, and so the lignocellulose biomass with higher quantity of extractives will cause lower ethanol yield.
Table 2 Chemical composition of Urochloa brizantha cv. Toledo grass at six harvesting frequencies.
| Frecuencia de cosecha (días) | Componente (%) | |||||
|---|---|---|---|---|---|---|
| Holocelulosa† | Celulosa† | Hemicelulosas† | Lignina† | Extractivos | Proteína | |
| 30 | 69.5cd | 39.4d | 30.1a | 16.8c | 10.1bc | 8.2a |
| 60 | 71.3cd | 41.3c | 30.0a | 16.2c | 10.8abc | 4.8b |
| 90 | 68.5d | 41.7c | 26.7c | 19.5a | 7.7d | 4.3bc |
| 120 | 71.5b | 42.6bc | 28.9ab | 16.8c | 9.4c | 4.3bc |
| 150 | 72.7ab | 44.1a | 28.5b | 17.1bc | 11.5ab | 2.4c |
| 180 | 73.6a | 43.7ab | 29.9ab | 18.3ab | 11.8a | 2.8c |
| Media | 71.1 | 42.1 | 29.0 | 17.4 | 10.2 | 4.5 |
| EE | 0.78 | 0.70 | 0.53 | 0.49 | 0.61 | 0.83 |
EE: Standard error. Different letters indicate differences between cuttings (Tukey, p≤0.05). †Values based on moisture-and extractive-free weight.
Holocellulose
The combination of cellulose and hemicelluloses makes up holocellulose. Between 60 and 80 % of the lignocellulosic biomass in a cell could be an energy source. Theoretically, a high content of carbohydrates means larger amounts of bioethanol. The quantity of holocellulose was different (p≤0.05) among cutting frequencies (Table 2) and the interval of values was 68.5 to 73.6 % (90 and 180 dauc). The lowest quantities were obtained with the frequencies of 30, 60 and 90 d. The quantity of holocellulose increased with the interval between cuttings up to a high of 73.6 % with the frequency of 180 days. At this cutting interval, the highest quantity of sugars for production of bioethanol was detected.
Holocellulose values for other C4 grass species are the following: 56.3 % Maralfalfa, 53.5 and 76.4 % Switchgrass, 59.7 % Bermuda and 76.5 % Miscanthus spp. (Sun and Cheng, 2002; Lee et al., 2009; Xu et al., 2010; Brosse et al., 2012; Mateus et al., 2012). The highest quantity of holocellulose in Toledo grass was 73.6 %, which was lower than Miscanthus, similar to Switchgrass and higher than Maralfalfa and Bermuda. The composition and percentage of polymers vary among plant species, growth stage and age.
Cellulose
Cellulose hydrolysis by cellulolytic enzymes or chemically with sulfuric acid or other acids produces glucose (Sues et al., 2005) and constitutes the main source of available glucose for fermentation. The content of cellulose was different (p≤0.05) among cutting frequencies. The lowest and the highest percentage corresponded to every 30 (39.4 %) and 150 days (44.1 %) (Table 2). The increase in cellulose with age of the grass is due to thickening of the secondary cell wall. The contents of cellulose in Switchgrass is 31 to 45 % (Sun and Cheng, 2002; Carroll and Somerville, 2009). Grasses contain between 25 and 40 % cellulose (Sun and Cheng, 2002), while broadleaf woods contain 40 to 44 % and conifers 45 to 50 % (Betts et al., 1991). The cellulose content of Toledo grass was lower than that of wood and higher than that of Switchgrass, Miscanthus and Maralfalfa.
Hemicelluloses
Hemicelluloses are also sources of carbohydrates in the lignocellulosic biomass. They are ramified pentose and hexose chains, which can potentially be hydrolyzed to sugars, such as xylose, arabinose, galactose, glucose and mannose. The hemicellulose contents were different (p≤0.05) among cutting frequencies. The lowest contents (26.7 and 28.5 %) were obtained with the frequencies of 90 and 150 days (Table 2). The highest proportion (30 %) was with frequencies of 30 and 60 d.
The average hemicellulose contents of the frequencies was 29 %. Sun and Cheng (2002) report 31.4 % hemicellulose in Swithchgrass and 35.7 % in Bermuda. Brosse (2012) obtained 30.3 % in Miscanthus spp. and in Maralfalfa 22.54 % (Mateus et al., 2012). In Toledo grass, the content was similar to that in Switchgrass and Miscanthus, lower than in Bermuda and higher than in Maralfalfa. Hemicellulose contents are homogeneous among grasses. Nevertheless, characterization of the sugars in each species is necessary to identify the enzymes suitable for increasing the efficiency of the fermentation process and bioethanol yield.
Lignin
The differences in lignin content among cutting frequencies were significant (p≤0.05) (Table 3). The values between 16.2 and 18.3 % had a variability of only 2.1 %, indicating that lignification is notably homogeneous among harvesting ages. The content of lignin in Switchgrass, Bermuda and Miscanthus is 15 and 28 % (Lee et al., 2009; Hodgson et al., 2010; Zhang et al., 2012), while in Maralfalfa (Pennisetum glaucum Pennisetum purpureum) it is 22.54 %. McKendry (2002) found 27 to 30 % in pinewoods and 20 to 25 % in broadleaf woods. The quantity of lignin in Toledo grass is lower than that in Switchgrass, Bermuda, Maralfalfa and woods, and is similar to that in Miscanthus. Lignin does not ferment to ethanol and its presence negatively affects fermentation. Therefore, a viable process for obtaining ethanol from lignocellulose should extract the lignin and use it for other products.
Table 3 Immediate analysis of Urochloa brizantha cv. Toledo grass at six harvesting frequencies.
| Frecuencia de cosechas) | Componente (%) | |||
|---|---|---|---|---|
| Humedad | Cenizas | Carbono fijo | Materia volátil | |
| 30 | 8.4a | 9.0a | 17.2ab | 73.6d |
| 60 | 7.9a | 8.1b | 16.6ab | 75.1c |
| 90 | 8.1a | 8.1b | 17.8a | 73.9cd |
| 120 | 8.1a | 6.4c | 16.8ab | 76.7b |
| 150 | 7.1b | 5.2d | 16.3b | 78.4a |
| 180 | 7.8a | 5.3d | 16.3b | 78.2a |
| Media | 7.9 | 7.0 | 16.8 | 75.9 |
| EE | 0.18 | 0.65 | 0.23 | 0.85 |
EE: Stan dard error. Different letters indicate differences between cuttings (Tukey, p≤0.05).
Protein
The amount of protein was different (p≤0.05) among cutting frequencies; as the plant matured, the content decreased. The highest content (8.2 %) was found in grass cut every 30 d (Table 2). Rincón et al. (2008) report 11.2 % crude protein in Toledo grass 28 d after regrowth. This value was higher than those of our study. Chacón-Hernández and Vargas-Rodríguez (2009) show 9.5, 8.7 and 8.4 % crude protein in King grass (Pennisetum purpureum) cut at: 60, 75 and 90 d. The three values were higher than those of Toledo grass in our study. The more protein in the biomass, the lower the bioconversion of total sugars into bioethanol because the proteins of the cell wall form bridges with ferulic acid and increase resistance and insolubility of the entire structure (Bidlack et al., 1992).
Immediate analysis
Immediate analysis indicates the chemical energy stored in the biomass in the form of volatile matter and fixed carbon. The analysis consists of separating the compounds of the fuel depending on their volatility by gradual heating and it provides the contents of moisture, ash, volatile matter and fixed carbon.
Moisture
Most processes of energy conversion require that the biomass contain moisture below 30 % (Valverde et al., 2007). In Toledo grass, moisture accounts for 7.1 to 8.4 % (Table 3). The value at 150 d (87.1 %) was different (p≤0.05) among cutting frequencies. Moisture of 11.5, 13 and 15, 20 and 6 % has been documented for Miscanthus, Switchgrass, wood and straw (McKendry, 2002). Moisture facilitates the formation of hydrogen but reduces thermal efficiency.
Ash
Ash content decreased with grass maturity and changed (p≤0.05) between cutting frequencies (Table 3). The highest content (9 %) corresponded to the frequency of 30 d and the lowest contents (5.2 and 5.3 %) to that of 150 and 180 d. The values determined in other grasses are similar to those of our study: 3.8 % Switchgrass, 4.8 % Bermuda and 2.5 % Maralfalfa (Lee et al., 2009; Xu et al., 2010; Mateus et al., 2012). These values contrast with those obtained by Chacón-Hernández and Vargas-Rodríguez (2009) in King grass (Pennisetum purpureum), at 60, 75 and 90 d after regrowth (14.5, 13.8 and 13.6 %).
Ash content differs among species, has no energy value and is higher in farm residues. Herbaceous plants have higher ash and silica contents than woods. In the process of combustion, it is necessary to know the ash concentration to improve efficiency of the operation and reduce costs associated with production of waste in the boilers or heaters. Alternatively, the ashes can be used to remedy acid soils since they are completely harmless.
Volatile matter
During initial combustion, CO, CO2, H2 and other gases derived from volatile compounds are released through an exothermic process that aids combustion (Lewandowski and Kicherer, 1997). Amounts of volatile matter were different (p≤0.05) among cutting frequencies. The highest percentages (78.4 and 78.2 %) were found for frequencies of 150 and 180 d; the lowest (73.6 %) were for to the 30 d frequency (Table 3). The amount of volatile matter in Miscanthus was 66.8 %, in cereal straws 79 %, in forest residues 79.8 %, in Switchgrass 76.6 %, and in sugarcane bagasse 83.6 % (McKendry, 2002; Vamvuka et al., 2003; Yin, 2011). The content of volatile matter in Toledo grass was similar to that reported for Switchgrass, cereal straw and forest residues; it was higher than in Miscanthus and lower than in sugarcane bagasse. Volatile matter content indicates reactivity, ease of ignition and rate of combustion of the biomass.
Fixed carbon
Fixed carbon is the residual mass after release of volatile compounds, excluding ash and moisture. It is indicative of partially burned combustible material in the ashes. The amount of fixed carbon among the cutting frequencies was different (p≤0.05) (Table 3). Fixed carbon accounted for 16.3 to 17.8 % and cutting frequencies of 150 and 180 days had lower quantities (16.3 %). McKendry (2002) quantified 15.9 and 21 % fixed carbon in Miscanthus and wheat straw, and Vamvuka et al. (2003) found 20 % in forest residues. The percentage of fixed carbon in Toledo grass was lower than that of wheat straw and forest residues and similar to that of Miscanthus. Fixed carbon is directly related to the mass of lignin because it is notably resistant to thermic decomposition. Higher quantities of fixed carbon cause lower bioethanol yields.
Theoretical bioethanol yield
Based on chemical reactions, the maximum theoretical yield of bioethanol is 0.51 kg and 0.49 kg carbon dioxide per kg of glucose or xylose (Balat et al., 2008). Stoichiometric calculations have determined that hydrolysis of cellulose and hemicelluloses to fermentable sugars is 76 and 90 %, and conversion efficiency of glucose to ethanol is 75 %, while that of xylose to ethanol is 50 % (Badger, 2002).
Cellulose and hemicellulose contents determine theoretical bioethanol yield per unit of biomass. The highest bioethanol yields per unit of biomass were 281.3 and 278.6 L Mg DM-1, for the 150and 180day frequencies. The estimated interval was between 262.6 and 281.3 L Mg DM-1 (Table 4). Annual bioethanol yield is directly related to harvested biomass. The maximum annual yield of bioethanol (7865.1 L Mg-1year-1) was at 180 d and different (p≤0.05) from the other cutting frequencies. Cutting every 30 d resulted in lowest production (2935.6 L Mg-1year-1).
Table 4 Theoretical yield of bioethanol from Urochloa brizantha cv. Toledo grass at six harvest frequencies.
| Frecuencia de cosecha (Días) | Producción de bioetanol | |
|---|---|---|
| L Mg-MS -1 | L ha -1 año -1 | |
| 30 | 264.3cd | 2935.6e |
| 60 | 271.6bc | 3720.0de |
| 90 | 262.6d | 4858.3bc |
| 120 | 273.1b | 5677.0b |
| 150 | 278.6ab | 4517.8cd |
| 180 | 281.3a | 7865.1a |
| Media | 271.9 | 4928.9 |
| EE | 3.04 | 702.1 |
EE: Standard error. Different letters indicate differences between cuttings (Tukey, p≤0.05).
Differences in bioethanol yield among cutting frequencies evidenced the inconvenience of harvesting at early stages of the crop since the annual yield of biomass and of bioethanol is low. Moreover, the cost of harvesting increases when cutting is more frequent during the year.
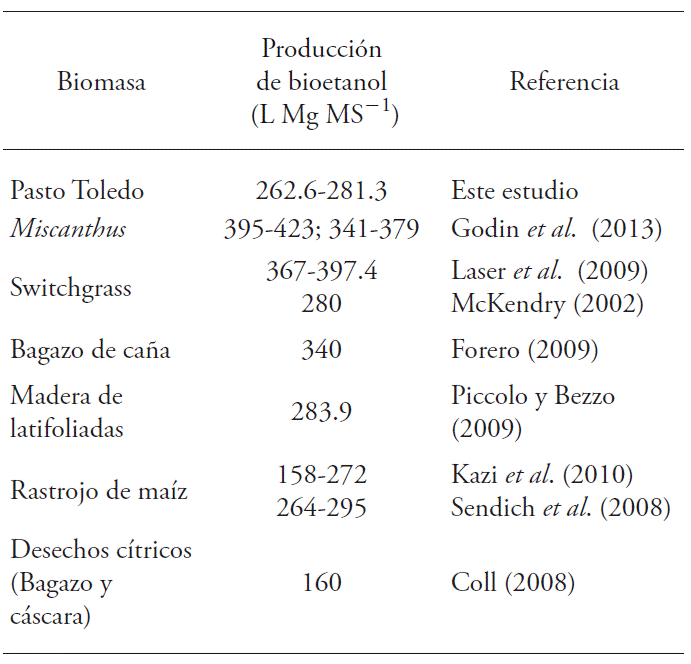
McKendry (2002) indicate that by enzyme hydrolysis up to 280 L Mg DM-1 ethanol can be obtained from Switchgrass and 205 L Mg DM-1 from wood. Bioethanol yield from Toledo grass (Table 5) is between these values and those obtained with maize stalks (Sendich et al., 2008). In contrast, yields above 340 L Mg DM-1 are reported for Miscanthus (Godin et al., 2013) and Switchgrass (Laser et al., 2009). The difference in theoretical bioethanol yield is due mainly to the efficiency values used in the calculations, mainly in hydrolysis and fermentation (Spatari et al., 2010), and in dry biomass yields and cellulose and hemicellulose contents (Godin et al., 2013).
Conclusions
Yield of U. brizantha cv. Toledo increases with the length of the interval between cuttings. Chemical composition also changes: total carbohydrates (holocellulose) and lignin increase with days to harvest and, consequently, the theoretical bioethanol yield increases as harvest frequency decreases. The highest values of dry matter, total carbohydrates and bioethanol were obtained with harvests at intervals of 180 days.
The results for dry matter yield and chemical characteristics of the biomass indicate that U. brizantha cv. Toledo is an option for production of bioethanol.
Literatura citada
Anderson, W., B. Dien, S. Brandon, and J. Peterson. 2008. Assessment of bermudagrass and bunch grasses as feedstock for conversion to ethanol. App. Biochem. Biotech. 145: 13-21. [ Links ]
AOAC (Association of Official Analytical Chemists). 1990. Protein (Crude) Determination in Animal Feed. Official Methods of Analysis. 15th ed. Helrich, K. (ed). AOAC. Arlington. VA, U.S.A. pp: 72-74. [ Links ]
ASTM (American Society for Testing and Materials). 1977. Method of Test for Alpha-Cellulose in Wood ASTM D110360. Annual Book of ASTM Standards. Part 22: Wood; Adhesives. ASTM, Easton, Md., USA. pp: 346-348. [ Links ]
ASTM (American Society for Testing and Materials). 2012. Annual Book of ASTM Standards. Vol. 11.06. Biological effects and environmental fate; biotechnology. ASTM International, West Cornshohocken, PA, USA. 1461 p. [ Links ]
Badger, P. C. 2002. Ethanol from cellulose: A general review. In: Janick, J. and A. Whipkey (eds). Trends in New Crops and New Uses, Vol. 1. ASHS Press, Alexandria, VA. pp: 17-21. [ Links ]
Balat, M., H. Balat, and S. Oz. 2008. Progress in bioethanol processing. Prog. Energ. Combust. Sci. 34: 551-573. [ Links ]
Betts, W. B., R. K. Dart, A. S. Ball, and S. L. Pedlar. 1991. Biosynthesis and Structure of lignocellulose. In: Betts, W. B. (ed). Biodegradation: Natural and Synthetic Materials. Springer-Verlag, Berlin, Germany. pp: 139-155. [ Links ]
Bidlack, J., M. Malone, and R. Benson. 1992. Molecular structure and component integration of secondary cell walls in plants. Proc. the Okla. Acad. Sci. 71: 51-56. [ Links ]
Brosse, N., A. Dufour, X. Meng, Q. Sun, and A. Ragauskas. 2012. Miscanthus: a fast-growing crop for biofuels and chemicals production. Biofuels. Bioprod. Bioref. 6: 580-598. [ Links ]
Cab, F. E. J., J. F. Enríquez Q., J. Pérez P., A. Hernández G., J. G. Herrera H., E. Ortega J. y A. R. Quero C. 2007. Potencial productivo de tres especies de Brachiaria en monocultivo y asociadas con Arachis pintoi en Isla, Veracruz. Técnica Pecuaria 46: 317-332. [ Links ]
Cardona M. E., L. A. Rios, y J. D. Peña. 2012. Disponibilidad de variedades de pastos y forrajes como potenciales materiales lignocelulósicos para la producción de bioetanol en Colombia. Información Tecnológica 23: 87-96. [ Links ]
Carroll, A., and C. Somerville. 2009. Cellulosic biofuels. Annual Review of Plant Biology 60: 165-182. [ Links ]
CEN (Comité Européen de Normalisation). 2005. EN149611:2005. Solid biofuels fuel specification and classes. Part 1 General requirements. London, UK. pp: 25-33. [ Links ]
Chacón-Hernández, P. A. y C. F. Vargas-Rodríguez, 2009. Digestibilidad y calidad del Pennisetum purpureum cv. King grass a tres edades de rebrote. Agron. Mesoam.. 20: 399-408. [ Links ]
Coll, L. C. 2008. La utilización de los residuos frutícolas para obtener bioetanol de Segunda Generación. Jornadas Técnicas de Frutas y Hortalizas. Palma de Mallorca-España. http:// www.agro-alimentarias.coop/ficheros/doc/02378.pdf . (Consultado 2 de noviembre de 2014). [ Links ]
Demirbas, A. 2004. Ethanol from cellulosic biomass resources. Int. J. Green Energ. 1: 79-87. [ Links ]
Dien, B. S. 2010. Mass balances and analytical methods for biomass pre-treatment experiments. In: Vertès, A. A., N. Qureshi, H. Blaschek and H. Yukawa (eds). Biomass to biofuels: strategies for global industries. Wiley, Chichester, United Kingdom. pp: 213-231. [ Links ]
Enríquez, Q. J. F., y Romero, M.J. 1999. Tasa de crecimiento estacional a diferentes edades de rebrote de 16 ecotipos de Brachiaria spp. en Isla, Veracruz. Agrociencia 33: 141-148. [ Links ]
Forero, O. 2009. El bagazo de caña de azúcar, petróleo verde del siglo. Documento URL. http://www.dinero.com/seccion-patrocinios/green/bagazo-cana-azucar-petroleoverde-del-siglo_62876.aspx (Consultado el 23 septiembre de 2014). [ Links ]
García E. 1988. Modificaciones al Sistema de Clasificación Climática de Koppen (para adaptarlo a las condiciones de la República Mexicana) 4a ed. Instituto de Geografía, UNAM; México. 217 p. [ Links ]
Godin, B., S. Lamaudiere, R. Agneessens, T. Schmit, J. P. Goffart, D. Stilmant, P. A. Gerin, and J. Delcarte. 2013. Chemical characteristics and biofuels potentials of various plant biomasses: influence of the harvesting date. J. Sci. Food Agric. 93: 3216-3224. [ Links ]
Hakkila, P. 1989. Utilization of residual forest biomass. Springer Series in Wood Science. Springer, Heildelberg. New York. 568 p. [ Links ]
Hernández-Salas, J. M., M. S. Villa-Ramírez, J. S. Veloz-Rendón, K. N. Rivera-Hernández., R. A. González-César., M. A. Plascencia-Espinoza, and S. R. Trejo-Estrada. 2009. Comparative hydrolysis and fermentation of sugarcane bagasse. Biores. Technol. 100: 1238-1245. [ Links ]
Hodgson, E. M., S. J. Lister, A. V. Bridgwater, J. Clifton-Brown, and I. S. Donnison. 2010. Genotypic and environmentally derived variation in the cell wall composition of Miscanthus in relation to its use as a biomass feedstock. Biomass Bioenerg. 34: 652-660. [ Links ]
Jenkins, B. M., L. L. Baxter, T. R. Miles Jr., and T. R. Miles. 1998. Combustion properties of biomass. Fuel Process. Technol. 54: 17-46. [ Links ]
Kazi, F. K., J. A. Fortman, R. Anex, D. D. Hsu, A. Dutta, and G. Kothandaraman. 2010. Techno-Economic Comparison of Process Technologies for Biochemical Ethanol Production from Corn Stover. Fuel 89: 20-28. [ Links ]
Laser, M., E. Larson, B. Dale, M. Wang, N. Greene, and L.R. Lynd. 2009. Comparative Analysis of Efficiency, Environmental Impact, and Process Economics for Mature Biomass Refining Scenarios. Biofuels, Bioprod. Bioref. 3: 247-270. [ Links ]
Lee, J. M., J. Shi, R. A. Venditti, and H. Jameel. 2009. Autohydrolysis pretreatment of coastal Bermuda grass for increased enzyme hydrolysis. Bioresource Technol. 100: 6434-6441. [ Links ]
Lewandowski, I., and A. Kicherer. 1997. Combustion quality of biomass: practical relevance and experiments to modify the biomass quality of Miscanthus x giganteus. Eur. J. Agron. 6: 163-177. [ Links ]
Lewandowski, I. , J. C. Clifton-Brown, B. Andersson, G. Basch, D. G. Christian, U. Jorgensen, M. B. Jones, A. B. Riche, K. U. Schwarz, K. Tayebi, and F. Teixeira. 2003. Environment and harvest time affects the combustion qualities of Miscanthus Genotypes. Agron. J. 95: 1274-1280. [ Links ]
Mateus, L., O. Hernández, M. Velásquez, y J. J. Díaz. 2012. Evaluación del pretratamiento con ácido sulfúrico diluido del pasto Maralfalfa (Pennisetum glaucumxPennisetum purpureum) para la producción de etanol. Revista Colombiana de Biotecnología XIV: 146-156. [ Links ]
McKendry, P. 2002. Energy production from biomass (Part 1): overview of biomass. Biores. Technol. 83: 37-46. [ Links ]
Murnen, H. K., V. Balan, S. P. S. Chundawat, B. Bals, L. D. C. Sousa, and B. E. Dale. 2007. Optimization of ammonia fiber expansion (AFEX) pretreatment and enzymatic hydrolysis of Miscanthusxgiganteus to fermentable sugars. Biotechnol. Prog. 23: 846-850. [ Links ]
Nava V., M. Morales, and S. Revah. 2007. Cometabolism of methyl tert-butyl ether (MTBE) with alkanes. Rev. Environ. Sci. Biotechnol. 6: 339-352. [ Links ]
Olanders, B., and B. M. Steenari. 1995. Characterization of ashes from wood and straw. Biomass Bioenerg 8: 105-115. [ Links ]
ORNL (Oak Ridge National Laboratory). 2006. Bioenergy conversion factors. Available from: Available from: http://www.localenergy.org/pdfs/Document%20Library/Bioenergy%20 conversion%20factors.pdf (Consultado el 30 septiembre de 2014.) [ Links ]
Parrish, D. J., and J. H. Fike. 2005. The biology and agronomy of Switchgrass for biofuels. Crit. Rev. Plant Sci. 24: 423-459. [ Links ]
Piccolo, C., and F. Bezzo. 2009. A techno-economic comparison between two technologies for bioethanol production from lignocellulose. Biomass and Bioenerg. 33: 478-491. [ Links ]
Rincón A. C., M. G. A. Ligarreto, y E. Garay. 2008. Producción de forraje en los pastos Brachiaria decumbens cv. amargo y Brachiaria brizantha cv. Toledo, sometidos a tres frecuencias y a dos intensidades de defoliación en condiciones del piedemonte llanero colombiano. Rev. Fac. Nal. Agr. Medellín 61: 4336-4346. [ Links ]
Robinson J., J. D. Keating, A. Boussaid, S. D. Mansfield and J. N. Saddler. 2002. The influence of bark on the fermentation of Douglas-fir whitewood pre-hydrolysates. Appl. Biochem. Biotech. 59: 443-448. [ Links ]
Roger, Q. L. M., A. N Souza, H. Ángelo, D. V. A. Teixeira e M. I. Soares 2011. Custo de produção das biomassas de eucalipto e capim-elefante para energia. Cerne Lavras 17: 417-426. [ Links ]
Rowell, R. M., R. Pettersen, J. S. Han, J. S. Rowell and M. A. Tshabalala. 2005. Cell Wall Chemistry. In: Rowell, R. M. (ed.) Handbook of wood chemistry and wood composites. CRC Press, Boca Raton, Florida, U.S.A. pp: 35-74. [ Links ]
Sarath, G., D. Akin, R. Mitchell, and K. Vogel. 2008. Cellwall composition and accessibility to hydrolytic enzymes is differentially altered in divergently bred switchgrass (Panicum virgatum L.) genotypes. Appl. Biochem. Biotech. 150: 1-14. [ Links ]
SAS Institute. 2002. Statistical Analysis System (SAS). Software v.9.0. User’s Guide. N. C., USA. 315 p. [ Links ]
Schmer, M. R., K. P. Vogel, R. B. Mitchell, and R. K. Perrin, 2008. Net energy of cellulosic ethanol from switchgrass. Proc. Natl. Acad. Sci. USA 105: 464-469. [ Links ]
Sendich, E. N., M. Laser, S. Kim, H. Alizadeh, L. Laureano Perez, B. Dale , and L. Lynd. 2008. Recent process improvements for the ammonia fiber expansion (AFEX). Process and resulting reductions in minimum ethanol selling price. Bioresource Technol. 99: 8429-8435. [ Links ]
Spatari, S., D. M. Bagley, and H. L. MacLean. 2010. Life cycle evaluation of emerging lignocellulosic ethanol conversion technologies. Bioresource Technol. 101: 654-667. [ Links ]
Sues A., R. Millati, and L. Edebo. 2005. Ethanol production from hexoses, pentoses, and dilute-acid hydrolyzate by Mucor indicus. FEMS Yeast Res. 5: 669-676. [ Links ]
Sun, Y., and J. Cheng. 2002. Hydrolysis of lignocellulosic material from ethanol production: A review. Bioresource Technol. 83: 1-11. [ Links ]
TAPPI (Technical Association of the Pulp and Paper Industry). 2007. TAPPI Test Methods. Fibrous Materials and Pulp Testing. Tests T 204 and T 222. Atlanta, GA. U.S.A. [ Links ]
Thomason W. E., W. R. Raun, G. V. Johnson, C. M. Taliaferro, K. W. Freeman, K. J. Wynn, and R. W. Mullen. 2004. Switchgrass response to harvest frequency and time and rate of applied nitrogen. J. Plant Nut. 27: 1199-1226. [ Links ]
Valverde, A. G., B. Sarria L., y J. P. Monteagudo Y. 2007. Análisis comparativo de las características fisicoquímicas de la cascarilla de arroz. Scientia et Technica 13: 255-260. [ Links ]
Vamvuka D., E. Kakaras, E. Kastanaki, and P. Grammelis. 2003. Pyrolysis characteristics and kinetics of biomass residuals mixtures with lignite. Fuel 82: 1949-1960. [ Links ]
Xu, J., J. J. Cheng, R. R. Sharma-Shivappa, and J. C. Burns. 2010. Lime pretreatment of Switchgrass at mild temperatures for ethanol production. Bioresource Technol. 101: 2900-2903. [ Links ]
Yacobucci, B. D. 2010. Intermediate-Level Blends of Ethanol in Gasoline, and the Ethanol “Blend Wall”. Congressional Research Service. http://fas.org/sgp/crs/misc/R40445.pdf (Consultado el 10 de diciembre de 2014). [ Links ]
Yin, C-Y. 2011. Prediction of higher heating values of biomass from proximate and ultimate analyses. Fuel 90: 1128-1132. [ Links ]
Zhang, T., C. Wyman, K. Jakob, and B. Yang. 2012. Rapid selection and identification of Miscanthus genotypes with enhanced glucan and xylan yields from hydrothermal pretreatment followed by enzymatic hydrolysis. Biotechnol. Biofuels 5: 2-14. [ Links ]
Received: May 01, 2015; Accepted: March 01, 2016











 text in
text in