Services on Demand
Journal
Article
Indicators
Related links
Share
Agrociencia
On-line version ISSN 2521-9766Print version ISSN 1405-3195
Agrociencia vol.50 n.4 Texcoco May./Jun. 2016
Animal science
Concentration of phenolic compounds in tropical forage fabaceae at different regrowth time
1 Áreas de Ciencia Vegetal y Ciencia Animal. Colegio de Posgraduados, Campus Tabasco. Periférico Carlos A. Molina s/n 86500 H. Cárdenas, Tabasco.
2 Programa de Forrajes. Campo Experimental Huimanguillo. INIFAP-Tabasco. 86600 Huimanguillo, Tabasco
Fabaceae are an option to increase the availability of protein in the prairies, but species of the Fabaceae family contain phenolic compounds, which depending on their number and type, benefit or harm livestock production. The aim of the study was to determine the concentration of phenolic regrowvth compounds of different ages of Arachis pintoi, Stylosanthes guianensis, Clitoria ternatea and Pueraria phaseoloides. The study was carried out in an experimental field, INIFAP-Tabasco, Mexico, from March to August 2013, covering the dry and rainy seasons, and in four ages of regrowth after uniformity cutting: 21, 42, 63 and 84 d. The variables evaluated were the concentrations of five types of phenolic compounds: total polyphenols (PFT), no tannins (FNT), condensed tannins (TC) hydrolysable (TH) and total tannins (TT). Sixteen treatments were evaluated in a design of completely randomly divided plots with three replications, the species as main plot, and regrowth age as a minor plot. Comparisons between means were performed using the Tukey test (p≤0.05). We observed the interaction species x age of regrowvth in the five types of compounds. The highest concentrations of phenolic compounds (CF) were obtained in all species at 42 d of regrowth. Clitoria ternatea presents the highest concentrations in the months without rain (155.4, 40.1, 16.9, 98.4 and 115.3 g kg-1 MS of PFT, FNT, TC and TH) and rainy (121.7, 39.6, 20.1, 61.9 and 82.0 g kg-1 MS of PFT, FNT, TC and TH). In contrast, S. guianensis presented lower concentrations of CF. The TC concentration varied between species, in the months without rain from 4.62 (Stylo) to 20.21 (Kudzú) g kg-1 MS and in rainy months from 3.66 (Stylo) to 20.10 (Clitoria) g kg-1 MS. No species reached the toxic level of 60 g kg-1 MS of TC. Results showed that the concentration of CT varied among species and age of regrowth. Among species, the highest concentrations were observed in C. ternatea and the lowest in S. guianensis. The interaction species-regrowvth age determined the concentration of phenolic compounds.
Keywords: Arachis pintoi; Stylosanthes guianensis; Pueraria phaseoloides; Clitoria ternatea; fabaceae; condensed tannins
Las fabáceas son una opción para incrementar la disponibilidad de proteína en las praderas pero especies de la familia Fabaceae contienen compuestos fenólicos que, según su cantidad y tipo, benefician o perjudican la producción pecuaria. El objetivo del estudio fue determinar la concentración de compuestos fenólicos del rebrote de diferentes edades de Arachis pintoi, Stylosanthes guianensis, Clitoria ternatea y Pueraria phaseoloides. El estudio se realizó en un campo experimental del INIFAP-Tabasco, México, de marzo a agosto del año 2013, abarcando las épocas seca y lluviosa, y en cuatro edades del rebrote después del corte de uniformidad: 21, 42, 63 y 84 d. Las variables evaluadas fueron las concentraciones de cinco tipos de compuestos fenólicos: polifenoles totales (PFT), no taninos (FNT), taninos condensados (TC) hidrolizables (TH) y totales (TT). Dieciséis tratamientos se evaluaron en un diseño de parcelas divididas completamente al azar con tres repeticiones, la especie como parcela mayor, y la edad del rebrote como parcela menor. Las comparaciones entre medias se realizaron con la prueba de Tukey (p≤0.05). La interacción especie x edad del rebrote se observó en los cinco tipos de compuestos. Las concentraciones mayores de compuestos fenólicos (CF) se obtuvieron en todas las especies a los 42 d del rebrote. Clitoria ternatea presentó las concentraciones mayores en los meses sin lluvias (155.4, 40.1, 16.9, 98.4 y 115.3 g kg-1 MS de PFT, FNT, TC y TH) y en los lluviosos (121.7, 39.6, 20.1, 61.9 y 82.0 g kg-1 MS de PFT, FNT, TC y TH). En contraste, S. guianensis presentó las concentraciones menores de CF. La concentración de TC varío entre especies, en los meses sin lluvias de 4.62 (Sytlo) a 20.21 (Kudzú) g kg-1 MS y en meses lluviosos de 3.66 (Stylo) a 20.10 (Clitoria) g kg-1 MS. Ninguna especie alcanzó el nivel tóxico de 60 g kg-1 MS de TC. Los resultados mostraron que la concentración de CT varió entre las especie y con la edad de rebrote. Entre especies, las concentraciones mayores se observaron en C. ternatea y las menores en S. guianensis. La interacción especie por edad del rebrote determina la concentración de compuestos fenólicos.
Palabras clave: Arachis pintoi; Stylosanthes guianensis; Pueraria phaseoloides; Clitoria ternatea; fabáceas; taninos condensados
Introduction
The use of forage fabaceae in association with grasses is important in the nutrition and sustainability of livestock production system (Martens et al., 2012). In tropical grasses the protein content varies from 6 to 14 % and in some cases is less than 6 %, depending on the time of year, age of regrowth, and species (Juárez-Hernández et al., 2004). In forage fabaceae protein content can reach up to 25 % (Heinritz et al., 2012); besides, these species fix N to the soil, which can benefit associated grasses (Onyeonagu and Eze, 2013). Fabaceae family contain secondary metabolites such as phenolic compounds, which benefit or adversely affect the animals that consume them (Jezierny et al., 2010). Phenolic compounds are related to the taste, smell and color of the food, and can modify the ruminal fermentation and reduce the emission of methane gas (Evans and Martin, 2000; Rochfort et al., 2008). Tannins are among the phenolic compounds, and their high concentration in plants make them less accepted by animals, and their effect depends on the amount ingested and the type of tannin (Mole et al, 1993; Patra et al, 2006).
There are condensed tanninsorproanthocyanidins, and the hydrolysable type (Hagerman and Butler, 1991). The condensed tannins participate in plant protection against fungi and bacteria (Scalbert, 1991), act against gastrointestinal parasites (Aerts et al., 1999), are antioxidants and can improve resistance to heat stress (Liu et al., 2011). In contrast, high concentrations depress feed intake by the animal (Miller and Ehlke, 1994). Low to moderate concentrations, between 5 and 55 g kg- 1 DM, contribute to the protection of proteins in the rumen, allowing their passage to the small intestine, where they are degraded and absorbed (Gebrehiwot et al., 2002; Berard et al, 2011;. Patra, and Saxena, 2011).
Most studies of forage fabaceae carried out in the tropical region of Mexico are based on their nutritionalvalue without including studies ofphenolic compounds, which could positively or negatively affect the nutritional value of the prairie, when associated, or provided as protein supply or cutting fodder. Furthermore, the concentration of phenolic compounds, particularly tannins, may vary depending on the temperature at which the fabaceae grows, soil fertility, state of maturity of the plant and species (Berard et al., 2011). Therefore, the determination of the concentration of tannins in forage fabaceae during plant development will help develop management strategies and use in animal feed, without causing adverse effects. The aim of this study was to determine the concentration of phenolic compounds in four tropical forage fabaceae with different ages of regrowth.
Materials and Methods
The field phase took place in the Garden of Fodder Genetic Resources of the Huimanguillo Experimental Field (17 ° 50' N, 93 ° 23' W) of the National Institute of Forestry, Agriculture and Livestock Research (INIFAP) in Tabasco, Mexico, from February to August 2013. This period covers the dry months of the year (March to May) and the rainy season (June to August) (Figure 1). The soil texture was loamy, with 41.1 % sand, 24.5 % clay, and 34.4 % silt, pH 7.0 and 21.5 meq 100 g-1 CIC. No fertilizer was applied, neither irrigation during the dry period of the year.

Figure 1 Cumulative rainfall and average temperature per month during the study in 2013. Huimanguillo, Tabasco, Mexico. Arrows indicate the month in which the uniformity cut was made, prior to the months with and without rain.
We evaluated 16 treatments in a design of completely randomly divided plots with three replications. The largest plot was the fabacea species: Cacahuatillo (Arachis pintoi Krapovickas & Gregory), Stylo (Stylosanthes guianensis (Aubl) Sw.), Clitoria, Conchita or Tehuana (Clitoria ternatea L.) and Kudzù (Pueraria phaseoloides Roxburgh Bentham). The smallest plot was that of regrowth: 21, 42, 63 and 84 d. The experimental unit was a plot of 2 X 1 m.
Planting took place on August 8, 2012 in plots of 2x4 m, density of 5 kg seed ha-1 in rows 50 cm apart, except Cacahuatillo, which was established by vegetative material (stems with roots), 25 cm spacing within each row.
To determine the age of regrowth, each experimental unit was randomly assigned one of the four ages of regrowth: 21, 42, 63 and 84 days. The days of regrowth ages were counted from uniformity cut. The uniformity cut was applied to all plots on February 26 for the dry months, and May 20 for rainy months of 2013.
Aboveground biomass samples collected by species and age of regrowth were dried at 50 °C, crushed, passed through a 1 mm mesh in a Wiley mill, and stored in dark at 4 °C until analysis.
The evaluated variables were the concentrations of total polyphenols, no tannin phenols, condensed, hydrolyzable and total tannins.
Total polyphenols (PFT)
The samples were degreased with petroleum ether (Muzquiz et al., 1993). Polyphenols were determined using the Folin-Ciocalteu method, and gallic acid as standard. Absorbance was measured at 765 nm in a spectrometer (ThermoElectron, Genesys 10 UV) (Makkar et al., 1993).
Non tannin phenols
Free fat samples were added polyvinylpyrrolidone to separate tannin phenols from non tannin phenols; after shaking them in vortex they were incubated for 15 min in dark at 4 °C and centrifuged (10 000 rpm) at 25 °C for 10 min (Makkar et al., 1993). In the supernatant we estimated the concentration of non tannin phenols with the Folin-Ciocalteu reagent at 725 nm. Standards were prepared from a standard solution of 0.5 mg mL-1 of gallic acid.
Condensed tannins (proanthocyanidins)
To quantify the condensed tannins, fat-free samples were extracted with 80 % methanol; to 250 µL of this extract 1500 µL of butanol were added: HCl (95: 5, v/v) and 50 µL of ferric reagent (ferric ammonium sulphate at 2 % in 2N HCl) were covered and maintained in boiling waterbath for 60 min and left to cool to room temperature.
We included a blank prepared with butanol:HCl and unheated (Porter et al., 1986); and measured absorbance at 550 nm. The concentration of condensed tannins was calculated as a leucocyanidin equivalent with the formula: TC (g kg-1 DM) = (A550nm x 78.26 x Dilution factor) / RMSP (g kg-1 DM), where 78.26 is a correction factor. The molar extinction coefficient (E 1 %, 1 cm, 550nm) of leucocyanidins is 460.
Total and hydrolysable tannins
We calculated total tannins (TT) with the difference of non tannin phenols from total polyphenols. Hydrolysable tannins (TH) were estimated as the difference of total tannins and condensed tannins (Singh et al., 2005; Rana et al., 2006).
Results and Discussion
Environmental Conditions
Monthly average temperatures and monthly accumulated rainfall were higher and lower in the dry months compared to the rainy season (Figure 1). Among the dry months March had the lowest temperature, 3.7 °C lower than the average of April and May. The difference in accumulated rainfall between dry and rainy months averaged 190 mm more rain in the latter. May had 98 mm more rain than the average in March and April.
Low or high rainfall and duration of the period are environmental conditions to which the tropical forage fabaceae are exposed to during their growth, which may limit the accumulation of biomass and modify the concentration of secondary metabolites (Garcia et al., 2005; Jayanegara et al., 2011).
Total polyphenols
In the dry months fabaceae species, regrowth age and their interaction had a significant effect (p≤0.05) on the concentration of total polyphenols (PFT) (Table 1). The four species of fabaceae showed initial increase between 21 and 42 d of regrowth; but this increase trend did not last (Table 2). Cacahuatillo, Stylo, Clitoria and Kudzú increased their concentration by 27.02, 31.01, 74.62 and 46.38 g kg-1 DM in that period. At 42 days, Clitoria accumulated the largest amount of PFT of the species tested (155.5 g kg-1 DM) and Cacahuatillo, Stylo and Kudzú showed no significant differences (average 79 g kg- 1 DM).
Table 1 Table 1. Effect of fabaceae species, regrowth age and their interaction in the concentration of phenolic compounds in the dry and rainy months of 2013, in Huimanguillo, Tabasco, Mexico.
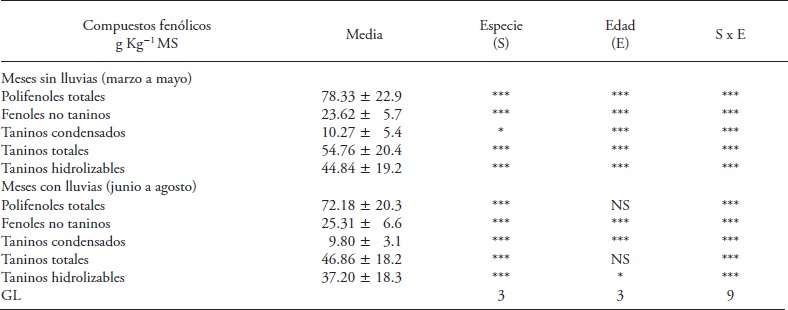
*, ** and *** Significance p<0.05, 0.01 and 0.001. NS: not significant. GL: degrees of freedom.
Table 2 Table 2. Phenol fractions (g kg-1 DM) in regrowths of tropical forage fabaceae between March and May (dry months) 2013. Huimanguillo, Tabasco, Mexico.

Means with different lowercase letters in a column and within each species are significantly different (Tukey, p≤0.05). Means with different capital letters in a column indicate significant difference (Tukey, p≤0.05) between species of the same age of re-growth.
Clitoria and Kudzú maintained unchanged PFT concentration between 42 and 84 d of regrowth, and Cacahuatillo and Stylo decreased their concentration (Table 2). In the rainy months PFT increased after 21 d with respect to the dry months, and decreased concentration with age of regrowth, except in Kudzú (Table 3). Th e interaction species x age of regrowth affected Kudzú since its PFT concentration increased with regrowth age, and changed from a low concentration, at 21 d together with Stylo, to a greater concentration at 84 old.
Table 3 Table 3. Phenols and their fractions (g kg-1 DM) in tropical forage fabaceae species in different ages of regrowth during June to August (rainy months) 2013. Huimanguillo, Tabasco, Mexico.

Means with different lowercase letters in a column and within each species are significantly different (Tukey, p≤0.05). Means with different capital letters in a column indicate significant differences (Tukey, p≤0.05) between species of the same age of regrowth.
Simultaneously Stylo concentration remained low throughout its growth. Cacahuatillo and Clitoria decreased the content of PFT, going from 21 to 84 d of regrowth.
During growth, Clitoria and Stylo showed the highest and lowest concentrations of PFT, except Clitoria at 84 d in the rainy months.
The PFT content changes during growth of tropical forage species have been documented (Rana et al., 2006; Jayanegara et al., 2011). The significant effect of the rainy season on the concentration of PFT in Morus alba (García et al., 2005) was probably due to the increase of the leaf area (Frutos et al., 2004). In this regard, the expanded leaf area may have increased PFT during the rainy months, in our study.
The content of Clitoria PFT during growth was significant, so that this plant could help reduce methane emissions by ruminants, since the concentration of PFT in plants shows negative correlation with the production of methane (Jayanegara et al, 2009; Jayanegara et al., 2011).
Non tannin phenols (FNT)
Simple non tannin phenols showed a similar pattern to the PFT in regrowth age, and a significant species x age of regrowth interaction (Table 1). Stylo had the lowest concentration of FNT between the species evaluated (Table 2 and 3). The FNT varied both in the rainy and non-rainy months. At 42 d Stylo, Clitoria and Kudzú increased the FNT, except Cacahuatillo inthe drymonths. Higherconcentrations of 14-56 g kg-1 MS FNT were observed in leaves of shrubby fabaceae (Jayanegara et al., 2011). These compounds are of interest because some of them exhibit antioxidant properties (Makkar et al., 2007) and could have a direct inhibitory action on the activity of methanogens (Jayanegara et al., 2011).
Total tannins (TT)
Total tannins concentration increased in the four species of fabaceae in the dry months, and only in Kudzú in the rainy months (Table 2 and 3). The species x regrowth age interaction was significant only in the dry months (Table 1). The TT ranged from 2.17 (Cacahuatillo) to 115.37 (Clitoria) g kg- 1 MS in the dry months and 9.97 (Stylo) to 92.63 (Clitoria) g kg-1 MS in the rainy season. Other studies show that tannins can act favorably on ruminal fermentation, as they form tannin-protein complexes that prevent the latter's degradation in the rumen, inhibit methanogenesis and improve reproductive efficiency, although the beneficial effects are not the same in all cases (Patra and Saxena, 2011). The beneficial effect on livestock reproductive efficiency depends on the chemical structure of tannins, their concentration and type of diet (Makkar et al., 2007). The effect of reducing methanogenesis with TT is not observed in all cases (Jayanegara et al., 2011). Only Stylo and Cacahuatillo showed average values below 60 g kg-1 DM of TT in all ages of regrowth (Tables 2 and 3). Clitoria and Stylo showed the highest and lowest concentrations along their growth during the year. Something similar was observed in Clitoria and Kudzú in the last 21 d during the rainy period, and in Stylo and Cacahuatillo in the dry months at 84 d of regrowth.
Condensed tannins (TC)
There is abundant information on the variation of condensed tannins in forage fabaceae species and their beneficial effect on livestock production when concentrations do not exceed 60 g kg-1 MS (Makkar et al., 2003; Rochfort et al., 2008). The TC of the species under study ranged, on average; in similar amounts in dry and wet months (Tables 2 and 3). Only in Clitoria we observed a TC increase in the rainy months at all ages of regrowth (Table 3). Kudzú, Stylo and Cacahuatillo showed higher concentrations from 63 d of regrowth and in Clitoria from 42 d in the dry months (Table 2).
Clitoria concentration decreased almost 10 times at 63 d compared to 42 d, and in the rainy months this species also increased the concentration of TC with regrowth age (Table 3). Berard et al. (2011) reported the content of TC in leaves of tropical fabaceae, Acacia angustissima and Calliandra calothyrsus (33 and 196 g kg-1 DM), as well as herbaceous fabaceae, Medicago sativa and Astragalus cicer (0.0 g kg-1 DM) and Daleapurpurea (68.7 g kg-1 DM). The authors also observed an increase in TC with the maturity of the plant. The species had levels that could be beneficial for feeding ruminants (Patra and Saxena, 2011), although their biological activity depends on their chemical structure and molecular weight. The binding of tannins with proteins depends on the number of phenolic groups in the molecule and those of high molecular weight (Frutos et al., 2004) are not absorbed.
Low concentrations of TC in the species under study compared to other forage fabaceae can be the result of the species and plant tissue analyzed. In this regard, Häring et al. (2007) indicated that the distribution of tannin in the tissues of plants is heterogeneous, and concentration are higher in leaves and lower in stems. On this account a dilution effect is observed on the concentration of TC when the sample included total aboveground biomass.
Hydrolyzable tannins (TH)
All species reached a higher concentration of TH at 42 d of regrowth age. From this age, Clitoria maintained the concentration of 102.2 g kg- 1 DM. In the other species concentration fluctuated. On the contrary, in the rainy months, the species showed no similar trends. Clitoria maintained an average concentration of 58.19 g kg-1 DM during growth. Kudzú continuously increased its concentration with regrowth age. Clitoria and Kudzú had a higher concentration between 42 and 84 d. Stylo showed lower concentrations, along with Kudzú at 21 d and Cacahuatillo at 63 and 84 d of regrowth age (Table 3).
The TH concentrations above 200 g kg-1 DM may cause toxicity to livestock (Mole et al., 1993; Rana et al., 2006). The species tested did not exceed the toxid levels in months with or without rain. Toxicity induced by TH may be due to TH degradation, so its molecular weight decreases and the absorption of products in the rumen can cause an increased metabolic burden with phenols, which may exceed the capacity of liver detoxification (Jayanegara et al., 2011).
The results of our study showed the effect of plant age on the concentration of phenolic compounds during their growth. But the interaction species x regrowth age was significant for the groups of compounds evaluated. Plants had the highest concentrations of phenolic compounds at 42 d of regrowth age. The highest concentrations of phenolic compounds were observed in Clitoria and the lowest in Stylo; these concentrations were lower than those identified as toxic by some authors. The highest levels of condensed tannins (16.91 and 20.1 g kg- 1 DM) in Clitoria, at the age of 42 d in months with and without rains, are lower than that considered (60 g kg- 1 DM) minimum for the rumen fermentation be affected (Makkar, 2003).
Conclusions
The fabaceae species and age of regrowth are a source of variation in the concentration of total polyphenols during growth; regrowth age (maturity of the plant) is the factor that most affects these concentrations. The maximum concentrations of phenolic compounds were observed at 42 d, and did not reach toxic levels for animals. Clitoria and Stylo presented the highest and lowest concentration of phenolics between species.
Literatura Citada
Aerts, R. J., T. N. Barry, and W. C. Mcnabb. 1999. Polyphenols and agriculture: beneficial effects of proanthocyanidins in forages. Agr. Ecosyst. Environ. 75: 1-12. [ Links ]
Berard, N. C., Y. Wang, K. M. Wittenberg, D. O. Krause, B. E. Coulman, T. A. McAllister, and K. H. Ominski. 2011. Condensed tannin concentrations found in vegetative and mature forage legumes grown in western Canada. Can. J. Plant Sci. 91: 669-675. [ Links ]
Evans, J. D., and S. A. Martin. 2000. Effects of thymol on ruminal microorganisms. Curr. Microbiol. 41: 336-340. [ Links ]
Frutos, P., G. Hervás, F. J. Giráldez, and A. R. Mantecón. 2004. Review. Tannins and ruminant nutrition. Spanish J. Agric. Res. 2: 191-202. [ Links ]
García D. E., M. G. Medina, y F. Ojeda. 2005. Efecto de la fertilización orgánica, la variedad y la época en el perfil polifenólico de Morus alba (L.). Rev. AIA 9: 53-68. [ Links ]
Gebrehiwot, L., P. R. Beuselinck, and A. R. Craig. 2002. Seasonal variations in condensed tannin concentration of three Lotus species. Agron. J. 94: 1059-1065. [ Links ]
Hagerman, A. E., and L. G. Butler. 1991. Tannins and lignins. In: Rosenthal G. A. and M. R. Berenbaum (eds). Herbivores: Their Interactions with Secondary Plant Metabolites. Vol. I. Academic Press, San Diego, CA. pp. 355-388. [ Links ]
Häring, D. A., D. Suter, N. Amrhein, and A. Lüscher. 2007. Biomass allocation is an important determinant of the tannin concentration in growing plants. Ann. Bot. (Oxford) 99: 111-120. [ Links ]
Heinritz, S. N., D. Martens S., P. Avila, and S. Hoedtke. 2012. The effect of inoculant and sucrose addition on the silage quality of tropical forage legumes with varying ensilability. Anim. Feed Sci. Technol. 174: 201-210. [ Links ]
Jayanegara, A., H. P. S. Makkar, and K. Becker. 2009. Methane reduction effect of simple phenolic acids evaluated by in vitro Hohenheim gas production method. Proc. Soc. Nutr. Physiol. 18: 98. [ Links ]
Jayanegara, A., E. Wina, C. R. Soliva, S. Marquardt, M. Kreuzer, and F. Leiber. 2011. Dependance of forage quality and methanogenic potential of tropical plants on their phenolic fractions as determined by principal component analysis. Anim. Feed Sci. Technol. 163: 231-243. [ Links ]
Jezierny, D., R. Mosenthin, and E. Bauer. 2010. The use of grain legumes as a protein source in pig nutrition: a review. Anim. Feed Sci. Technol. 157: 111-128. [ Links ]
Juárez-Hernández, J., E. D. Bolaños-Aguilar, y M. Reinoso. 2004. Contenido de proteína por unidad de materia seca acumulada en pastos tropicales. Época de Nortes. Rev. Cubana Cienc. Agric. 38: 423-430. [ Links ]
Liu, H. W., X. F. Dong, J. M. Tong, and Q. Zhang. 2011. A comparative study of growth performance and antioxidant status of rabbits when fed with or without chestnut tannins under high ambient temperature. Anim. Feed Sci. Technol. 164: 89-95. [ Links ]
Makkar, H. P. S. 2003. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Ruminant Res. 49: 241-256. [ Links ]
Makkar, H. P. S., M. Blummel, N. K. Borowy, and K. Becker. 1993. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric. 61: 161-165. [ Links ]
Makkar, H. P. S., G. Francis, and K. Becker. 2007. Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems. Animal 1: 1371-1391. [ Links ]
Martens, S. D., T. T. Tiemann, J. Bindelle, M. Peters, and C. E. Lascano. 2012. Alternative plant protein sources for pigs and chickensin the tropics - nutritional value and constraints: a review. J. Agric. Rural Dev. Tropics and Subtropics 113: 101-123. [ Links ]
Miller, P. R., and N. J. Ehlke. 1994. Condensed tannins relationships with in vitro forage quality analysis for birdsfood trefoil. Crop Sci. 34: 1074-1079. [ Links ]
Mole, S., J. C. Roglers, and L. G. Butler. 1993. Growth reduction by dietary tannins: different effects due to different tannins. Biochem. Syst. Ecol. 21: 667-677. [ Links ]
Muzquiz, M., C. Burbano., Cuadrado C., and C. De la Cuadra. 1993. Determination of thermo resistant antinutritional factors in legumes. I. Alkaloids. Invest. Agrar. Prod. Prot. Veg. 8: 351-361. [ Links ]
Onyeonagu, C. C., and S. M. Eze. 2013. Proximate compositions of some forage grasses and legumes as influenced by season of harvest. African J. Agric. Res. 8: 4033-4037. [ Links ]
Patra, A. K., D. N. Kamra, and N. Agarwal. 2006. Effect of plant extracts on in vitro methanogenesis, enzyme activities and fermentation of feed in rumen liquor of buffalo. Anim. Feed Sci. Technol. 128: 276-291. [ Links ]
Patra, A. K., and J. Saxena. 2011. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 91: 24-37. [ Links ]
Porter, L. H., L. N. Hrstich, and B. C. Chan. 1986. The conversion of procyanidins and prodelphinidins to cyaniding and delphinidin. Phytochemistry 25: 223-230. [ Links ]
Rana, K. K., M. Wadhwa, and M. P. S. Bakshi. 2006. Seasonal variations in tannin profile of tree leaves. Asian-Aust. J. Anim. Sci. 19: 1134-1138. [ Links ]
Rochfort, S., A. J. Parker, and F. R. Dunshea. 2008. Plant bioactives for ruminant health and productivity. Phytochemistry. 69: 299-322. [ Links ]
Scalbert, A. 1991. Antimicrobial properties of tannins. Phyto-chemistry. 30: 3875-3883. [ Links ]
Singh, B., A. Sahoo, R. Sharma, and T. K. Bhat. 2005. Effect of polyethylene glicol on gas production parameters and nitrogen disappearance of some tree forages. Anim. Feed Sci. Technol. 123-124: 351-364. [ Links ]
Received: April 2015; Accepted: November 2015











 text in
text in 


