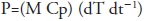Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Agrociencia
On-line version ISSN 2521-9766Print version ISSN 1405-3195
Agrociencia vol.50 n.4 Texcoco May./Jun. 2016
Food Science
Ultrasound-assisted extraction of phenolics compounds from chia (Salvia hispanica L.) seeds and their antioxidant activity
1 Facultad de Ingeniería Química. Benemérita Universidad Autónoma de Puebla. Avenida San Claudio y 18 Sur. C.P. 72570. Ciudad Universitaria. Puebla, Puebla. Mexico.
2 Departamento de Tecnología de Alimentos, Universitat Politècnica de València, Camí de Vera s/n, E46022 Valencia, Spain.
3 Departamento de Nutrición. Universidad Autónoma de Zacatecas, Carretera Zacatecas-Guadalajara Km. 6, Ejido "La Escondida", Zacatecas, Zacatecas. C.P. 98160, Mexico.
Extraction of phenolic compounds from chia (Salvia hispanica L.) seeds using two solvents (methanol and n-hexane) was performed with or without high intensity ultrasound assistance. Conventional solid-liquid extraction and ultrasonic-assisted extraction at 50, 75 and 100 % of maximum power (400 W) were performed using chia seeds mixed with solvent (1:10 w/v) at three extraction times (5, 10 and 15 min). The highest phenolic concentration and the best antioxidant capacity were obtained using methanol as extraction solvent for both methods. When using the regular procedure, higher phenolic contents in both methanol and hexane, extracts were obtained at shorter extraction times, whereas antioxidant capacity increased with time. Efficiency of ultrasound-assisted extraction of phenolics increased with time and power output. Methanolic extracts obtained at the maximum power (100 %, 15 min) did not exhibit the best antioxidant capacity. The ultrasound-assisted process showed better extraction ability than conventional methods, which means that the extraction yield of phenolic compounds from chia seeds increased. This increase may lead to a wider application of S. hispanica L. as a high-quality functional ingredient in several industrial processes. Therefore, the ultrasound-assisted technique could be a reliable and novel method for phenolic compounds and antioxidants extraction from chia seeds, due to increases and improves extraction yield.
Key words: Salvia hispanica L; processing; phenolic compounds; antioxidant capacity; solvent extraction
La extracción de compuestos fenólicos se realizó desde semillas de chía (Salvia hispanica L.) con dos disolventes (metanol y n-hexano) con o sin ultrasonido de alta intensidad. Esta se realizó en forma convencional sólido-líquido y con ultrasonido a 50, 75 y 100 % de potencia máxima (400 W) con las semillas de chía mezcladas con disolvente (1:10 p/v), en tres tiempos de extracción (5, 10 y 15 min). La concentración mayor fenólica y la mejor capacidad antioxidante se obtuvieron con metanol como disolvente de extracción con ambos métodos. Los contenidos fenólicos mayores se obtuvieron en ambos extractos con el procedimiento convencional, en los tiempos de extracción cortos y la capacidad antioxidante aumentó con el tiempo. La eficiencia de la extracción de fenólicos asistida por ultrasonido aumentó con el tiempo y potencia de salida. Extractos metanólicos obtenidos con la potencia mayor (100 %, 15 min) no mostraron la capacidad antioxidante mejor. El proceso asistido por ultrasonido mostró mayor capacidad de extracción que los métodos convencionales; es decir, el rendimiento de extracción de compuestos fenóli-cos con semillas de chía aumentó. Este aumento puede conducir a una aplicación más amplia de S. hispanica L. como un ingrediente funcional de calidad alta en varios procesos industriales. Por lo tanto, la técnica asistida por ultrasonido podría ser un método fiable para la extracción de compuestos fenólicos y antioxidantes desde semillas de chía, porque aumenta y mejora el rendimiento de la extracción.
Palabras clave: Salvia hispanica L.; procesamiento; compuestos fenólicos; capacidad antioxidante; extracción con disolventes
Introduction
Chia (Salvia hispanica L.) is herbaceous plant native from southern Mexico and northern Guatemala that belongs to the Lamiaceae family (Marineli et al., 2014). Chia seeds are good sources of fiber, oil rich in polyunsaturated fatty acids and natural antioxidants including tocopherols, phytosterols, carotenoids and phenolic compounds, such as chlorogenic acid, caffeic acid, myricetin, quercetin and kaempferol (Capitani et al., 2012). Along with corn (Zea mays L.), beans (Phaseolus vulgaris L.) and amaranthus (Amaranthus caudatus), chia was a core component in the diet of many pre-Colombian civilizations in America, including the Mayan and Aztec; its seeds were valued as a source of oils for medicinal use (Cahill, 2003). In Mexico, chia seeds are used for their nutritional and medicinal properties, i.e. endurance for athletes, appetite suppressor, weight loss agent, blood glucose control, and intestinal regulation. The potential use of chia seeds as a good source of proteins with a remarkable thermal stability was reported by Sandoval-Oliveros and Paredes-López (2013).
Phenolic compounds are basically cataloged into several classes, of which phenolic acids, flavonoids and tannins are the main dietary phenolic compounds (Balasundram et al., 2006). The antioxidant properties of phenolic compounds are attributed to their ability to scavenge free-radicals and to chelate metal ions involved in their production. Thus, the antioxidant activity of phenolic acids is due to their ability to donate a hydrogen anion, i.e., an unpaired electron, and relocate it within the aromatic structure (Fernández-Panchón et al., 2008; Cabrera-Soto et al., 2009; Ignat et al., 2011). Antioxidants are compounds that delay the oxidation process by inhibiting the initiation or propagation of oxidizing chain reactions, thus preventing many oxidative stress-related conditions, cardiovascular diseases, diabetes (Vuksan et al., 2010), atherosclerosis, stroke, cancer and neurodegenerative diseases. In order to isolate these bioactive components, conventional and solvent-based extraction techniques are used. However, these methods have limitations including elevated solvent consumption and long extraction times. Thus, alternative ways are needed to improve the extraction process, thereby increasing both yield and quality of the extracts (Rodríguez-Bernaldo et al., 2010). Methods proposed are the use of supercritical fluids (Zulkafli et al., 2014), microwaves (Setyaningsih et al., 2015) and high-power ultrasound (Hussam et al., 2013). There is an ongoing search for defining the adequate process variables of these emergent technologies to achieve the above defined goals.
Ultrasound-assisted extraction (UAE) is used for isolating compounds (Hussam et al., 2013). Certain benefits in terms of solvent penetration arise from using UAE of food components including mass transfer intensification and sonocapillary effects. Extraction might also be improved as a consequence of the collapse of cavitation-formed bubbles near the cell walls (Toma et al., 2001). Both extraction rate and yield might be improved by the optimal ultrasonic variables combination, such as intensity and time (Rodríguez-Bernaldo et al., 2010). The extraction of phenolic compounds from chia seed was reported by Reyes-Caudillo et al. (2008) and they compared the effect of pressing and solvent. The application of the UAE has not been explored yet. Moreover, different solvents for extraction could lead to different compositions of phenolic compounds in extracts, because the solubility of each compound in a given solvent would be quite different, this implies that the phenolic compounds with more hydrophobic characteristics might occur in lower amounts than those with hydrophilic characteristics. Consequently, the bioactivity of an extract might also be affected (Lou et al., 2014). The aim of this work was to quantify the UAE on the antioxidant capacity and the total phenolic content from chia seed using a polar (methanol) and a non-polar (hexane) solvent.
Materials and Methods
Raw material, reagents and standards
Fresh chia seeds were purchased in a city local market (Zacatecas City, Mexico). The seeds were cleaned, ground and sieved to a particle size of 500 μm, packed in hermetic plastic bags and stored in the dark at room temperature (25 ºC). Folin-Ciocalteu reagent (2N), 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), 6-hydroxy-2,5,7,8-tetramethylchromane-2 carboxylic acid (Trolox), gallic acid, potassium persulphate and sodium carbonate were analytical grade (Sigma Chemical Co.; St. Louis, MO, USA); n-hexane and methanol were HPLC-grade (JT Baker).
Conventional extraction
Chia seeds flour (5 g) and 50 mL of n-hexane or methanol (1:10 w/v) was thoroughly mixed for 5, 10 or 15 min using an Ultraturrax homogenizer (T-25, IKA, Staufen, Germany) and then centrifuged (2701 x g; 4 °C) for 10 min. The supernatants were stored at 4 ºC until their analysis (24 h).
Ultrasonic device set-up
An ultrasonic probe device (UP400S, Hielscher, Teltow, Germany) working under controlled temperature conditions (25 ºC) was used at 24 kHz, with maximum power output of 400W, and an emitter surface of 3.8 cm2 (Figure 1). The ultrasonic probe was immersed 1.5 cm into the solution and the vessel was kept in the dark in order to avoid potential light-induced damages of the extract.
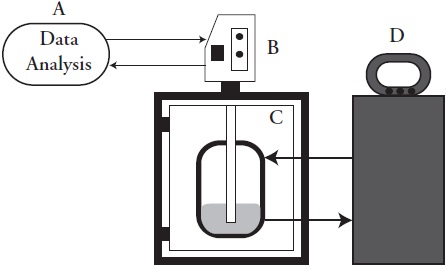
Figure 1 Experimental set-up for ultrasonic assisted extraction of chia (Salvia hispanica L.) seeds phenolic compounds. A: Portable Computer; B: ultrasonic probe system; C: jacketed extraction vessel; D: chiller.
The effective ultrasonic power transferred into the medium (P) was determined using a calorimetric procedure (Hussam et al., 2013) (Equation 1) registering the temperature rise each second, for the first 3 min of ultrasonic application. P was calculated as:
where M (kg) is the solvent mass, Cp (J kg-1 °C) is the heat capacity of solvent and dT dt-1 is the slope of the logged temperature-time curve. Determinations were carried out in triplicate.
Ultrasonic extraction
Experiments were carried out using the same chia seed flour (solvent ratio as before). Extract was placed in a jacketed extraction vessel and submitted to ultrasonic processing for 5, 10, and 15 min at amplitudes corresponding to 50, 75 and 100 % of power output (400 W). After extraction, the mixture was centrifuged (2701 x g 4 ºC) for 10 min. The supernatant obtained was stored (24 h) in opaque vials at 4 ºC until analyzed.
Determination of total phenolic compounds (TPC)
Total phenolic content (TPC) was determined using the Folin-Ciocalteu method described by Hussam et al. (2013) with some modifications. Briefly, 250 μL of extract was mixed with 15 mL deionized water and 1.25 mL of Folin-Ciocalteu phenol reagent. After 5 min, 3.75 mL of Na2CO3 (7.5 %) and bring to 25 mL with deionized water. Absorbance was measured at 765 nm in a spectrophotometer UV-Vis (Thermo Scientific 10S, Thermo Fisher Scientific Inc, USA). Response variable was the TPC in the extracts expressed as mg gallic acid equivalents (GAE) per sample (100 g) using a regression equation and a gallic acid calibration curve. At least three replicates were made for each extract.
Antioxidant capacity (AC) and ABTS +⋅ scavenging ability
ABTS scavenging ability was determined as described by Fu et al. (2011). An ABTS radical cation (ABTS+⋅) was generated by reacting an ABTS aqueous solution (7 mmol L-1) with K2S2O8 (2.45 mmol L-1, final concentration) in the dark for 16 h, adjusting its absorbance at 734 nm to 0.700±0.1 with ethanol. After addition of 900 mL of diluted ABTS⋅+ solution to 100 mL of extract, the absorbance reading was taken exactly 1 min after initial mixing. The percentage inhibition of absorbance at 734 nm is calculated and plotted as a function of concentration of antioxidants and of Trolox for the standard reference data. The Trolox Equivalent Antioxidant Capacity (TEAC) was subsequently calculated. Results were expressed as mg Trolox 100 g-1. Experiments were run in triplicate.
Experimental design and statistical analysis
The experimental design was factorial (3x3x2) and three replicates. All values expressed are means ± standard deviation. The data were used for to one-way and multifactorial ANOVA, and the differences between means was determined by Tukey's test (p≤0.05) using Statgraphics® Centurion XV (Statpoint Technologies Inc., Warrenton, VA, USA). Pearson's correlation test was carried out to determine the linear correlations between TPC and AC using the same software.
Results and Discussion
Conventional extraction
A significant difference (p<0.05) was observed between TPC of methanolic extracts and those obtained with hexane at all extraction times, with an average increment of 64.56 %; thus, phenolics in chia are thought to be polar in nature (Figure 2a). These results are in agreement with those of González-Jiménez et al. (2010). In ethanol-extracted chia samples, Reyes-Caudillo et al. (2008) obtained TPC values of 88.00 mg per 100 g of sample, while Marineli et al. (2014) reported TPC of 94 mg 100 g-1. A significant (p<0.05) difference in AC of methanol-extracted or hexane-extracted samples were observed (Figure 2B).

Figure 2 Effect of the type of solvent (n-hexane or methanol) at three different conventional extractions times on the average (A) TPC and (B) AC from samples of chia seeds. Average ± HSD (p≤ 0.05).
In order to compare the effect of solvent, average values of total phenolics and antioxidant activity were obtained from all values by conventional extraction. Methanolic extracts showed an AC of 8.27±0.15 mg Trolox 100 g-1 of chia (average value), whereas hexane extracts had an AC of 0.44±0.05 mg Trolox 100 g- 1 of chia (average value), which is consistent with TPC results. Hexane extracts are rich in highly-unstable polyunsaturated fatty acids (PUFA) (González-Jiménez et al. , 2010). The oxidation-prone PUFAs would be responsible for such low AC values. These results agree with those obtained by González-Jiménez et al. (2010), who reported that oil fractions of chia seeds showed a minor AC related to the low TPC obtained with hexane and the high PUFAs content. Results showed (Figure 2A) a significant difference (p≤0.05) between methanol extractions for 5 and 10 min in comparison with the 15 min extraction. Meanwhile, for hexane samples, a significant (p≤0.05) increase of TPC was observed in 5-min hexane-extracted samples, whereas AC (Figure 2B) did not exhibit a significant influence (p>0.05) of extraction time for both solvents used.
There is an influence of solvent polarity in the extraction process. Thus the higher TPC and AC were obtained in samples with methanol, while for the conventional extraction time, the TPC was decreasing in methanol samples and increasing in hexane samples, and the AC remained constant in both samples.
Ultrasonic assisted extraction
The effective ultrasonic power was in the range from 29.002 to 37.534 W. As expected, the ultrasound applied into the medium increased almost linearly (R2 Methanol = 0.8833; R2 n-Hexane = 0.9972) with supply of electric power to the transducer (Table 1).
Table 1 Ultrasonic power (W) applied to the medium as function of the percentage of the total electric power (400 W) and time (3min) of ultrasonic application.
Data are means ± standard deviation (n=3)
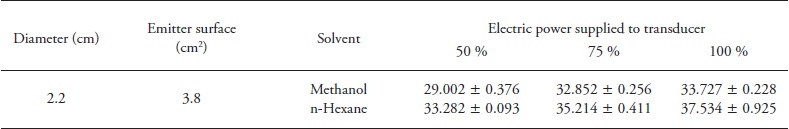
Similar to conventional extraction, in order to compare the effect of solvent, average values of total phenolics and antioxidant activity were obtained from all values by UAE. Net ultrasonic power applied was always higher for hexane than methanol; this was attributed to differences in heat capacity. UAE results (Table 2) showed a significant difference (p≤0.05) on TPC and AC between methanolic extracts (149.95±20.39 mg of GAE 100 g- 1 and 9.90±0.05 mg Trolox 100 g-1 of chia, average value) and hexane (60.31 ±5.99 mg of GAE 100 g-1 and 1.11 ± 0.38 mg Trolox 100 g-1 of chia, average value). From other studies (Cárcel et al., 2007; Hussam et al., 2013), the ultrasonic power applied was considered one of the key factors affecting phenolics extraction efficiency. A higher ultrasonic power applied generates more intense cavitation which facilitates solvent penetration into the matrix and increases the extraction efficiency (Priego and Luque, 2004).
Table 2 Influence of ultrasonic assisted extraction process parameters on the TPC and AC of Chia seeds with two different solvents (methanol and hexane).
Data are means ± standard deviation (n=3). Different lower case letters in the same cell and the same column indicate significant differences between amplitudes at a time of extraction (p≤0.05); while upper case letters in a different cell within a single column indicate statistically significant difference between times for the same amplitude (p≤0.05)
Best extraction parameters of TPC were obtained at 100 % ultrasonic power for 15 min using methanol (194.06± 11.11 mg of GAE 100 g-1 of chia); meanwhile, no influence (p>0.05) of ultrasonic application was observed for 5 and 15 min hexane extraction (68.14± 0.14 and 66.13± 2.14 mg of GAE 100 g-1 of chia, respectively). Again, this was attributed to non-affinity of hexane polarity towards hydrophilic compounds of chia, as well as with the irradiation power, which might reduce the efficiency of ultrasonic energy transmitted into the medium due to an increase in the bubble numbers in solvent during cavitation (Filgueiras et al., 2000; Zhao et al., 2007). As expected, methanolic extracts exhibit the highest AC ((9.90±0.05 mg Trolox 100 g-1 of chia) (Table 2); however, no significant differences in ultrasonic power applied (p> 0.05) were observed. Besides, there was correlation between TPC and AC of chia seeds for both solvents used (R=0.939; n=66; p=p 0.000) (Figure 3).
Conclusions
Ultrasound-assisted extraction with methanol could be used as an efficient alternative for obtaining phenolics from chia seeds with high antioxidant capacity. The ultrasonic effect was dependent on the effective ultrasonic power applied to the medium and extraction time.
The best total phenolic content extraction conditions were 100 % of total electric power for 15 min. Ultrasound-assisted extraction still presents some challenges for its industrial scale-up. Therefore, ultrasonic-assisted processes must be evaluated in order to increase the probabily of applications in the food processing field.
REFERENCES
Balasundram, N., K. Sundram, and S. Samman. 2006. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 99: 191-203. [ Links ]
Cabrera-Soto, M. L., Y. Salinas-Moreno, G. A. Velázquez-Cardelas, and E. Espinosa-Trujillo. 2009. Contenido de fenoles solubles e insolubles en las estructuras del grano de maíz y su relación con propiedades físicas. Agrociencia 43: 827-839. [ Links ]
Cahill, J. P. 2003. Ethnobotany of chia, Salvia hispanica L. (Lamiaceae). Econ. Bot. 57: 604-618. [ Links ]
Capitani, M. I., V. Spotorno, S. M. Nolasco, and M. C. Tomás. 2012. Physicochemical and functional characterization of by-products from Chia (Salvia hispanica L.) seeds of Argentina. Food Sci. Technol-Leb. 45: 94-102. [ Links ]
Cárcel J., A., J. Benedito, J. Bon, and A. Mulet. 2007. High intensity ultrasound effects on meat brining. Meat Sci. 76: 611-619. [ Links ]
Fernández-Panchón, M.S., D. Villano, A. M. Troncoso, and M.C. García-Parrilla. 2008. Antioxidant activity of phenolic compounds: from in vitro results to in vivo evidence. Crit. Rev. Food Sci. 48: 649-671. [ Links ]
Filgueiras, A. V., J. L. Capelo, I. Lavilla, and C. Bendicho. 2000. Comparison of ultrasound-assisted extraction and microwave-assisted digestion for determination of magnesium, manganese and zinc in plant samples by flame atomic absorption spectrometry. Talanta 53: 433-441. [ Links ]
Fu, L., B. T. Xu, X. R. Xu, R. Y. Gan, Y. Zhang, E. Q. Xia, and H. B. Li. 2011. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 129: 345-350. [ Links ]
González-Jiménez, F. E., M. C. Beltrán-Orozco, and M. G. Vargas. 2010. The antioxidant capacity and phenolic content of chía's (Salvia hispánica L.). Integral seed and oil. J. Biotechnol. 150: S315. [ Links ]
Hussam Ahmad-Qasem, M., J. Cánovas, E. Barrajón-Catalán, V. Micol, J. A. Cárcel, and J. V. García-Pérez. 2013. Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innov. Food Sci. Emerg. 17: 120-129. [ Links ]
Ignat, I., I. Volf, and V. I. Popa. 2011. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 126: 1821-1835. [ Links ]
Lou, S-N, Y-S. Hsu, and C-T. Ho. 2014. Flavonoid compositions and antioxidant activity of calamondin extracts prepared using different solvents. J. Food Drug Anal. 22: 290-295. [ Links ]
Marineli, R.S., É. Aguiar-Moraes, S. Alves-Lenquiste, A. Teixeira-Godoy, M. Nogueira-Eberlin, and M. R. Maróstica Jr. 2014. Chemical characterization and antioxidant potential of Chilean Chia seeds and oil (Salvia hispanica L.). Food Sci Technol.-Leb. 59: 1304-1310. [ Links ]
Priego-Capote, F., and M. D. Luque De C.. 2004. Analytical uses of ultrasound I. Sample preparation. Tra-Trend Anal. Chem. 23: 644-653. [ Links ]
Reyes-Caudillo, E., A. Tecante, and M. A. Valdivia-López. 2008. Dietary fibre content and antioxidant activity of phenolic compounds present in Mexican Chia (Salvia hispanica L.) seeds. Food Chem. 107: 656-663. [ Links ]
Rodríguez-Bernaldo De Q., A., M. A. Lage-Yusty, and J. López-Hernández. 2010. Determination of phenolic compounds in macroalgae for human consumption. Food Chem. 121: 634-638. [ Links ]
Sandoval-Oliveros, M. R., and O. Paredes-López. 2013. Isolation and characterization of proteins from chia seeds (Salvia hispanica L.). J. Agric. Food Chem. 61: 193-201. [ Links ]
Setyaningsih, W., I. E. Saputro, M. Palma, and C. G. Barroso. 2015. Optimization and validation of the microwave-assisted extraction of phenolic compounds from rice grains. Food Chem. 169: 141-149. [ Links ]
Toma, M., M. Vinatoru, L. Paniwnyk, and T. J. Mason. 2001. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason Sonochem. 8: 137-142. [ Links ]
Vuksan, V., A. L. Jenkins, A. G. Dias, A. S. Lee, E. Jovanovski, and A. L. Rogovik. 2010. Reduction in postprandial glucose excursion and prolongation of satiety: possible explanation of the long-term effects of whole grain Salba (Salvia hispanica L.). Eur. J. Clin. Nutr. 64: 436-438. [ Links ]
Zhao, S., K. C. Kwok, and H. Liang. 2007. Investigation on ultrasound assisted extraction of saikosaponins from Radix Bupleuri. Sep. Purif. Technol. 55: 307-312. [ Links ]
Zulkafli, Z. D., H. Wang, F. Miyashita, N. Utsumi, and K. Tamura. 2014. Cosolvent-modified supercritical carbon dioxide extraction of phenolic compounds from bamboo leaves (Sasa palmata). J. Supercrit Fluid. 94: 123-129. [ Links ]
Acknowledgements
Authors acknowledge financial support from Consejo Nacional de Ciencia y Tecnología (CONACyT) (through the funds of Project 207279) and also show their appreciation to Tania Martínez- Ramos for their technical support in the UAE procedure.
Received: March 2015; Accepted: November 2015











 text in
text in