Services on Demand
Journal
Article
Indicators
Related links
Share
Agrociencia
On-line version ISSN 2521-9766Print version ISSN 1405-3195
Agrociencia vol.46 n.8 Texcoco Nov./Dec. 2012
Recursos naturales renovables
Seedlings of cashew trees of the brazilian cerrado inoculated with arbuscular mycorrhizal fungi and phosphate–solubilizing microorganisms
Plántulas de anacardo del cerrado brasileño inoculadas con hongos micorrízicos arbusculares y microorganismos solubilizadores de fosfato
J. Silva Rodrigues–Cabral1*, Kerlley Cristina–de Assis2, Fabiano Guimarães–Silva1, Edson Luiz–Souchie1, M. Aurélio Carbone–Carneiro3
1 IF Goiano – Câmpus Rio Verde. Rod. Sul Goiana Km 01, Cx. Postai 66. 75.901–970. Rio Verde – Goiás, Brasil. *Author for correspondence: (jsrcabral@gmail.com).
2 Embrapa Clima Temperado Rodovia BR 392, Km 78, Cx. Postal 403. 96.010–971. Pelotas – Rio Grande do Sul, Brasil.
3 Universidade Federal de Lavras, DCS / Laboratório de Microbiologia do Solo, 37200–000, Lavras – Minas Gerais, Brasil.
Received: September, 2011.
Approved: September, 2012.
Abstract
Soil mictobiota catties out important functions in ecosystems, since it influences growth, mineral nutrition and plant health. Phosphorus (P) is the most limiting nutrient in tropical soils and P–solubilizing microorganisms (PSMs) and the arbuscular mycorrhizal fungi (AMF) are the most important groups of the soil microbial community. The aim of this study was to evaluate the effect of inoculating PSMs and AMF on the development of cashew trees (Anacardium othonianum Rizzini) from the Brazilian Cerrado growing on different substrates. An experiment was carried out in a greenhouse and the experimental design was completely randomized with a factorial 4 × 2 arrangement of treatments: PSMs, AMF, PSMs + AMF and the control. In addition, two substrates were used: a pure one with a sandy loam texture and a mixture of clay loam and clayish textures. Twelve replicates were performed. Data were subjected to an analysis of variance, and means were compared with the Tukey test (p<0.05). The co–inoculation with PSMs and AMF resulted in greater height and shoot dry matter of seedlings compared to isolated inoculations with these organisms. Seedlings increased height and shoot fresh and dry matter when grown in a mixture of substrates (soil with clay loam and clay textures). The use of a mixture of substrates also resulted in a greater symbiotic efficiency of Glomus etunicatum.
Keywords: Anacardium othonianum, promotion of plant growth, mycorrhiza.
Resumen
La microbiota del suelo desarrolla importantes funciones en los ecosistemas, ya que influye en el crecimiento, la nutrición mineral y la salud de las plantas. El fósforo (P) es el nutrimento más limitante en los suelos tropicales, y los microorganismos solubilizadores de P (MSP) y los hongos micorrízicos arbusculares (HMA) son los grupos más importantes de la comunidad microbiana del suelo. El objetivo de este estudio fue evaluar el efecto de la inoculación de MSP y HMA en el desarrollo de árboles de anacardo (Anacardium othonianum Rizzini) de el Cerrado Brasileño con distintos sustratos. Se realizó un experimento en invernadero y el diseño experimental fue completamente aleatorio con un arreglo factorial de 4 × 2 de los tratamientos: MSP, HMA, MSP+HMA y el control. Además, se usaron dos sustratos: uno puro, con textura franco arenoso, y una mezcla de texturas franco arcillosa y arcillosa. Se realizaron 12 réplicas. Los datos se procesaron con un análisis de varianza y las medias se compararon con la prueba de Tukey (p<0.05). La coinoculación con MSP y HMA resultó en mayor altura y materia seca del vástago de las plántulas comparado con las inoculaciones aisladas con estos organismos. Las plántulas aumentaron su altura y materia húmeda y seca del vástago al cultivarse en una mezcla de sustratos (suelo con texturas franco arcillosa y arcillosa). El uso de una mezcla de sustratos también resultó en una eficiencia simbiótica mayor con Glomus etunicatum.
Palabras clave: Anacardium othonianum, fomento de crecimiento vegetal, micorrizas.
INTRODUCTION
Inorganic P–solubilizing microorganisms (PSMs) play an important role in supplying phosphorus (P) to plants while the solubilizing action is mainly associated with the production of organic acids (Whitelaw, 1999). Inoculation with PSMs either in combination with other beneficial soil microorganisms or by itself may enhance plant development (Silva Filho and Vidor, 2001; Narloch et al., 2002; Souchie et al., 2006). In the soil, PSMs contribute to an increased P concentration in solution, which can be absorbed directly by roots or hyphae of symbiotic arbuscular mycorrhizal fungi (AMF; Moreira and Siqueira, 2006). Likewise, P–solubilizing bacteria may play an important role in interactions between roots and AMF acting as mycorrhiza helper bacteria (Fester et al., 1999). Similarly, there are fungal species that can also enhance plant growth through phosphate solubilization (Soares et al., 2010).
Arbuscular mycorrhizal fungi are recognized by the various positive effects on plant growth, namely, better uptake of nutrients, particularly P, increased volume of exploited soil and higher tolerance to biotic and abiotic factors (Locatelli and Lovato, 2002). The use of these microorganisms may benefit the seedling development of tree species in nurseries, maximizing their ability for establishment in the field (Souchie et al., 2005).
The genus Anacardium is composed of 10 species of tropical trees and shrubs, in particular, Anacardium othonianum Rizzini, the cashew tree of the Cerrado, is distinguished from other species due to its tree scale and its economic importance, because of this is the principal cashew species of the Midwest region of Brazil (Vieira et al., 2006). The trees of this species reach heights between 3 and 6 m, with boles measuring 1–2 m in height and 20–40 cm in diameter. The fruit is an achene that develops into a pseudofruit, which has several forms and colors, ranging from yellow to red. Therefore, this double fruit is a characteristic of this genus and consists of the combination of the fruit (nut) and the pseudofruit (Correa et al., 2008). This species is found in Brazilian Campo Sujo and Cerradao, and it is a productive species whose seeds germinate easily. Flowering occurs between June and October, with a yield of 200–600 fruits per plant. Its development under environments associated with concretionary soils with high slopes denotes its high potential for the exploration, preservation and management of large areas of the Cerrado (Vieira et al, 2006).
Among the factors that influence the production of seedlings of some species, the type of substrate is important because it directly reflects the quality of the final product. An ideal growth substrate should present homogeneity, low density, good porosity, suitable field capacity and cation exchange as well as be free of pests, pathogens and weeds. In a nursery, substrates must exhibit resistance to the development of pests and diseases, be operational at any time, be abundant and affordable and present good adhesion among particles or adherence to roots. A single material that meets all of these requirements is difficult to find. Therefore, a mixture of two or more materials is preferred to obtain a suitable substrate of good quality (Santos et al., 2000; Cunha et al, 2005; Lacerda et al., 2006).
Selection of materials to produce a substrate should take into consideration the species to be grown, the production conditions (irrigation system, type of fertilization and container size), the availability and price of the material as well as technical issues related to their use. Substrates influence on the architecture of root systems, nutritional status of plants and translocation of water in the soil–plant–atmosphere system (Santos et al., 2000). The most important physical aspect of a substrate is a porous structure for storing and supplying water to plant roots and, at the same time, providing adequate aeration. In addition, the most important chemical characteristics are pH and total concentration of soluble salts because they influence the supply of fertilizers (Santos et al., 2000; Lacerda et al., 2006; Pacheco et al. , 2006).
The objective of this study was to evaluate the effect of inoculation of cashew trees of the Cerrado (Anacardium othonianum Rizzini) with PSMs and AMF grown on two different substrates.
MATERIALS AND METHODS
The experiment was carried out in greenhouse, at the Instituto Federal Goiano (IF Goiano–Campus Rio Verde) using plastic pots with a capacity of 300 cm3. Seeds of cashew trees of the Cerrado were obtained from ripe fruits collected at the Gameleira Farm in the municipality of Montes Claros de Goiás, Goiás, Brazil.
Two types of non–sterile substrates were used; a pure substrate with sandy loam texture (Quartz–Sand Neosol) collected between 10 and 40 cm of depth at the Gameleira Farm; and a mixture 1:1 (v:v) of a red Argisol (clay loam texture), collected at the Rio Preto Farm, and a dystrophic red Latosol(clay texture), collected at the same depth in an area of the IF Goiano–Campus Rio Verde. Substrates were not fertilized, and no lime was applied.
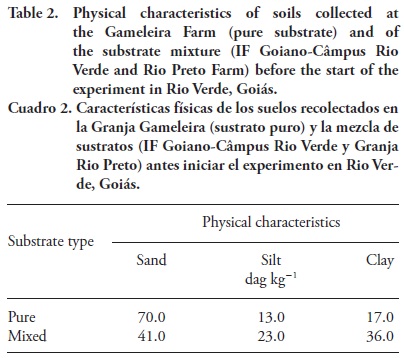
Analyses of chemical and physical characteristics of the soils were performed at the Laboratory of Soil Analyses at the Universidade Federal de Lavras according to the Embrapa methodology (Empresa Brasileira de Pesquisa Agropecuaria, 1997; Tables 1 and 2).
Five isolates of PSMs were utilized: three of them were P–solubilizing bacteria (PSB) and two were P–solubilizing fungi (PSF) obtained from the rhizosphere of wheat, sunflower, murici (Byrsonima verbascifolia), rice (Oryzasativa) and guapeva (Pouteria gardineriana). Wheat, rice and sunflower were cultivated for 20 d in a distroferric Red Latosol of medium texture, collected at a depth of 10 to 40 cm in a region of the IF Goiano–Campus Rio Verde. To isolate PSMs from tree species (murici and guapeva), three samples were collected from the rhizosphere soil of each tree in areas of the Cerrado preserved in Montes Claros de Goiás.
To obtain PFMs, 10 g sample of rhizosphere soil were mixed with 90 mL of a saline solution (0.85 %), followed by successive dilutions until obtaining a 10–5 final dilution. Aliquots (200 mL) from each dilution were transferred to sterilized petri dishes, followed by the addition of GL medium (2 g yeast extract, 10 g glucose and 15 g agar) at 45 °C containing CaHPO4 (10 %), formed by the addition of CaCl2 (10 %) and K2HPO4 (10 %) according to the method of Sylvester–Bradley et al. (1982). The appearance of a transparent halo in contrast to opaque medium around a colony of PSB or PSF isolates was indicative of phosphate solubilization. To confirm the P–solubilizing capacity, a sample of fungal and bacterial colonies that showed a clear halo around their colonies was removed using a platinum loop to replicate it in another petri dish containing solid GL medium. Colonies were considered to be positive when, after purification, they were able to solubilize CaHPO4 present in the medium (Barroso and Oliveira, 2001). To standardize the five isolates of PSMs, each was grown in a separate 125 mL Erlenmeyer flask containing liquid GL medium and incubated at 28 °C for 72 h. For direct counting of colony forming units (CFU), successive dilutions were performed until reaching a final dilution of 10–5. Then, three replicates of dilutions 10–4 and 10–5 were plated and incubated at 28 °C for 72 h. The inoculum was standardized to 108 CFU mL–1 and mixed (1:1; v:v) at the time of seed inoculation.
The mycorrhizal inoculum was formed with Glomus etunicatum acquired from the Laboratório de Solos–UFG/Campus Jatai. At sowing time the tubes that received AMF were inoculated in the planting orifice with 3.3 g of AMF inoculum (10 spores mL–1 of soil). For the inoculation with PSMs, seedlings were inoculated with 1 mL of liquid inoculant mixture (108 CFU mL–1) at the base of each seedling 62 d after emergence (DAE). During the experiment, seedlings were watered twice a day.
The seedlings were harvested at 120 DAE, and the stem diameter, shoot height, fresh and dry matter of shoots and roots, root volume, percentage of colonization by AMF, P available in the substrate and shoot N, P, K, Ca, Mg, S, Na, Cu, Fe, Mn, Z, Co, Mo and B were evaluated.
For the determination of root colonization, fractions of approximately 1 g of roots were separated and preserved in a 50 % ethanol alcohol solution. Afterwards, to evaluate the colonization by AMF, the roots were cleared and stained according to the method of Phillips and Hayman (1970), and the percentage of root colonization was measured with an optical microscope at a 200 x magnification (McGonigle et al., 1990).
The experimental design was completely randomized with a 4 x 2 factorial arrangement of treatments: 1) inoculation (PSMs; AMF; PSMs + AMF; control no inoculation); 2) substrates (pure substrate; a substrate mixture). There were 12 replicates per treatments. Data were subjected to an analysis of variance, and means were compared with the Tukey test (p<0.05) using SISVAR (Ferreira, 2008).
RESULTS AND DISCUSSION
Height and shoot fresh and dry matter in the substrate mixture were higher compared with the pure substrate (Table 3). Co–inoculation with PSMs and AMF resulted in greater increases in those variables compared with the control only in the mixed substrates group.

The fact that seedlings showed better development in the mixture of substrates (loamy clay texture with clay texture) might be explained by its lower natural fertility (Table 1) compared to the pure substrate. Because this tree species generally adapts to low soil fertility (Cardoso Filho et al., 2008) the increased presence of nutrients might not stimulate plant growth, similar to a plant species subjected to a genetic improvement process. The mixture of substrates presented greater clay content (Table 2) that enhanced the retention of water available to plants, which might have reduced the effect of water stress.
The co–inoculation of PSMs and AMF resulted in an increased growth possibly due to synergism between these microbial groups. In this sense, PSMs possibly solubilize P adsorbed to clay colloids, while AMF increase the absorption and translocation to the seedlings. Tarafdar and Marschner (1995); and Niranjan et al., (2007) show an increased benefit of combined inoculation with these organisms compared to a single inoculation.
Caldeira et al. (2003) evaluated Glomus clarum and G. macrocarpum separately inoculated in leguminous trees and report an increase in the dry weight of shoots and roots. Likewise, the inoculation with Gigaspora margarita and G. clarum subject to low P levels (50 and 100 mg kg–1) resulted in an increased plant height, stem diameter and number of leaves of Jaracatia spinosa seedlings (Silva et al., 2009). According to Santos et al. (2008), red mimosa seedlings inoculated with Glomus etunicatum and rhizobia showed higher height and dry shoot matter content, compared with seedlings inoculated only with G. etunicatum. Similarly, co–inoculation with a mixture of AMF (G. margarita, G. etunicatum and Scutellospora nigra) and Rhizobium directly contributed to the biological fixation of N and nodulation of Leucaena seedlings, maximizing seedling formation (Araujo etal., 2001). In addition, co–inoculation of indigenous AMF (Glomus macrocarpum, G. etunicatum and Entrophospora colombiana) and Rhizobium in Acacia mangium Willd resulted in greater dry matter production and N content in shoots (Schiavo and Martins, 2003). According to Tristao et al. (2006), the development of coffee seedlings in conventional substrate (soil + manure) inoculated with G. margarita caused greater growth and biomass production.
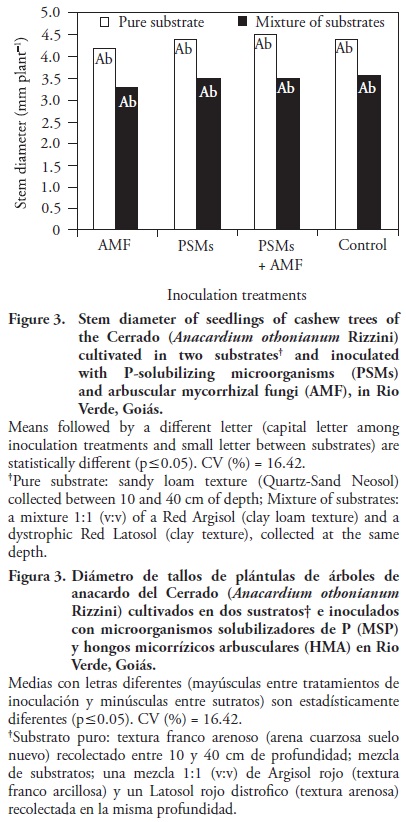
The soil mixture also increased root fresh matter and root volume (Figures 1A and 2). No differences were detected between substrates when comparing root dry matter (Figure 1B). In contrast, seedlings presented a greater stem diameter with the pure substrate (Figure 3).

The method used for assessing root colonization by AMF showed root fragments with good color and clarity from seedlings of cashew trees of the Cerrado grown in the pure substrate. However, similar coloration did not occur with root fragments of seedlings grown in the mixture because these were more pigmented and ligneous. The production of phenolic compounds, which increase the pigmentation of roots, is a common response of Cerrado plants during growth and development. These compounds resist the process of clarification with KOH (Detmann et al., 2008), requiring additional methods to clarify the roots for the assessment of mycorrhizal colonization.
In the pure substrate, treatments with PSMs + AMF and the control produced higher percentages of mycorrhizal colonization, (16.61 and 20.71 %), while the treatment with PSMs had the lowest value, 5–17 % (Table 4). The control treatment did not significantly differ from the treatments with AMF and PSMs + AMF. This result indicates that there was no response from seedlings to the inoculation with G. etunicatum and that the colonization detected in the control was due to the presence of AMF propagules in the substrate because it was not sterilized– Despite indigenous AMF species have not been identified in the substrate used for the control treatment, some AMF species were present and responsible for this plant mycorrhization result.

Regarding symbiotic efficiency (i.e. the difference in percentage of shoot dry matter production between mycorrhizal and non–mycorrhizal plants), the highest values were observed in treatments with AMF, while the inoculation had a negative effect on treatments with PSMs and PSMs + AMF in the pure substrate– For the mixture of substrates, the greatest result was obtained with the PSMs + AMF (Table 5).

The co–inoculation with PSMs + AMF resulted in a higher fresh root and shoot matter ratio in the pure substrate, while the highest values corresponded to the treatments only with AMF and the control– In the mixture, the highest ratio was obtained with the AMF treatment and the lowest with PSMs and PSMs + AMF (Table 6).

The highest values of fresh root matter and dry shoot matter corresponded to treatments with PSMs and PSMs + AMF in the pure substrate. Similarly, with substrates mixture, the AMF treatment showed the highest value.
At the end of the study, the substrates mixture showed higher content of available P (3.4 mg dm–3) compared with the pure substrate (0.5 mg dm–3). This outcome might be explained by the higher content of Ca and Fe in the pure substrate (Table 1). This characteristic favored the phosphate complexation reactions and resulted in lower availability of P in the soil solution.
No effects of the inoculation treatments were observed in the N content in the shoots grown in pure substrate (Table 7). However, for the mixture of substrates, a higher N content was observed in shoots inoculated with AMF compared with the control. Chu et al. (2001) and Weber et al. (2004) also report higher N contents in shoots of early dwarf cashew and soursop (Annona muricata L.) when inoculated with AMF. No difference was observed between the inoculation treatments and control in the levels of P, K, Ca, Mg, S, Na, Cu, Fe, Mn, Zn, Co, Mo and B in shoots of seedlings grown in the mixture of substrates (Table 7). Similarly, in the pure substrate there was no difference between the inoculation treatments regarding the levels of most of the nutrients in shoots. Cashew trees of the Cerrado, until the present, have not undergone any type of genetic improvement; therefore, it was more difficult to detect the effect of microbial inoculation treatments. Silva et al., (2003) point out the benefits of inoculation with AMF on the nutrition and growth of Eucalyptus, one of the few genetically improved tree species.
CONCLUSIONS
The co–inoculation with PSMs + AMF resulted in an increased height and shoot dry matter content of seedlings of cashew trees of the Cerrado compared to the control. Seedlings of cashew trees of the Cerrado demonstrated better performance if grown in a mixture of substrates (soil with loamy clay texture and clay). However, there was no preference when inoculating with Glomus etunicatum in seedlings grown in pure substrate. It seems that the mixture of soil substrates resulted in a greater symbiotic efficiency of Glomus etunicatum.
LITERATURE CITED
Araújo, A. S. F., H. A. Burity, and M. do C. C. P. Lyra. 2001. Influência de diferentes níveis de nitrogênio e fósforo em Leucena inoculada com Rhizobium e fungo micorrízico arbuscular. Ecossistema 26: 35–38. [ Links ]
Barroso, C. B., and L. A. Oliveira. 2001. Ocorrências de bacterias solubilizadoras de fosfato de cálcio nas raízes de plantas na Amazônia Brasileira. Rev. Bras. Ciênc. Solo 25: 575–581. [ Links ]
Caldeira, M. V. W., E. M. R. da. Silva, A. A. Franco, and L. F. Watzlawick. 2003. Influência de fungos micorrízicos arbusculares sobre o crescimento de três leguminosas arbóreas. Rev. Acad. Ciênc. Agrar. Ambient. 1: 27–32. [ Links ]
Cardoso Filho, J. A., E. E. P. de. Lemos, T. M. C. dos. Santos, L. C. Caetano, and M. A. Nogueira. 2008. Mycorrhizal dependency of mangaba tree under increasing phosphorus levels. Pes. Agrop. Bras. 43: 887–892. [ Links ]
Chu, E. Y., M. de R. F. Moller, and J. G. de. Carvalho. 2001. Efeitos da inoculação micorrízica em mudas de gravioleira em solo fumigado e não fumigado. Pes. Agrop. Bras. 36: 671–680. [ Links ]
Correa, G. de C., R. V. Naves, M. R. da. Rocha, L. J. Chaves, and J. D. Borges. 2008. Determinações físicas em frutos e sementes de Baru (Dipteryx alta Vog.), Cajuzinho (Anacardium othonianum Rizz.) e Pequi (Caryocar brasiliense Camb.), visando melhoramento genético. Biosc. J. 24: 4247. [ Links ]
Cunha, A. O., L. A. de. Andrade, R de L. A. Bruno, J. A. L. da. Silva, and V. C. de. Souza. 2005. Efeito de substratos e das dimensões dos recipientes na qualidade das mudas de Tabebuia impetiginosa (Mart. Ex D. C.) Standl. Rev. Árvore 29: 507–516. [ Links ]
Detmann, K. da S. C., Delgado, V. P. A. Rebello, T. de S. Leite, A. A. Azevedo, M. C. M., Kasuya, and A. M. de Almeida. 2008. Comparação de métodos para a observação de fungos micorrízicos arbusculares e endofíticos do tipo Dark septate em espécies nativas do Cerrado. Rev. Bras. Ciênc. Solo 32: 1883–1890. [ Links ]
Empresa Brasileira de Pesquisa Agropecuaria. 1997. Manual de Métodos de Análises de Solo. Embrapa. Rio de Janeiro. 212 p. [ Links ]
Ferreira, D. F. 2008. SISVAR: um programa para análises e ensino de estatística. Rev. Symposium 6: 36–41. [ Links ]
Fester, T., W. Maier, and D. Strack. 1999. Accumulation of secondary compounds in barley and wheat roots in response to inoculation with an arbuscular mycorrhizal fungus and co–inoculation with rhizosphere bacteria. Mycorrhiza 8: 241–246. [ Links ]
Lacerda, M. R. B., M. A. A. Passos, J. J. V. Rodrigues, and L. P. Barreto. 2006. Características físicas e químicas de substratos à base de pó de coco e resíduo de sisal para produção de mudas de sabiá (Mimosa caesalpiniaefolia Benth). Rev. Árvore 30: 163–170. [ Links ]
Locatelli, L. M., and P. E. Lovato. 2002. Inoculação micorrízica e aclimatização de dois porta–enxertos de macieira micropropagados. Pes. Agrop. Bras. 37: 177–184. [ Links ]
Mc Gonigle, T. P., M. H. Miller, D. G. Evans, G. L. Farchild, J. A. Swan. 1990. A new method which gives an objective measure of colonization of roots by vesicular–arbuscular mycorrhizal fungi. New Phytol. 115: 495–501. [ Links ]
Moreira, F. M. S., and J. O. Siqueira. Microbiologia e Bioquímica do Solo. UFLA. Lavras. 2006. 729 p. [ Links ]
Narloch, C., V. L. de. Oliveira, J. T. dos. Anjos, and G. N. Silva Filho. 2002. Respostas da cultura do rabanete à inoculação de fungos solubilizadores de fosfatos. Pes. Agrop. Bras. 37: 841–845. [ Links ]
Niranjan, R., V. Mohan, and V. M. Rao. 2007. Effect of indole acetic acid on the synergistic interactions of Bradyrhizobium and Glomus fasciculatum on growth, nodulation, and nitrogen fixation of Dalbergia sissoo Roxb. Arid Land Res. Manag. 21: 329–342. [ Links ]
Pacheco, M. V., V. P. Matos, R. L. C. Ferreira, A. L. P. Feliciano, K. M. S. Pinto. 2006. Efeito de temperaturas e substratos na germinação de sementes de Myracrodruon urundeuva Fr. All. (Anacardiaceae). Rev. Árvore 30: 359–367. [ Links ]
Phillips, J. M., and D. S. Hayman. 1970. Improved procedures for clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. British. Mycol. Soc. 55: 158–161. [ Links ]
Santos, C. B. dos., S. J. Longhi, J. M. Hoppe, and F. A. Moscovich. 2000. Efeito do volume de tubetes e tipos de substratos na qualidade de mudas de Cryptomeria japonica (L. F.) D. Don. Ciência Forestal 10: 1–15. [ Links ]
Santos, D. R. dos., M da C. S. Costa, J. R. P. de. Miranda, and R. V. dos. Santos. 2008. Micorriza e rizóbio no crescimento e nutrição em N e P de mudas de Angico–vermelho. Rev. Caatinga 21: 76–82. [ Links ]
Schiavo, J. A., and M. A. Martins. 2003. Produção de mudas de acácia colonizadas com micorrizas e rizóbio em diferentes recipientes. Pes. Agrop. Bras. 38: 173–178. [ Links ]
Silva, A. P. D., T. L. Garcia, O. Machineski, P. V. Truber, and E. L. Balota. 2009. Resposta do Jaracatia spinosa à inoculação de fungos micorrízicos arbusculares em diferentes níveis de fósforo. Synergismus Scyentifica 4:1–3. [ Links ]
Silva Filho, G. N., and C. Vidor. 2001. Atividade de microrganismos solubilizadores de fosfatos na presença de nitrogênio, ferro, cálcio e potássio. Pes. Agrop. Bras. 36: 1495–1508. [ Links ]
Silva, R. F. da., Z. I. Antoniolli, R. Andreazza, and S. J. Longhi. 2003. Fungos ectomicorrízicos na produção de mudas de Eucalyptusgrandis Hill ex Maiden. Bios. J. 19: 9–17. [ Links ]
Soares, A. C. F., A. da S. Sousa, M. da S. Garrido, and F. S. Lima. 2010. Isolados de estreptomicetos no crescimento e nutricao de mudas de tomateiro. Pes. Agrop. Trop. 40: 447–453. [ Links ]
Souchie, E. L., E. F. C. Campello, O. J. Saggin–Júnior, and E. M. R. da. Silva. 2005. Mudas de espécies arbóreas inoculadas com bactérias solubilizadoras de fosfato e fungos micorrízicos arbusculares. Floresta 35: 329–334. [ Links ]
Souchie, E. L., R. Azcón, J. M. Barea, O. J. Saggin–Júnior, and E. M. R. Silva. 2006. Phosphate solubilization and synergism between P–solubilizing and arbuscular mycorrhizal fungi. Pes. Agrop. Bras. 41: 1405–1411. [ Links ]
Sylvester–Bradley, R., N. Asakawa, S. La Torraca, F. M. M. Magalhaes, L. A. Oliveira, R. M. Pereira. 1982. Levantamento quantitativo de microrganismos solubilizadores de fosfatos na rizosfera de gramíneas e leguminosas forrageiras na Amazonia. Acta Amaz. 12: 15–22. [ Links ]
Tarafdar, J. C., and H. Marschner. 1995. Dual inoculation with Aspergillus fumigatus and Glomus mosseae enhances biomass production and nutrient uptake in wheat (Triticum aestivum L.) supplied with organic phosphorus as Naphytate. Plant Soil 73: 97–102. [ Links ]
Tristão, F. S. M., S. A. L. de. Andrade, and A. P. D. da. Silveira. 2006. Fungos micorrízicos arbusculares na formação de mudas de cafeeiro, em substratos orgânicos comerciais. Bragantia 65: 649–658. [ Links ]
Vieira, R. F., T. S. A. Costa, D. B. Silva, F. R. Ferreira, and S. M. Sano. 2006. Frutas nativas da região Centro–Oeste. Embrapa Recursos Genéticos e Biotecnologia. Brasília. 320 p. [ Links ]
Weber, O. B., C. C. M. de Souza, D. M. F. Gondin, F. N. S. Oliveira, L. A. Crisóstomo, A. L. Caproni, and O. J. Saggin–Júnior. 2004. Inoculação de fungos micorrízicos arbusculares e adubação fosfatada em mudas de cajueiro–anão–precoce. Pes. Agrop. Bras. 39: 477–483. [ Links ]
Whitelaw, M. A. 1999. Growth promotion of plant inoculated with phosphate–solubilizing fungi. Adv. Agron. 69: 99–151. [ Links ]














