Highlights
● The phenological growth stages of soursop trees were described using the extended BBCH-scale.
● A total of eight main phenological stages and 37 secondary ones were identified.
● Adequate descriptions and the quantification of some stages were given so that suitable agronomic practices can be established.
Introduction
The soursop (Annona muricata L.) is a fruit tree native to Central America, the West Indies, and northern South America (Pinto et al., 2005), though its current distribution also includes tropical areas in both Africa and Asia (Coria-Téllez, Montalvo-González, & Obledo-Vázquez, 2018). In Latin America, the fruit is widely cultivated in Colombia, Brazil, and Venezuela; however, Mexico is the leading producer and consumer worldwide (Anaya-Dyck et al., 2021), with a total cultivated area of 3,612 ha, average yield of 9.7 t·ha-1, and total production value of 248.1 million MXN (11.8 million USD) (Servicio de Información Agrícola y Pesquera [SIAP], 2021).
In Mexico, A. muricata L. is grown mostly in the states of Tabasco, Veracruz, Colima, Guerrero, and Nayarit (the latter concentrating ~71 % of the total cultivated land area, particularly in Compostela. a region responsible for 81 % of the total value of production nationally) (Andrés-Agustín & Andrés-Hernández, 2011; SIAP, 2021; Anaya-Dyck et al., 2021). Despite this, the agronomic management of this species is deficient, both in this region (Nayarit), and in the rest of Mexico. For instance, orchards are generally established using trees germinated from seeds as grafting is limited. Also, the planting density is low (280 plants∙ha-1), the irrigation system deficient or lacking, and phytosanitary control absent despite anthracnose and the seed borer (Bephratelloides cubensis) being serious issues. Furthermore, the pollination of flowers (which occurs naturally, and is not manually assisted) is deficient, resulting in fruits of a variable shape and size (Hernández-Fuentes, Nolasco-González, & Cruz-Gutiérrez, 2017; Berumen-Varela, Hernández-Oñate, & Tiznado-Hernández, 2019; Anaya-Dick et al., 2021). As a result, soursop losses are high (up to 3 t∙ha-1, with those attributed to handling and transportation constituting an additional 25-35 % of the total volume produced), yields low and irregular (in certain orchards, as low as 6-8 t∙ha-1), fruit quality variable, and postharvest life quite short (Anaya-Dick et al., 2021).
In this context, an aspect scarcely studied is the phenology of the crop itself, which can be used to implement suitable agronomic practices (e.g. pruning, defoliation, fertigation, etc.) and understand its life cycle (Liu et al., 2015). Phenology has previously been studied in numerous herbaceous plants of economic importance (e.g. rice, corn, sunflower, beans, peas, etc.) using the extended BBCH-scale, i.e., the Biologische Bundesantalt, Bundessortenamt, und Chemische Industrie, a descriptive system that assigns uniform codes to similar stages of growth in both mono- and dicotyledonous species (Meier et al., 2009). In fruits, the BBCH-scale can also be used to establish harvest indices that do not rely on external coloration, which is currently the main indicator used by most producers (Serrano, Guillén, Martínez-Romero, Castillo, & Valero, 2005).
Earlier studies have described the phenological stages of A. muricata L. under various climatic conditions in Brazil (Falcão, Lleras, & Leite, 1982; Nascimento, Filho, & Santos, 2002) and Venezuela (Yamarte et al., 2004; Yamarte, Marín, Baustista, & Avilán, 2006), but not in Mexico. Thus, the objective of this work was to study these stages in two soursop-producing regions of the state of Nayarit. For this, the extended BBCH-scale was consulted and applied.
Materials and methods
Location and plant material
Twenty ungrafted trees of A. muricata L. were selected for this study: ten from each of the following two locations: a 15-year-old commercial orchard located in Venustiano Carranza, Tepic (state of Nayarit), Mexico (21° 32’ 2.77’’ NL, 104° 58’ 39.73’’ WL, 893 m a. s. l.) and a 30-year-old orchard located in El Tonino, Compostela (also in the state of Nayarit) (21° 03’ 25.7’’ NL, 105.11’ 54.63’’ WL, 425 m a. s. l.). The climate in Tepic is classified as semi-warm, sub-humid and the one in Compostela as warm, sub-humid. The average yearly temperature in the two locations differs by 2 °C, with Tepic being generally rainier than Compostela (Table 1). All trees were established from seeds, which yielded a high level of morphological and size variability in the fruits produced (Hernández-Fuentes, Gómez-Jaimes, & Andrés-Agustín, 2013). The ultimate origin of their parent trees, however, could not be determined.
Table 1 Climatic conditions in the regions of study.
| Locality | Weather | Temperature (°C)* | Altitude (m) | Annual rainfall (mm)* | ||
|---|---|---|---|---|---|---|
| Average | Average max. | Average min. | ||||
| Tepic | AC(w) | 21.1 | 28.8 | 13.3 | 963 | 1451.9 |
| Compostela | A(w) | 22.9 | 30.2 | 15.7 | 834 | 971.4 |
Source = Servicio Meteorológico Nacional (SMN, 2019); Instituto Nacional de Estadística y Geografía (INEGI, 2017). *Period 1951-2010.
Four branches per tree (127.8-131.5 cm in length and 6.2-10.3 cm in diameter) were marked in each of the four cardinal directions, as were 15 resting buds in each branch (single subterminal flowers and grouped flowers appeared later on these branches). The markings were performed in order to study the phenological stages in these structures for approximately one year, from October, 2016 to November, 2017. For each phenological stage, observations were made either every eight days or twice a week. In both locations, the orchards lacked an irrigation system; thus, agronomic management was focused on sanitation, pruning, and the elimination of dead branches or shoots from the base of the main trunks.
Phenological development stages (BBCH-scale)
The BBCH-scale uses a decimal code system, which is divided into principal and secondary growth stages (Meier et al., 2009). The first digit of the scale corresponds to the principal growth stage, and its interval goes from 0 to 9. The second digit varies from 1 to 9 and specifies the mid-stages (i.e. mesostages) between the principal and secondary stages. Finally, the third digit is a numerical value between 0 and 9 that may refer to the percentage of growth that occurs in a particular organ/structure (e.g. leaf buds, leaves, shoots, flower buds, flowers, fruits, etc.) or to the percentage of progress in a particular physiological process (e.g. defoliation during senescence) (Liu et al., 2015).
The phenological stages of each sample were determined using eight of the ten principal ones outlined in the BBCH-scale (Cautín & Agustí, 2005; Meier et al., 2009), starting with bud development and ending with branch and leaf senescence. Additionally, the percentage of vegetative shoots, floral structures, fruit development, and overall defoliation was also quantified. Temperature and relative humidity (RH) were recorded with a data logger (U14-001, HOBO®, USA) that remained active during the entire period of study. Photographs were taken of each structure analyzed and were selected to illustrate the different phenological stages observed.
Statistical analysis
A comparison between the phenological stages of trees in the two orchards (i.e. in Compostela and Tepic) was performed using Student's t-test. These data were previously transformed using an angular method (Townend, 2002). Using the statistical software SAS v. 9.1 (Castillo-Márquez, 2011), simple correlations were also made between the presence of plant structures and the average temperature, RH, and precipitation.
Results and discussion
Climatic conditions in the regions of study
Monthly average temperature
In Compostela, the average temperature varied between 22 and 28.2 °C, with the average maximum (37 °C) occurring in October, 2017 and the average minimum (15°C) in December, 2016 (Figure 1A). The monthly average temperature in Tepic fluctuated between 19.8 and 23.6 °C, with the average maximum (34 °C) recorded in May, 2017 and the average minimum (10.7 °C) in January, 2017 (Figure 1B). These differences in temperature influenced both vegetative and reproductive growth, especially in leaves, shoots, and flowers (110-119, 311-319, and 510-519, respectively). As a result, the duration of these stages was shorter in Compostela (25, 51, and 36 d) than in Tepic (47, 73, and 42 d) (Table 2).
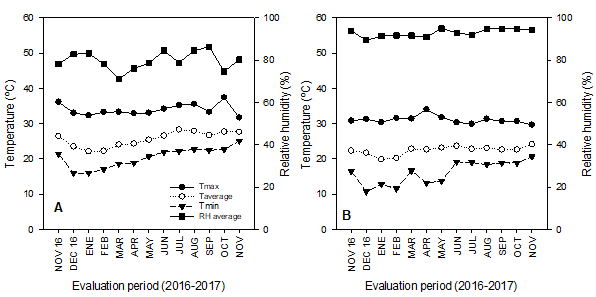
Figure 1 Temperature (T) and relative humidity (RH) in Compostela (A) and Tepic (B), state of Nayarit, Mexico.
Table 2 Phenological stages of soursop trees (A. muricata L.) cultivated in two regions of Nayarit, Mexico (Tepic and Compostela).
| Phenological stage | Main period | Duration (days) | |
|---|---|---|---|
| Tepic | Compostela | ||
| Leaf bud development (010 – 019) | April – July | 46 | 42 |
| Leaf development (110 – 119) | April – July | 47 | 25 |
| Shoot development (311 – 319) | April – July | 73 | 51 |
| Flower development (510 – 519)/ | September – December | 42 | 36 |
| Flowering (610 – 619) | September – December | 132 | 153 |
| Fruit development (710 – 719) | November 2016 – February (Tepic) July – September (Compostela) | 119 | 109 |
| Fruit maturity (810 – 819) | May – July | 8-10 | 8-10 |
| Senescence of leaf / senescence of branches (910 – 919) | October - November (Tepic) November – December 2016 (Compostela) | 20 | 17 |
According to Pinto et al. (2005) soursops are best cultivated between 21 and 30 °C as the fruit is susceptible to sudden changes in temperature, especially those below 12 °C. In Tepic, temperatures below 12 °C were recorded for approximately two months (i.e. from January to part of February) while in Compostela, minimum temperatures did not fall below 15 °C (Figure 1A). These data indicate that the best conditions for soursop development occurred in Compostela, and that ecotypes adapted to low temperature (prevalent during the winter season) may exist in Tepic.
Monthly average relative humidity
The monthly average RH registered in Compostela and Tepic fluctuated between 71-86 and 89-95 %, respectively (Figure 1A and B). The latter is important as RH is among the main determinants for pollination. For instance, Cárdenas-Torres (2002) reports that when RH is greater than 80 %, the percentage of pollination and fertilization increases so that more flowers turn into fruits, whereas Nakasone and Paull (1998) find that values lower than 30 % result in deficient pollination. Furthermore, the russeting of the peel (rind) in fruits close to physiological maturity can increase when RH is less than 60 % (Nakasone & Paull, 1998). The production system at both locations did not include irrigation, and under these conditions, RH remained close to the recommended interval (≥ 80 %). Only in Compostela were values lower than the 80 % threshold during the months of October and March (Figure 1A).
Annual pluvial precipitation
The total volume of this parameter (1,377.3 mm in Tepic; 1,403.2 mm in Compostela) as well as its temporal distribution (light precipitations from October-December, 2016; heavy precipitations from June 2016-September, 2017) were similar in the two regions of study (Figure 2A and B). This pattern appears to be acceptable for the phenological development of soursops as moderate fluctuations are conducive to adequate growth in A. muricata L. (Yamarte et al., 2006). Nevertheless, other studies insist that optimal development depends upon uniform rainfall year-round (Pinto et al., 2005).
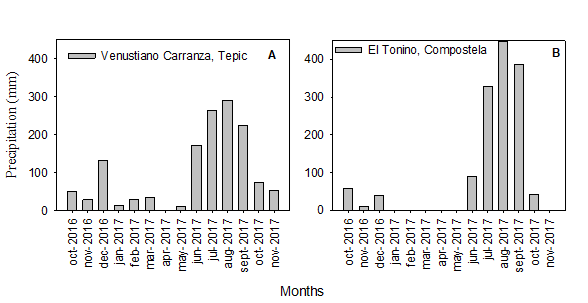
Figure 2 Annual precipitation in Tepic (A) andCompostela (B), state of Nayarit, Mexico (Source: SMN, 2019).
Description of phenological stages
In the life cycle of A. muricata L., eight main stages of growth were typified using the extended BBCH-scale: vegetative growth, which included the development of buds (stage 0), leaves (stage 1), and shoots (stage 3); flower growth, which included their emergence (stage 5) and opening (stage 6); fruit development, which included both fruit setting/growth (stage 7) and fruit maturity (stage 8); and lastly, the senescence of branches (implied by defoliation or the falling of leaves, stage 9). All of these are described in Table 3 and illustrated with photographs in Figure 3.
Table 3 Phenological stages of soursop (Annona muricata L.) cultivated in two regions of Nayarit, Mexico. 2016-2017*.
| Stage 0: Bud development |
| First vegetative flush (mesostage 1) |
| 010 Dormancy: buds are completely closed and covered by green-brown scales. A small ostiole (<2 mm in diameter) is visible. |
| 011 Leaf bud swelling starts: buds begin to swell and are more visible. |
| 013 Leaf bud swelling ends: bud scales are completely separated, lighter sections of the inner bud scales are visible. |
| 017 Bud burst starts: The apices of the leaves begin to be visible. |
| 019 Bud burst ends: shoot apices about 5 mm long are clearly visible above bud scales. |
| Stage 1: Leaf development |
| First vegetative flush (mesostage 1) |
| 110 First leaves start to separate: green scales slightly open. |
| 111 First leaves unfolded: first leaves visible and separated. |
| 115 More leaves unfolded: petioles become visible. |
| 117 First leaves completely expanded. |
| 119 All leaves unfolded and completely expanded. |
| Stage 3: Shoot development |
| First vegetative flush (mesostage 1) |
| 311 Shoot growth starts: approximately 10 % of their final length, axes become visible. |
| 313 Shoots are approx. 30 % of their final length. |
| 315 Shoots are approx. 50 % of their final length. |
| 317 Shoots are approx. 70 % of their final length. |
| 319 Shoots are approx. 90 % of their final length. |
| Stage 5: Flower emergence in stems and branches |
| Principal flowering (mesostage 1) |
| 510 Inflorescence bud swelling: buds closed and covered with yellow scales, without peduncle. |
| 514 Petals begin to elongate. |
| 515 Petals continue to elongate. |
| 517 Flowers still closed, but the petals continue to elongate. |
| 519 Most flowers closed, with fully elongated petals. |
| Stage 6: Flowering on stems and branches |
| Principal flowering (mesostage 1) |
| 610 First flowers open: petals begin to separate. |
| 611 Flowers partially opened. |
| 613 Early flowering: about 30 % of the flowers opened. |
| 615 Full flowering: more than 50 % of the flowers are completely opened. |
| 617 Flower fading: most of the petals fall off or dry out. |
| 619 Flowering ends: all petals have fallen off or dried out. |
| Stage 7: Fruit development on stems and branches |
| Main season of fruit development (mesostage 1) |
| 711 Fruit set: ovary growth starts. |
| 712 Fruit growth: approximately 10 % of its final size with green pericarp visible; round, oval, conical or heart-shaped |
| 713 Fruit is approximately 30 % of its final size. |
| 715 Fruit is approximately 50 % of its final size. |
| 717 Fruit is approximately 70 % of its final size. |
| 719 Fruit is approximately 90 % of its final size. |
| Stage 8: Maturity of the fruit on stems and branches. |
| Main season of fruit development (mesostage 1) |
| 815 Fruit ripening starts: average size is reached (full fruit), green opaque color and seeds become black. |
| 819 Fruit ripening is completed: the typical rind color, firmness and flavor (taste and aroma) are acquired. |
| Stage 9: Senescence of branches |
| Principal vegetative flush (mesostage 1) |
| 912 Senescence starts: old leaves begin to fall. |
| 915 Senescence continues: fall of the leaves increases. |
| 917 Most of the leaves have fallen. |
*Description of growth stages are illustrated with photographs in Figure 3.

Figure 3 The eight main phenological stages of soursop (Annona muricata L.) as determined with the extended BBCH-scale. The trees were cultivated in orchards located in Compostela and Tepic, state of Nayarit, Mexico.
The stages of vegetative growth (i.e. the development of buds [010-019], leaves [110-119], and shoots [311-319]) occurred in high proportion from November, 2016 to June, 2017 in both orchards; however, they decreased from August-November in Compostela, and to a lesser extent, in Tepic, with a more active growth phase (P ≤ 0.05) occurring from October-November in the latter’s case (Figure 4A). Thus, vegetative growth in Tepic was apparently continuous and required almost 166 days to complete (compared to the 118 days in Compostela, a region with higher overall temperatures) (Table 2). Although reports on the duration of vegetative growth in A. muricata L. are scarce and not strictly comparable with those of this study, similar time frames to those in Tepic (182 days) are also observed in Annona squamosa L. (Liu et al., 2015).
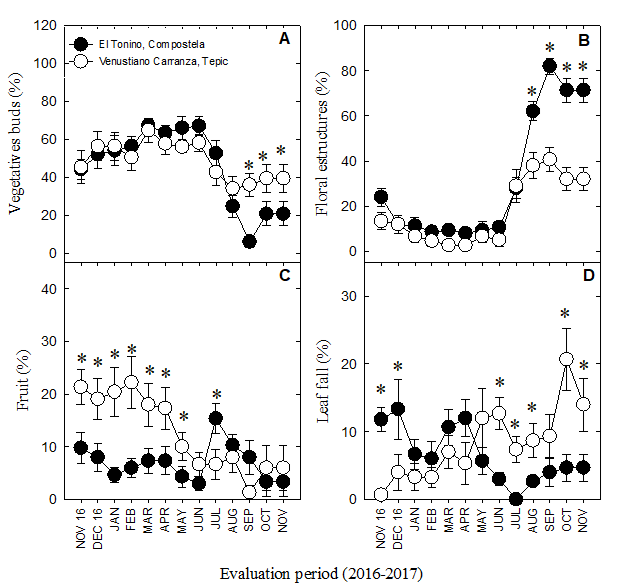
Figure 4 Year-long progress of the phenological stages in soursop trees (A. muricata L.). The trees were cultivated in two regions of the state of Nayarit, Mexico (Tepic and Compostela). Vegetative buds (A), floral structures (B), fruit growth (C), defoliation (D). * Significant differences (P ≤ 0.05) between regions according to the least significant difference test.
According to Yamarte et al. (2006), growth and branching are scarce during periods of drought, with shoot formation occurring mostly during the rainy season. Despite this, we found active growth of vegetative buds during the dry season (i.e. from January to May; Figure 2). Cruz-Castillo et al. (2002) indicate that in Puerto Rico the growth of the branches is continuous during the months of January and August; this is similar to what was found in the present work. These authors mention the hypothesis that the greater partition of carbohydrates in soursop is not directed towards the formation of fruits, but towards vegetative growth, which is observed in excessive vegetative growth of this species.
On the other hand, the percentage of floral structures decreased slightly in both Compostela and Tepic from November to January, though they later remained stable until June. At this point, they began to increase, reaching a peak in September before decreasing once more (Figure 4B). Significant differences between both orchards were detected only in the last four months (August-November), with higher percentages in Compostela (P ≤ 0.05). Overall, the period from the emergence of floral buds (stage 5) to the end of flowering (stage 6) lasted 174-189 days in both orchards (Table 2); however, Yamarte et al. (2004) report only 98 days from the time of floral differentiation until fruit setting (i.e. also in ungrafted soursop trees). Cruz-Castillo et al. (2002) and, Rosas and Becerra (2012) note that 31-90 days are necessary from the beginning of floral bud development until the start of anthesis. This discrepancy in the duration of flowering could be a result of the age of the trees themselves, their access to minerals and nutrition, and to water relations (Avilán-Rovira, Leal-Pinto, & Bautista-Arellano, 1992).
Falcão et al. (1982) report the presence of flowers throughout most of the year in soursops cultivated in Brazil, with a greater occurrence from September-January. Similarly, in Zulia (Venezuela) peak flowering occurs at the end of the rainy season (July-August) (Yamarte et al., 2004), while in Veracruz (Mexico) two main periods of increased production are present: one from May-July and the other from September-November (Rosas & Becerra, 2012). For their part, Escobar, Zarate, and Bastida (1986) note that flowering in humid regions occurs from November- May, while in drier climates it takes place from June-August.
In this study, we additionally found a positive correlation between average temperature and the presence of floral structures in soursops from Compostela (r = 0.56*), suggesting that flowering might also be favored by warm climates. This is not unreasonable, as 25-28 °C temperatures do appear to favor this process in A. muricata L. (Yamarte et al., 2004), and the average temperature in Compostela fluctuated between 26.7 and 28.2 °C from August-November (Figure 1A), coinciding with the greatest number of flowers (Figure 4B). Generally, flowering is observed year-round in Nayarit due to: 1) the presence of favorable temperatures across the state (including those in Tepic and Compostela) and 2) the overlap of phenological stages (e.g. flowering, fruit development, fruit maturity) in genetically-variable, seed-propagated trees (Hernández-Fuentes et al., 2017).
In both orchards, the period from fruit setting to physiological maturity took 114 d on average (Table 2). Similar times are also reported in Colombia (100 d) for this same phenological period (Escobar et al., 1986), with the difference again residing in the age of the trees themselves, their varietal character, and the specific location of the fruit evaluated (among various other environmental factors). Likewise, Rosas and Becerra (2012) note 77-174 d from the time of fruit setting to harvest in 10 selections of A. muricata L. from Veracruz (Mexico), whereas in Manaus (Brazil), the main fructification period occurs from January-March (Falcão et al., 1982). In this last case, fructification times are likely linked to local climatic factors, especially the water regiment.
Defoliation occurred during the entire study period. For instance, from November 2016 to January 2017, percentages were highest in Compostela, while from February-March they were comparable between the two regions. This trend later reversed, however, with Tepic attaining the highest values (Figure 4D). Falcão et al. (1982) also note year-round defoliation in A. muricata L., with higher intensities from January-March. Likewise, Nascimento et al. (2002) report leaves falling year-round, though in this case, the periods of highest intensity are February-April and October-November. Generally, defoliation is attributed to low temperatures and a lack of water (Nascimento et al., 2002). In the latter’s case (lack of water), Falcão et al. (1982) and Nascimento et al. (2002) agree that the periods of highest intensity overlap with the dry season (though this climactic condition is also intrinsically prompted by lower temperatures). Indeed, in the present work, senescence was negatively correlated with both lower precipitation (r = 0.76**) and RH (r = 0.74**).
Phytosanitary pruning should therefore occur from May-July (after the main fruiting period harvest), followed by the application of contact fungicides as this would limit the development of certain diseases. The latter would especially be necessary in regions like Compostela, where A. muricata L. trees grow surrounded by leaf litter and fallen fruit, potentially exposing them to warm, humid, and poorly ventilated environments (Hernández-Fuentes et al., 2017; Anaya-Dyck et al., 2021). On the other hand, irrigation applied during flowering, fruit development, or the dry season, would increase fruit yields and quality, as well as avoid the severe defoliation that occurs in rainfed orchards as a result of water stress (Hernández-Fuentes et al., 2017). Light defoliation by chemical agents would likewise increase yields, especially if applied at the beginning of the dry season or as needed in times of low production (Cruz-Castillo & Cedeño-Maldonado, 1989).
Lastly, manual pollination of receptive stigmas (spanning anthesis to stigma fall) using pollen from semi- or fully-open flowers would help standardize fruit size and shape (Escobar et al., 1986), facilitating postharvest management. This could also replace natural pollination (either by autogamy or by insects such as beetles) which, despite being inefficient, still persists in many regions (e.g. Compostela).
Conclusions
Eight phenological stages of Annona muricata L., cultivated in two of the most important production regions in the world, are quantified and described here for the first time. For this purpose, the extended BBCH-scale was used, which was designed specifically for this type of work. Despite the similarity of climatic conditions, the phenological behavior of trees varied in terms of vegetative growth (166 d in Tepic vs 118 d in Compostela) and in the presence of floral structures (higher percentages in Compostela).











 text in
text in 



