Services on Demand
Journal
Article
Indicators
Related links
Share
Revista mexicana de ciencias geológicas
On-line version ISSN 2007-2902Print version ISSN 1026-8774
Rev. mex. cienc. geol vol.25 n.3 Ciudad de México Dec. 2008
Geochemical assessment of Simav geothermal field, Turkey
Evaluación geoquímica del campo geotérmico Simav, Turquía
Yildiray Palabiyik and Umran Serpen*
Istanbul Technical University, Faculty of Mines, Petroleum and Natural Gas Engineering Department, 34469 Maslak, Istanbul, Turkey.* serpen@itu.edu.tr
Manuscript received: April 11, 2008
Corrected manuscript received: June 4, 2008
Manuscript accepted: June 4, 2008
ABSTRACT
In this study, geochemical methods are used to reliably analyze and understand the Simav geothermal field –whose thermal –water is rich in terms of Na–HCO3–SO4 and is affected by groundwater which is low in Cl. The water is of meteoric origin and belongs mostly to the immature water group. Quartz and Na–K geothermometers are used to calculate the reservoir temperatures as 70–195 °C and 167–249 °C, respectively, and the Na–K–Mg geothermometer indicated temperatures of approximately 230–240 °C. The isotopic evaluation of the geothermal system indicates that the water in the Simav geothermal reservoir is 18O enriched, is fed by cold water from Nadargam and that the age of the water is older than 50 years. The alteration mineralogy of the field points out to reservoir temperatures between 160 °C and 250 °C in the thermal water. The activity diagrams of the thermal water indicate the existence of fluid–rock interaction and show that the water is in equilibrium with K–feldspar, muscovite, albite (Na–feldspar), Mg–chlorite and epidote minerals at a temperature range of 150–250 °C. The activity diagrams also point to a potential source that might be located in a deeper zone that is hotter than the reservoir currently used for production, which is consistent with the alteration mineralogy of the field and Na–K geothermometers. The mineral equilibrium diagrams yield reservoir temperature values that are in harmony with the values obtained from the production zone and silica geothermometers. According to the mineral equilibrium diagrams, it is probable that calcite precipitates at high temperatures and that silica precipitates at low temperatures.
Keywords: water chemistry, geothermometry, isotopic evaluation, fluid–rock interaction, fluid geochemistry, Simav geothermal field, Turkey.
RESUMEN
En este trabajo se usan métodos geoquímicos para analizar y entender el campo geotérmico Simav, cuyas aguas getérmicas son ricas en Na–HCO3–SO4 y son afectadas por aguas subterráneas con bajo contenido de Cl. El agua es de origen meteórico y pertenece principalmente al grupo de aguas inmaduras. Se usaron los geotermómetros de cuarzo y Na–K para calcular las temperaturas del reservorio en 70–195 °C y 167–249 °C, respectivamente, mientras que el geotermómetro de Na–K–Mg indicó temperaturas de aproximadamente 230–240 °C. La evaluación isotópica del sistema geotérmico indica que el agua del reservorio geotérmico de Simav está enriquecida en 18O, es alimentada por agua fría de Nadargam y tiene una edad mayor a 50 años. La mineralogía de alteración del campo indica temperaturas entre 160 C y 250 °C en el agua termal. Los diagramas de actividad de las aguas termales indican la existencia de interacción agua–roca y muestran que el agua está en equilibrio con feldespato potásico, muscovita, albita (feldespato sódico), clorita de Mg y epidota, en un rango de temperatura de 150 a 260 °C. Los diagramas de actividad también señalan una fuente potencial quepodria estar localizada en una zona más profunda y más caliente que el reservorio que actualmente está en producción, lo cual es consistente con la mineralogía de alteración del campo y los geotermómetros de Na–K. Los valores de temperatura del reservorio derivados de los diagramas de equilibrio mineral concuerdan con los valores obtenidos de la zona de producción y de geotermómetros de sílice. De acuerdo con los diagramas de equilibrio mineral es probable que calcita precipite a altas temperaturas y que sílice precipite a bajas temperaturas.
Keywords: Química de aguas, geotermometría, evaluación isotópica, interación agua–roca, geoquímica de fluidos, campo geotérmico de Simav, Turquía.
INTRODUCTION
The Simav geothermal field, one of Turkey's most important fields, is located in Kütahya's Simav graben system of western Anatolia. Known for the Eynal, Çitgöl and Nasa hot springs, the Simav geothermal field is primarily utilized forbalneotherapy and heating for greenhouses and residences. The geothermal fluid produced from the field has been used in a district heating system for the equivalent of 6,000 residences. In the near future, heating for the equivalent of 4,000 residences will be added to that figure. The utilization of the field for power generation is being favorably debated due to the fact that the reservoir temperature exceeds the temperature needed to heat the residences. There are two primary geothermal occurrences in the field: Eynal and Çitgöl–Nasa (Figure 1). Geologic, isotopic and water chemistry data from previous periods are used to generate a general geochemical evaluation that covers the time periods before and after the field began to be used for production, which has been going on since 1993. In addition, various geochemical softwares are used to interpret and evaluate the fluid–rock interaction as well as the current alteration mineralogy. Investigation is carried out to determine whether the geothermal system has a deeper and hotter component. The geochemical investigation is conducted to determine the origin, residence time and probable route of the geothermal fluid as well as to evaluate the issue of whether the temperature is sufficient to enable power generation from the geothermal system.

The purpose of this study is to use various geological, water chemistry and isotopic data to investigate the characteristic geochemical features of a hydrothermal system and to study the fluid–rock interaction with geochemical models.
GEOLOGICAL SETTING
The Simav geothermal field is located in the eastern part of Simav graben, approximately 4 km north of Simav town and on the NE edge of the Simav plain, which is separated from the mountain by a high and steep escarpment (Figures 1 and 2). The plain covers an area of about 70 km2 and is at an altitude of about 780 m asl. In contrast, Simav Mountain, which is a horst structure located to the south of the plain, reaches an altitude of 1,780 m asl.
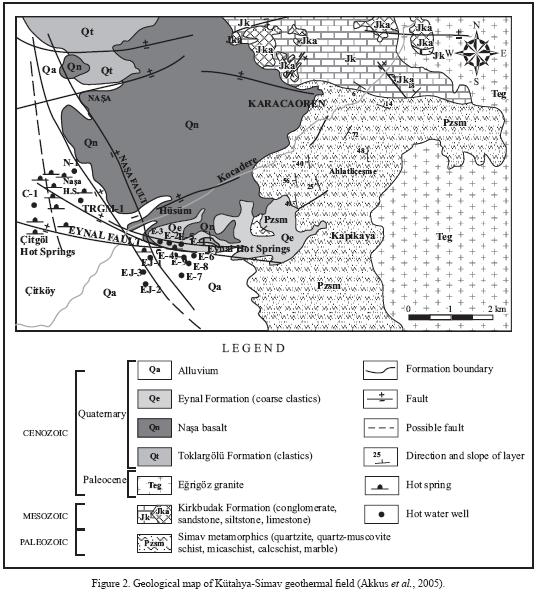
Geology
The stratigraphic sequence of the formations in the Simav region is given in Figure 2. Paleozoic metamorphic rocks are located at the base of the rock strata in the region. These rocks form the mountains that border the graben on both sides, and outcrop frequently in these mountains. Also, it is known that under the graben these rock units are underlain by younger sedimentary rocks. The metamorphic rocks are covered by a layer of lower Mesozoic rocks and Jurassic carbonates that have not been metamorphosed, which also overlay the metamorphites in the Simav horst to the south of the graben as well as in the relatively lower Akdag horst to the north. These rocks are overlain by volcanic rocks and lake sediments of Miocene age that were deposited in the graben along a NNE–SSW axis or formed in relation to those grabens. These formations appear on the Simav horst to the south of the graben as well as on ridges of the arm to the north of the graben, which has risen to a lower altitude. These are followed by younger formations which precipitated or formed together with the Simav graben or at a latertime (Akdeniz and Konak, 1979). These are spread out over the graben interior areas, which have been downthrown significantly compared with the horsts on both sides of the Simav graben. The coarse–grained terrestrial sediments, basaltic lava deposits and thick alluvium have created a layer in the graben that is hundreds of meters thick (Öngür, 2004). Based on the existing wells drilled in the region, the fractured reservoir rocks producing hot fluids in the field largely consist of Nasa Basalt, Simav metamorphics and Mesozoic limestones, while the cap rock consists of Tertiary strata of volcano–sedimentary rock.

Western Turkey and the southern Basin and Range, USA, are two of the best examples of large continental extension and exhibit remarkable similarities and some unique differences. The two regions contain structural features unique to extension such as metamorphic core complexes, extensional folds, shear zones, and detachment surfaces, although the origin of extension is different (Cernen et al., 2002). In extensional tectonic regimes like the Basin and Range province and the Anatolia Belt of western Turkey, extensional detachments have created favorable structures for geothermal occurrences.
Three crustal segments, namely central core, southern and northern submassifs, differing in structure and cooling history have been identified in Menderes Massif of western Turkey by Ring et al. (2003). The Simav detachment, formed later, reactivated the Eocene Cyclades–Menderes thrust and its initial movement was synchronous with the intrusion of Egrigoz granites (Ring et al., 2003).
After Miocene, western Anatolia has undergone the extensional regime that helped build the actual form. This process produced the Simav graben, which has an asymmetric structure. The southern part of the graben is limited by the Simav fault, which is roughly extended in west–east direction for more than 80 km and separates Simav Mountain from Simav plain. In addition to the Simav geothermal system, there are geothermal occurrences at both extremes of the Simav fault (Düvertepe and Saphane). Extending in a corridor–like narrow structure, the Simav fault is expanded around the Simav plain gaining the features of a typical graben–like structure with a triangular shape that resembles a pull–apart type basin, which is delimited by a few extensional faults (Eynal, Nasa) in the northern section.
The Simav fault with a slip of 1,000 m is located at the southern flank of Simav graben and formed at a later phase, probably during Pliocene, together with major grabens within the Menderes massif. Although the Eynal, Qitgol and Nasa hot springs discharge at the northern flank of the Simav graben along the Eynal and Nasa faults, deep conductive anomalies identified by geophysical studies are located at southern flank close to the Simav fault. These deep conductive anomalies are also related to shallower conductive anomalies found close to Eynal and Nasa faults. Therefore, it is believed that the deeply slipped Simav fault plays a primordial role in the formation of the Simav geothermal system.
Heat source
Hochstein et al. (1990) and Zhongke et al. (1990) described and studied low temperature fracture–zone systems in SE Asia. Turkey is also known to have many low to moderate temperature geothermal resources (Serpen and Mihcakan, 1999) and most of these resources are related to important fracture systems. The major geothermal systems of western Turkey are all structurally similar geothermal occurrences. A conceptual hydrological model may be hypothesized to explain the likely heat and mass transfer of the Simav geothermal system. The Simav fault penetrates very deep to communicate a deep heat sweep of naturally convecting meteoric waters in the metamorphic crust. Hochstein et al. (1990) pointed out that fracture zone systems, which are driven by higher than normal heat flow, occur in areas underlain by thinner continental type of crust (i.e., systems in the Basin and Range tectonic province of USA or Menderes massif of Turkey). An earlier theoretical study by Kassoy and Zebib (1978) also showed that fluid convection can occur in a narrow fracture zone which stands in a crustal environment with normal temperature. The Simav geothermal system is thought to be controlled by the active Simav fault and probably driven by higher than normal heat flow, 110 mW/m2 (Ilkisik, 1995), in the Aegean region of Turkey, and terrain–induced forced convection. With prevailing temperatures of 160 °C at economic depths (less than 1 km) within the fracture zone reservoir, the Simav geothermal system resembles "the fracture zone systems with high temperatures at sweep base" described by Hochstein (1990).
Alteration mineralogy
Unfortunately, we do not have access to any core sample or cuttings from drillings that have been taken from wells in the Simav geothermal field. But surface geological studies indicate alteration minerals alunite (Burcak et al., 2007) and kaolinite along the Simav fault (from Düvertepe to Saphane). Although the alteration mineralogy of the Simav geothermal field is not well known, the mineralogical data from 14 rock samples were presented in a study by Öktü (1984). The rock samples that have been taken from Simav, Kütahya and the surrounding areas were used for an evaluation of the alteration mineralogy in this study. In the light of these mineralogical data, the primary alteration minerals observed in the Simav geothermal field include chlorite, albite, K–feldspar, epidote, muscovite, illite, and montmorillonite. Of these minerals, the ones that are most notable with regard to hydrothermal alteration are chlorite, K–feldspar, illite, montmorillonite and epidote. This is because epidote (Ca[Al,Fe]3Si3O12OH) generally occurs in geothermal fields with temperatures between 200 and 250 °C (Bird et al., 1984). On the other hand, illite is a clay mineral that is identified with X–ray diffraction data and is generally observed infields with temperatures over 180 °C (Browne, 1996). From this information, itcanbe concluded that the Simav geothermal field is a high–temperature one, with alteration mineralogy that should be carefully studied in future.
HOT SPRINGS AND WELLS
There are hot water springs in the region that existed before the wells were drilled, although some of these springs still appear during the summer. According to Yücel et al. (1983), there are four large hot springs that outflow from alluvium and debris from slopes in the Eynal area and ten large springs that outflow from the alluvium in the Çitgöl–Nasa area. Öktü (1984) reported that there were a total of 89 hot water springs in the region, consisting of 34 in the Eynal area and 55 in the Çitgöl–Nasa area. Springs in the Eynal area are generally hotter than those in the Çitgöl–Nasa area and outflow in a E–W linear fashion along faults, with flow rates that vary between 0.02 L/s and 0.2 L/s, with a total flow of 2.1 L/s and temperatures that vary between 51 °C and 96 °C. The flow rates of springs in the Çitgöl–Nasa area vary between 0.15 L/s and 0.86 L/s, while temperatures vary between 34 °C and 86 °C.
Drilling in the region was started in 1985 by the General Directorate of Mineral Research and Exploration in Turkey (MTA). Currently there are a total of 11 exploration and production wells that are active in the region, including five in the Eynal hot springs area (E–1, E–2, E–3, E–7 and E–8) and three in the Çitgöl–Nasa hot springs area (C–1, C–2 and N–1). Later, three deeper exploration and production wells (EJ–1, EJ–2 and EJ–3) were drilled to the south of Eynal in conjunction with the Simav District Heating Project. The depth of the wells in the Simav field varies between 65.8 m (E–1) and 958 m (EJ–2) while the bottom–hole temperatures varied between 105 °C (C–1) and 162 °C (EJ–1). Information regarding the depth, level of production, temperature, production flow rate, reservoir rock type and date of drilling for certain wells in the region are shown in Table 1.
WATER CHEMISTRY
In the regional framework, the majority of west Anatolian geothermal waters are either Na–HCO3 or Na–Cl in nature, although SO4–type waters are also present. The waters are weakly acidic to alkaline with pH values ranging from 6.1 to 9.6, and have total dissolved solids (TDS) contents between 550 and 54,884 ppm (Mutlu and Gulec, 1998).
Chemical compositions of waters from thermal springs and wells from Eynal and Çitgöl–Nasa areas of Simav region are summarized in Table 2. The pH of most of the waters in the study area is between 7 and 9 giving them a slightly alkaline character that is close to neutral. The concentration of total dissolved solids in water vary between 1,400 and 3,000 mg/L. The waters from the Simav region can be classified as Na+>K+>Ca+ according to dominant cations and as HCO3–>SO42>C1– according to dominant anions. With HCO3– as the dominant anion and Na+ as the dominant cation, these waters are also classified as soda waters. Similar types of waters seem to occur in different parts of the Simav region. As is true with thermal springs like Gediz–Abide and Saphane in the surrounding regions, waters in Simav region are high in SO42– and F, and low in Cl– (Burcak et al, 2007). The primary source of high sulfate concentrations in waters is thought to be alunite that has formed from the alteration of tuffs that outcrop over a large area around Saphane, which is situated about 20 km east of Simav. It is very likely that thermal water in this region leaches sulfate from these rocks. On the other hand, the source of high F concentration in waters is probably the fluorite in the alteration zones of migmatitic and granitic rocks.
Thermal and cold springs in the Simav region are compositionally different. Underground waters in the region are rich in Ca+ with variable Mg2+ levels and generally low in alkali contents. Thus, Simav cold springs can be classified as CaMg(HCO3) waters. Chemistry of hot springs of the Simav region varies with distance from the Eynal fault. While concentrations of Ca+ and Mg2+ of hot springs and wells increase, Na+, K+ and SO42– concentrations decrease toward Çitgöl and Nasa areas. Since the chemical compositions of thermal springs and wells in the Eynal area are close to each other, a deep rising fluid component through the Eynal fault seems to be important in this area.
Instead of using ternary diagrams to describe trends for data, a simple approach is preferred to investigate bi–variate correlations for some chemical parameters utilized in ternary diagrams using conventional x–y regression analysis. Computer program OYNYL by Verma et al. (2006) was used for this purpose. This computer program has the unique feature of a built–in algorithm for discordant outliers detection following the methodology of Barnett and Lewis (1994). Table 3 presents the results of regression analysis for selected chemical parameters in fluids from the Simav geothermal field. As seen in Table 3, of ten pairs of data investigated, a statistically valid correlation at 99% confidence level is obtained for seven pairs: SO4–C1, Ca–Mg, Na+K–Ca, Na+K–Mg, Cl–B, HCO3–B, and HCO3–Cl. Of these statistically valid correlations, the highest correlation coefficients (around0.81) are obtained for the pairs SO4–C1 and Ca–Mg. Strong association of Ca–Mg may be attributed to the dissolution of carbonate rocks of Mesozoic and Paleozoic age. Marbles of metamorphic origin in the Menderes massif are known to contain abundant dolomite; therefore, their association might be expected. On the other hand, inverse relation in Na+K–Ca and Na+K–Mg pairs might be attributed to the enrichment of Na+K and depletion of Ca+Mg as a result of interaction of CO2 with water and rocks (Fametal, 1999). As for the association of Cl–B, they originate from deep waters circulating through Paleozoic age metamorphites.
GEOTHERMOMETERS
The computer program SolGeo (Verma et al., in press) was used for processing water chemistry data for solute geothermometry. From the Excel output file of this program, the most important results were extracted and are discussed below.
Silica geothermometers
The silica geothermometers used on the Simav thermal water samples are presented in Table 4. The silica concentrations for some well samples (E–2, E–3, C–l, N–l) taken in 1985 were very low (28–56 ppm), probably as result of sampling errors or precipitation, and were thus not included in this evaluation. Of the silica geothermometers, Fournier's (1977) quartz–maximum evaporation (88–177 °C), Founder and Potter's (1982) quartz–adiabatic cooling (86–179 °C), and Arnorsson's (2000) quartz (70–184 °C) geothermometers yielded a temperature range of 70–184 °C, indicating temperatures that are likely to be close to actual reservoir and bottom–hole temperatures, particularly on deep wells in the field. On the other hand, Fournier's (1977) quartz (85–191 °C), Fournier and Potter's (1982) quartz (85–192 °C), Verma and Santoyo (1997) quartz (85–192 °C) and Verma (2000) quartz (78–195 °C) geothermometers reported a temperature range of 78–195 °C, indicating a reservoir temperature approximately 10 °C higher than the aforementioned temperature range.
Cation geothermometers
For almost all of the water samples, the reservoir temperatures calculated using cation geothermometers (except for Na–Li and K–Mg geothermometers) were higher than temperatures calculated with silica geothermometers (Table 5). This is because silica geothermometers indicate the supply water temperature for the reservoir while the Na–K geothermometers in particular indicate deeper and hotter systems (see also Verma et al, in press).
Of the Na–K geothermometers, the highest temperatures (221–249 °C) were predicted by the geothermometer developed by Giggenbach (1988). Arnorsson's (1983), Fournier's (1979), Can's (2002) and Verma and Santoyo's (1997) Na–K geothermometers indicate temperature ranges (181–237 °C) that are similar to each other but higher than other geothermometers.
Temperatures calculated with the Na–Li geothermometer developed by Kharaka and Mariner (1989) indicated a reservoir temperature of 173–174 °C, which was similar to the quartz geothermometer results for Eynal area water samples. On the other hand, Na–Li geothermometer by Verma and Santoyo (1997) pointed out to bottom hole temperatures of 114–116 °C. Temperatures calculated with Giggenbach's (1988) K–Mg geothermometer, which reflects temperatures at shallow depths before the geothermal fluid reaches the surface, indicated a reservoir temperature range of 141–178 °C. Na–K geothermometers indicate higher temperatures than expected for geothermal fluids that have a high concentration of calcium (Ca). Since water in Eynal has a relatively low calcium content, the Na–K–Ca geothermometer developed by Fournier and Truesdell (1973) was applied for those samples. Reservoir temperatures in the range of 193–234 °C were estimated for those water samples, which are in harmony with the temperatures obtained by other Na–K geothermometers.
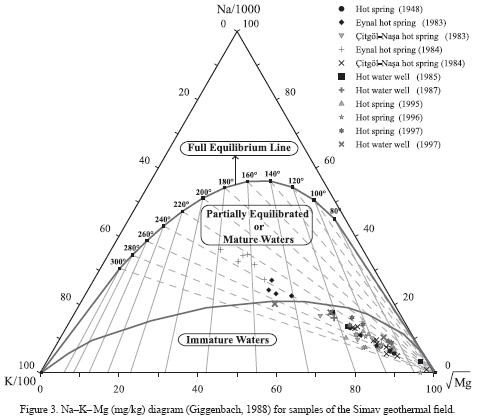
Na–K–Mg diagram
Figure 3 presents an evaluation of all of the Simav thermal water samples in a Na–K–Mg diagram developed by Giggenbach (1988). Caution is however required to use ternary diagrams (Butler, 1979; Philip et al, 1987). It is evident from the diagram that none of the Simav thermal water samples shows fluid–rock equilibrium and that the water is probably not fully equilibrated. It can be said that most of the water samples in the diagram fall in the region of immature water and on the border that separates the partially equilibrated water region from the immature water region. Aside from these samples, most of the spring water samples taken from the Eynal area in 1983 and 1984 fall in the region of partially equilibrated water. From this, it is possible to conclude that when compared with water in the Çitgöl–Nasa area, water in the Eynal area is more mature, less affected by processes caused by underground water (such as mixing, dilution, etc.) and is characterized as water that is closer to the conditions in the reservoir. In addition, because these samples fall in the region of partially equilibrated water or equilibrated and mature water, the application of the cation geothermometers on the samples will give somewhat reliable results. For this reason, the cation geothermometer temperatures for the water samples that fell in the region of partially equilibrated water were calculated (Table 5). On the other hand, the linear trend formed by all of the water samples on the diagram might indicate a reservoir temperature that is equilibrated at approximately 230–240 °C. This temperature is within the reservoir temperature range (205–249 °C) recommended by Verma and Santoyo (1997), Fournier's (1979) and Giggenbach's (1988) Na–K geothermometers.
K–Mg–Ca geoindicator diagram
If we assume that the geothermal fluid is equilibrated with calcite (CaCO3), it is possible to evaluate the partial pressure for CO2 (PCO2) on the K–Mg–Ca geoindicator diagram (Figure 4). In the diagram developed by Giggenbach (1988), almost all of the thermal water samples are under the line of full equilibrium and are located in the region of water that has not matured with the formation of calcite. In addition, most water samples tend to fall between the line for granitic rock dissolution and the line for average crust rock dissolution. From this diagrma, it is evident that water was formed as a result of rock dissolving into thermal water. This diagram shows that the water composition at final equilibrium temperatures (tkm) is limited by calcite precipitation due to CO2.
Na–K–Mg–Ca diagram
Another evaluation of temperature and fluid–rock equilibrium based on cation ratios can be performed with the Na–K–Mg–Ca diagram recommended by Giggenbach (1988). Figure 5 shows a diagram that evaluates 10Mg2+ /(10Mg2++Ca2+) versus 10K+ /(10K++Na+) for all of the Simav thermal water samples.
As is the case with the models in Figures 3 and 4, this model also does not indicate that the water samples have reached equilibrium between water and rock. However, if we investigate this diagram carefully, it is evident that the water samples from the Eynal area are closest to the complete equilibrium line for fluid–rock equilibrium, which is consistent with the model in Figure 3. Another conclusion drawn from the diagram is that most of the thermal water in the Simav region has become saline due to simple rock leaching or mixing with other water. In addition, by looking at the diagram it is easy to understand that the change in the ratio [10K+ /(10K++Na+)] (0.4–0.6) is significantly less than the change in the ratio [10Mg2+ /(10Mg2++Ca2+)] (0.1–0.9). This situation indicates that the K/Na ratio in the water is not strongly affected by the processes of dissolution and precipitation in the system. On the other hand, the fact that the Mg/Ca ratio is spread out over a large area indicates that the minerals that contain Ca and Mg (most likely carbonate minerals) are significantly affected by the dissolution and precipitation processes in the system.
Although the composition of the water samples do not indicate equilibrium between fluid and rock, it is evident that when the progressive trend of the group of water samples in the center of the diagram (which includes most of the water samples) is extended to the full equilibrium curve, a reservoir temperature consistent with the model in Figure 3 (230–240 °C) is indicated, pointing also to a deeper equilibrated source with a temperature of approximately 240 °C, which is hotter than the reservoir from which production is currently being carried out (approximately 160 °C).
ISOTOPE CHEMISTRY
The isotope chemistry of a geothermal system provides information about the origin, dating and residence time of the geothermal fluid, the physical processes to which the water is subjected, the fluid–rock interaction and the reservoir.
Origin and recharge of Simav thermal waters
Figure 6a shows the δ2H–δ18O relationship for Simav water. From the diagram it is evident that the isotopic components of hot water samples fall below the line of local meteoric water (Imbach, 1997). The cold water sample from Nadarçam, however, falls near the line of local meteoric water. In addition, when compared with the wells (E–l and E–7) and spring in the Eynal area, it is evident that the Nasa–2 spring in the Çitgöl–Nasa area has undergone more dilution with shallow water. As can be recalled from the previous models, water in the Çitgöl–Nasa area was more affected by dilution than water in the Eynal area. Figure 6a shows that Simav geothermal water is enriched in 18O. This situation points to the existence of fluid–rock interaction in the system and/or boiling due to the high temperature in the reservoir. Also, the change of δ2H content in the water may indicate that the system is fed by water from a high altitude and/or comes from a long distance away.
Because the amount of tritium in meteoric water is higher than that in geothermal water, the increase in tritium at geothermal surface manifestations indicates that the system is being fed by meteoric water. In this regard, 3H (tritium)–Cl diagrams can be used to identify the direction of recharge and the mixture between geothermal water and underground water. Figure 6b shows the 3H–C1 relationship for Simav water. Based on the diagram in Figure 6b and tritium and chloride data (Table 6), the cold water sample from Nadarçam has the highest 3H (10.57 TU) and the lowest Cl (8.9 ppm) content. A line drawn from the Nadarçam cold water to the geothermal well and hot spring indicates the mixing line for the Simav field. As a result, it can be said that the thermal water is being fed by meteoric water in the Nadarçam area.

Age of Simav thermal waters
Tritium (3H) is the isotope that is most widely used to identify the age and residence time of geothermal water. Table 6 shows the tritium data and the calculated ages for thermal water of Simav. From Table 6, it is apparent that the age of the water varies between about 35.7 and 60.6 years old. If we note that water containing 1–3 TU of tritium is considered to be 40–50 years old and that the tritium content of Simav geothermal water is between 0.36 and 1.44 TU, and if the error in the tritium measurement is taken into consideration, it can be said that Simav geothermal water is over 50 years old. However, it must not be forgotten that if geothermal water mixes with surface waters, this can cause errors in these age estimations.
FLUID–MINERAL EQUILIBRIA
An evaluation of the chemical equilibrium between fluid and rock in geothermal systems requires information about the solubility of minerals in rocks that have undergone hydrothermal alteration and about the activity of the mineral types in the solution. Because of the large number of ions, ion pairs and complexes in the solution, generating the activity values for each type using existing analytic data (particularly at increasing temperatures) requires the use of a computer program. In this study, we made use of WATEQ4F (Ball and Nordstrom, 1991) and SOLVEQ (Reed and Spycher, 1990). Another computer program, SUPCRT–92 (Johnson et al, 1992), was used to generate activity diagrams for minerals.
Activity diagrams
Activity diagrams were used to investigate the fluid–mineral equilibrium of the Simav geothermal system. Diagrams were plotted for reservoir temperatures between 150 °C and 250 °C based on bottom–hole temperature data and temperatures estimated with silica and cation geothermometers (Figures 7a–d).
The generated activity diagrams show that as temperature increases, the stability fields of albite (Figure 7c), epidote (Figure 7b), Mg–chlorite (Figure 7d), gibbsite (Figure 7a), and muscovite (Figure 7a, c, d) increase. This indicates that these minerals are more stable at higher temperatures. On the other hand, the stability of K–feldspar at increasing temperatures is limited by the higher activity of K and the lower activity of Na, Mg and Ca. In addition, at higher temperatures, muscovite has the broadest activity range for K, but has the lowest activity for Mg and Ca. From Figure 7c, it can be seen that paragonite (Na–montmorillonite) is stable at temperatures of 150 °C and above.
When we inspect each of the activity diagrams, it is apparent that almost all of the thermal water (except for Figure 7d) is in equilibrium with K–feldspar, which is congruent with the findings from the other models. In Figure 7d, however, it is apparent that the water is in equilibrium with Mg–chlorite. When the temperature rises over 175 °C, Figure 7c indicates that the water is in equilibrium with K–feldspar, albite and muscovite; Figure 7b indicates that the water equilibrated with K–feldspar at 150 °C and equilibrated with zoisite (epidote) at temperatures of 175 °C and above; while Figure 7a indicates that the water equilibrated with K–feldspar and muscovite. On the other hand, the fact that samples from spring Ey–2 and well EJ–1 are distinct from the other water samples and located in the upper portions of the diagrams is due to the high pH values (8.9 and 9.2) of these samples. In conclusion, since chlorite, albite, K–feldspar, epidote, muscovite and montmorillonite minerals were found in rock samples reported to be taken from the Simav environs, the activity diagrams concur with the field's alteration mineralogy and indicate that a potential source exists, which is a deeper and hotter than the reservoir from which production is currently being carried out.
Thermodynamic saturation states
Temperature–saturation index diagrams for minerals in the Simav thermal water (chalcedony, quartz, calcite, and muscovite) are shown in Figure 8a–d. The temperatures in the diagrams have been specified from the temperature at which each sample was measured (<100 °C) up to 250 °C in 25 °C increments such that they cover the temperature range calculated with chemical geothermometers and other models.
The diagram in Figure 8a was generated for chalcedony, a silica (SiO2) mineral, and indicates reservoir saturation temperatures of 125–150 °C. The diagram in Figure 8b was generated for quartz and indicates reservoir saturation temperatures of 150–175 °C, which correspond with the values obtained with the quartz geothermometers. The slope of the curves indicates that precipitation occurs for chalcedony and quartz from low temperatures until the saturation temperature is reached, but after saturation has occurred, the minerals dissolve in the geothermal fluid from the equilibrium temperature to higher temperatures. Figure 8c was generated for calcite, which is a calcium carbonate mineral (CaCO3), and indicates that the water is oversaturated in calcite at all temperatures and that precipitation of calcite is probably occurring in the Simav geothermal field. Figure 8d, generated for muscovite, indicates reservoir saturation temperatures of 135–170 °C, which is congruent with values obtained with silica and K–Mg geothermometers. The figure also indicates that the solubility behavior of muscovite is similar to that of chalcedony and quartz. The same diagrams were also generated using the hot water spring data provided by Öktü (1984), resulting in similar conclusions.
CONCLUSIONS
Thermal water in the Simav region can be classified as water that is affected by peripheral underground water that is rich in NaHCO3, contains high amounts of SO42– and low amounts of Cl–. Thermal waters in the Simav region are all related and have mixed close to the surface with underground water of meteoric origin. Thermal water from the Eynal area is less affected by dilution or mixing processes than water in the Çitgöl–Nasa area. Cation geothermometers applied to these samples will yield more reliable results than other samples.
Silica geothermometers indicate temperatures a little bit higher than the measured bottom hole temperatures (70–195 °C), and on the other hand, cation geothermometers and geoindicators give deep thermal water temperatures of 173–249 °C, which are consistent with the temperatures indicated by the field geological model and alteration mineralogy.
The delta deuterium (δ2H) –delta oxygen–18 (δ18O) diagram indicates 18O enrichment for Simav geothermal water. This situation points to the existence of fluid–rock interaction in the system and/or boiling due to the high temperature in the reservoir. On the other hand, tritium (3H) – chloride (Cl) diagram shows that the Simav thermal water is partly fed by meteoric water from the Nadarçam area. The tritium content of the samples indicates that the age of Simav geothermal water is older than approximately 50 years.
The alteration mineralogy of rock samples collected the region indicates that chlorite, albite, K–feldspar, epidote, muscovite, illite and montmorillonite are probably in equilibrium with geothermal water and that the reservoir temperature could be between 160 °C and 250 °C. The activity diagrams concur with the field alteration mineralogy and the cation geothermometers, pointing to the existence of a source that is deeper and hotter than the reservoir from which production is currently being carried out.
Saturation index diagrams generated for Simav geothermal water using certain alteration minerals generally indicate reservoir temperatures that are similar to those given by silica geothermometers. On the other hand, chalcedony, quartz and muscovite precipitate from low temperatures until the equilibrium temperature is reached but dissolve in the geothermal fluid as the temperature increases. Calcite, however, is oversaturated at almost every temperature and precipitates.
ACKNOWLEDGEMENTS
The authors would like to thank Ege Energy for providing data and would like to express special gratitude to the CEO, Muharrem Balat. Thanks are due especially to Dr. Halim Mutlu for his valuable help and to Tahir Öngür in particular for his valuable comments. We would like to extend our gratitude to Dr. Surendra P. Verma for his valuable efforts and help.
REFERENCES
Akdeniz, N., Konak, N., 1979, Geology of Simav–Emet–Tavsanli–Dursunbey–Demirci regions [in Turkish): Ankara, Mineral Research and Exploration of Turkey (MTA), 6547, 9–96. [ Links ]
Akkus, I., Akilli, H., Ceyhan, S., Dilemre, A., Tekin, Z., 2005, Turkey's Geothermal Resource Inventory (in Turkish): Ankara, Mineral Research and Exploration of Turkey (MTA), Inventory Series, 201, 521 p. [ Links ]
Arnorsson, S., 1983, Chemical equilibria in Icelandic geothermal systems, Implications for chemical geothermometry investigations: Geothermics, 12, 119–128. [ Links ]
Arnorsson, S., 2000, Isotopic and chemical techniques in geothermal exploration, development and use: Vienna, International Atomic Energy Agency, 156–187. [ Links ]
Ball, J.W., Nordstrom, D.K., 1991, User's manual for WATEQ4F, with revised thermodynamic data base and test cases for calculating speciation of major, trace and redox elements in natural waters: United States Geological Survey (USGS), Open–File Report, 91–183. [ Links ]
Barnett, V., Lewis, T, 1994, Outliers in statistical data: Chichester, John Wiley & Sons, 584 p. [ Links ]
Bayram, A.F., Simsek, S., 2005, Hydrogeochemical and isotopic survey of Kütahya–Simav geothermal field, in World Geothermal Congress, Antalya, Turkey, 843, 3–5. [ Links ]
Bevington, P.R., Robinson, D.K., 2003, Data reduction and error analysis for the physical sciences: Boston, McGraw Hill, 320 p. [ Links ]
Bird, D.K., Schiffman, P., Elders, W.A., Williams, A.E., McDowell, S.D., 1984, Calcsilicate mineralization in active geothermal systems: Economic Geology, 79, 671–695. [ Links ]
Browne, P.R.L., 1996, Hydrothermal Alteration: Auckland, University of Auckland, Geothermal Institute, Lecture Notes, 665.611, 32 p. [ Links ]
Burcak, M., Sevim, F., Hacisalihoglu, O., 2007, Discovering a new buried geothermal field which have been found out using geological–geophysical and geochemical methods in Uchbash–Shaphane, Kutahya western Anatolia, Turkey, in Thirty–Second Workshop on Geothermal Reservoir Engineering, Proceedings, Stanford: California, Stanford University, SGP–TR–183, 2–3. [ Links ]
Butler, J.C., 1979, Trends in ternary petrologic variation diagrams – fact or fantasy?: American Mineralogist, 64, 1115–1121. [ Links ]
Can, I., 2002, A new improved Na/K geothermometers by artificial neural networks: Geothermics, 31, 751–760. [ Links ]
Cemen, I., Seyitoglu, G., Isik, V., 2002, Extensional tectonics in southern Basins and Ranges,United States, and in western Turkey: A review of similarities, differences and problems, in Geological Society of America Annual Meeting, Denver Colorado, USA, Paper 79–1. [ Links ]
Caglar, K.Ö., 1948, Turkey's mineral waters and hot springs (in Turkish): Ankara, Mineral Research and Exploration of Turkey (MTA) Publications, 11,461–468. [ Links ]
Erisen, B., Can, A.R., Yildirim, N., 1989, EJ–I and EJ–II geothermal wells completion reports of Simav–Eynal (Kütahya) geothermal region (in Turkish): Ankara, Mineral Research and Exploration of Turkey (MTA), 8916, 60 p. [ Links ]
Fara, M., Chandrasekharam, D., Minissale, A., 1999, Hydrogeochemistry of Damt thermal springs, Yemen Republic: Geothermics, 28, 241–252. [ Links ]
Fournier, R.O., 1977, Chemical geothermometers and mixing models for geothermal systems: Geothermics, 5, 41–50. [ Links ]
Fournier, R.O., 1979, A revised equation for the Na/K geothermometer: Geothermal Resources Council (GRC) Transactions, 3, 221–224. [ Links ]
Fournier, R.O., Potter, R.W., 1982, A revised and expanded silica (quartz) geothermometer: Geothermal Resources Council (GRC) Bulletin, 11(10), 3–12. [ Links ]
Fournier, R.O., Truesdell, A.H., 1973, An empirical Na–K–Ca geothermometer for geothermal waters: Geochimica et Cosmochimica Acta, 37, 1255–1275. [ Links ]
Giggenbach, W.F., 1988, Geothermal solute equilibria. Derivation of Na–K–Ca–Mg geoindicators: Geochimica et Cosmochimica Acta, 52, 2749–2765. [ Links ]
Güven, M., Taskin, I., 1985, Simav–Nasa (Kütahya) hot water well (N–l) completion report (in Turkish): Ankara, Mineral Research and Exploration of Turkey (MTA), 7903, 12 p. [ Links ]
Hochstein, M.P., 1990, Geothermal resources: classification and assessment of geothermal resources: Pisa, Italy, International Institute for Geothermal Research, International School of Geothermics, 2, 31–57. [ Links ]
Hochstein, M.P., Zhongke, Y., Ehara, S., 1990, The Fuzhou geothermal system (People's Republic of China): Modelling study of a low temperature fracture–zone system: Geothermics, 19(1), 43–60. [ Links ]
Ilkisik, M., 1995, Regional heat flow in Western Anatolia using silica temperature estimates from thermal springs: Tectonophysics, 244, 175–184. [ Links ]
Imbach, T, 1997, Geology of Mount Uludag with emphasis on the genesis of the bursa thermal waters, northwest Anatolia, Turkey. Active tectonics of northwestern Anatolia, in Schindler, C, Pfister, M., Aksoy, A., Marmara Poly–Project (eds.), The Marmara Poly–Project: a Multidisciplinary Approach by Space–geodesy, Geology, Hydrogeology, Geothermics and Seismology: Zurich, vdf Hochschulverlag AG an der ETH, 253 p. [ Links ]
Johnson, J.W., Oelkers, E.H., Helgeson, H.C., 1992, A software package for calculating the standard molal thermodynamic properties of mineral, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000 °C: Computers and Geosciences, 18(7), 899–947. [ Links ]
Kassoy, D.R., Zebib, A., 1978, Convection fluid dynamics in a model of a fault zone in the earth crust: Journal of Fluid Mechanics, 88, 769–792. [ Links ]
Kharaka, Y.K., Mariner, R.H., 1989, Chemical geothermometers and their application to formation waters from sedimentary basins, in Naeser, N.D., McCulloh, T.H., (eds.), Thermal History of Sedimentary Basins: Methods and Case Histories: New York, Springer–Verlag, 99–117. [ Links ]
Mutlu, H., Gulec, N., 1998, Hydrogeochemical outline of thermal waters and geothermometry applications in Anatolia (Turkey): Journal of Volcanology and Geothermal Research, 85, 495–515. [ Links ]
Oygür, V., Erler, A., 2000, Metalogeny of Simav graben (in Turkish): Geological Bulletin of Turkey, 43(1), 7–19. [ Links ]
Öktü, G., 1984, Hydrological investigation of Eynal and Çitgöl–Nasa (Simav) hot springs (in Turkish): Ankara, Mineral Research and Exploration of Turkey (MTA), 7737, 15–44. [ Links ]
Öngür, T., 2004, Capacity of Simav geothermal region. Geological Evaluation (in Turkish): Ege Enerji Company, Report, 3 p. [ Links ]
Philip, G.M., Skilbeck, C.G., Watson, D.F., 1987, Algebraic dispersion fields on ternary diagrams: Mathematical Geology, 19, 171–181. [ Links ]
Reed, M.H., Spycher, N.F., 1990, User's guide for SOLVEQ: A computer program for computing aqueous–mineral–gas equilibria: Eugene: Oregon, University of Oregon, Department of Geological Sciences, 4–37. [ Links ]
Ring, U., Johnson, C., Hetzel, R., Gessner, K., 2003, Tectonic denudation of a Late Cretaceous–Tertiary collisional belt: Regionally symmetric cooling patterns and their relation to extentional faults in Anatolide belt of western Turkey: Geological Magazine, 140(4), 421–441. [ Links ]
Serpen, U., Mihcakan, M., 1999, Heat flow and related geothermal potential of Turkey, in Geothermal Resources Council (GRC) Annual Meeting, GRC Transactions, 23, 485–490. [ Links ]
Verma, M.P., 2000, Revised quartz solubility temperature dependence equation along the water–vapor saturation curve, in World Geothermal Congress, Kyushu–Tohoku, Japan, 1927–1932. [ Links ]
Verma, S.P., Santoyo, E., 1997, New improved equations for Na/K, Na/Li and SiO2 Geothermometers by outlier detection and rejection: Journal of Volcanology and Geothermal Research, 79, 9–23. [ Links ]
Verma, S.P., Quiroz–Ruiz, A., 2006a, Critical values for six Dixon tests for outliers in normal samples up to sizes 100, and applications in science and engineering: Revista Mexicana de Ciencias Geológicas, 23(2), 133–161. [ Links ]
Verma, S.P., Quiroz–Ruiz, A., 2006b, Critical values for 22 discordancy test variants for outliers in normal samples up to sizes 100, and applications in science and engineering: Revista Mexicana de Ciencias Geológicas, 23(3), 302–319. [ Links ]
Verma, S.P., Díaz–González, L., Sánchez–Upton, P, Santoyo, E., 2006, OYNYL: A new computer program for ordinary, York, and New York least–squares linear regressions: World Scientific and Engineering Academy and Society (WSEAS), Transactions on Environment and Development, 2(8), 997–1002. [ Links ]
Verma, S.P, Pandarinath, K., Santoyo, E., in press, SolGeo: A new computer program for solute geothermometers and its application to Mexican geothermal fields: Geothermics. [ Links ]
Yurtseven, D., Özgür, R., Uzel, Ö.F., 1998, Kütahya–Simav Eynal E–7, E–8 and EJ–3 Wells Completion Report (in Turkish): Ankara, Mineral Research and Exploration of Turkey (MTA), 18–57. [ Links ]
Yücel, B., Coskun, B., Demirci, S., Yildirim, N., 1983, Geology and geothermal potential of Simav (Kütahya) Region (in Turkish): Ankara, Mineral Research and Exploration of Turkey (MTA), 8219, 27 p. [ Links ]
Zhongke, Y, Shengbiao, H., Hochstein, M.P., 1990, Conceptual model of the Zhangzhou low temperature system and its surrounding catchment (Fujian Province, P.R. China), in 15th Workshop on Geothermal Reservoir Engineering, Proceedings, Stanford: California, Stanford University, 97–102. [ Links ]














