Services on Demand
Journal
Article
Indicators
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias geológicas
On-line version ISSN 2007-2902Print version ISSN 1026-8774
Rev. mex. cienc. geol vol.24 n.2 Ciudad de México Aug. 2007
Acid rock drainage and metal leaching from mine waste material (tailings) of a Pb–Zn–Ag skarn deposit: environmental assessment through static and kinetic laboratory tests
Drenaje ácido de roca y lixiviación de metales de desechos mineros (jales) derivados de un depósito tipo skarn de Pb–Zn–Ag: evaluación ambiental por medio de pruebas de laboratorio estáticas y cinéticas
Blanca Adriana Méndez–Ortiz1,2, Alejandro Carrillo–Chávez3,*, and Marcos Gustavo Monroy–Fernández1
1 Instituto de Metalurgia, Universidad Autónoma de San Luis Potosí, Sierra Leona 550, Col. Lomas 2a. Sección, 78210 San Luis Potosí, S.L.P., Mexico.
2 Posgrado en Ciencias de la Tierra, Centro de Geociencias, Universidad Nacional Autónoma de México, Campus Juriquilla, Apartado Postal 1–752, 76230 Querétaro, Qro., Mexico.
3 Centro de Geociencias, Universidad Nacional Autónoma de México, Campus Juriquilla, Apartado Postal 1–752, 76230 Querétaro, Qro., Mexico.* ambiente@geociencias.unam.mx
Manuscript received: September 9, 2005
Corrected manuscript received: February 28, 2007
Manuscript accepted: March 13, 2007
ABSTRACT
In this work, the processes and products involved in the generation of acid rock drainage – metal leaching (ARD–ML) from mine waste material (tailings) derived from the exploitation of an ore type Pb–Zn–Ag skarn were characterized. Laboratory tests (static and kinetic) of historic and recent tailings were conducted along with the mineralogical characterization of solids, and chemical analyses of solids and leachates. Pyrite (FeS2) is the most abundant sulfide phase, and one of the main minerals promoting ARD–ML generation, followed by pyrrhotite (Fe1–xS) and arsenopyrite (FeAsS). Carbonates are present as calcite (CaCO3) and lesser amounts of ankerite [Ca(Fe,Mg)(CO3)2]. Also, smaller concentrations of quartz and feldspar were identified. Results of the static tests (acid base accounting: ABA) indicate that both, recent and historic tailings, have a likely ARD–ML potential (neutralization potential, NP <1). Kinetic tests were performed in humidity cells to simulate the natural oxidation of primary mineral samples; the obtained leachates had circum–neutral pH values (4.5–7.9) and relatively low heavy metals contents. Nevertheless, oxidation–neutralization curves suggest that oxidation capacity in both sample types is higher than their neutralizing capacity, and that sulfide oxidation is more important in historic than in recent tailings. The obtained information, along with statistical tests (F ratio and t–student), demonstrated that the tailings have a strong capacity to generate ARD–ML, and that the particular characteristics of the historic and recent mine waste materials created different conditions for the generation of ARD–ML.
Key words: acid rock drainage, metal leaching, neutralization potential, kinetic tests, static tests, Pb–Zn–Ag skarn.
RESUMEN
En este trabajo se caracterizaron los procesos y productos involucrados en la generación de drenaje ácido de roca – lixiviación de metales (DAR–LM) de desechos mineros (jales) derivados de la explotación de una mineralización tipo skarn de Pb–Zn–Ag. Se realizaron pruebas de laboratorio (estáticas y cinéticas) en muestras de jales históricos y recientes, además de la caracterización mineralógica de los sólidos y análisis químicos de sólidos y lixiviados. La pirita (FeS2) es la fase mineral más abundante –y uno de los principales minerales promotores de DAR–LM–, seguida de la pirrotita (Fe1–×S) y arsenopirita (FeAsS). Los carbonatos se encuentran presentes como calcita (CaCO3) y en cantidades menores de ankerita [Ca(Fe,Mg)(CO3)2]. Se identificó también la presencia de cuarzo y feldespatos en pequeñas cantidades. Los resultados de las pruebas estáticas, (cuantificación ácido–base: ABA) indicaron que tanto los jales históricos como los recientes tienen potencial de generación de DAR–LM (potencial de neutralización, PN <1). Las pruebas cinéticas fueron realizadas en celdas húmedas para simular la oxidación natural de muestras minerales primarias. En los lixiviados obtenidos se detectaron valores de pH cercanos a la neutralidad (4.5–7.9) y una limitada presencia de metales en solución. Sin embargo, las curvas de oxidación–neutralización sugieren que la capacitad de oxidación en ambas muestras es mayor que su capacidad de neutralización; además, la oxidación de sulfuros fue más significativa en los jales históricos que en los recientes. La información obtenida, junto con las pruebas estadísticas (prueba F y t de Student), demostró que los jales tienen capacidad de generación de DAR–LM y que las características particulares de los jales históricos y recientes crearon condiciones distintas para la generación de DAR–LM.
Palabras clave: drenaje ácido de roca, lixiviación de metales, potencial de neutralización, pruebas cinéticas, pruebas estáticas, skarn de Pb–Zn–Ag.
INTRODUCTION
Intense mining operations of copper, lead, and zinc ore deposits have existed in Mexico for several centuries. These operations have generated considerable amounts (millions of tons) of sulfide–rich waste material, generally disposed as tailings impoundment. Most of the historic tailings impoundments present strong evidences of weathering, mostly oxidation of sulfide minerals such as pyrrhotite (Fe1–×S), pyrite (FeS2), and arsenopyrite (FeAsS). Mine tailings containing metal sulfides could have an important environmental impact if control strategies imposed to prevent the oxidation of sulfide exposed to weathering conditions are not established. Natural oxidation of metal sulfides may generate the so–called "acid rock drainage" and "metal leaching" (ARD–ML). The ARD–ML is often characterized by high concentrations of metals and sulfates in solution, and by generally low pH values (pH = 2–4). Such solutions can potentially contaminate surface water and groundwater, as well as soils (Skousen, 1995; Morin and Hutt, 1997). The most commonly used procedures to predict ARD–ML consist of both static and kinetic tests (Morin and Hutt, 1997). The static tests are used to evaluate the balance between acid producing and acid consuming (neutralizing) minerals, and thus to determine the potential ARD–LM generation. Samples identified as potential ARD–ML generators according to the static tests are submitted to kinetic tests. In these tests, the conditions of acidity production and metal dissolution in mining wastes subjected to alteration are simulated over long periods of time (at least for 30 weeks) in humidity cells.
The objective of this work was to assess the potential generation of ARD–ML in recent and historic mine waste materials through static and kinetic tests. Wastes come from a flotation processing plant for a Pb–Zn–Ag skarn ore characterized by high acidic and neutralizing potentials. The static and kinetic tests were complemented with scanning electron microscope (SEM) imaging in order to visualize the state of sulfide minerals. The data were statistically treated using different parameters to test and assure their validity. The generation of ARD–ML under the geoclimatic conditions of Mexico (semiarid) is also considered. By special request of the mining unit, who facilitated and financed the sampling stage, the origin of the tailings considered in this study has been omitted.
MATERIALS AND METHODS
In this work, two kinds of samples from the same mining unit were studied: the first one was a representative composite sample obtained directly from the recent flotation tailings of the concentration plant; the second sample was a composite sample collected from a historic tailings impoundment to a depth of 3 m, where there was no evidence of oxidization. Approximately 500 kg of each sample was collected.
Chemical and mineralogical characterization of each solid sample (recent and historic tailings) was performed before the leaching test. Mineralogical observations were carried out using a Leica DM EP polarization microscope and a Philips XL30 scanning electron microscope (SEM). Microanalyses were performed with an energy dispersion X–ray fluorescence spectrometer (EDAX 4 Dix) coupled to the SEM. Particle size distribution of each sample was determined using Tyler series sieves and a Shimadzu SALD–1100 size analyzer.
The acid–base accounting (ABA) test is the most commonly used method to perform static tests to predict ARD–ML. With this method, the balance between acid–producing (acid potential, AP), and acid–consuming (neutralizing) minerals (neutralization potential, NP) in a given sample can be determined. The acid potential (AP) was calculated from the sulfur content in sulfide, which was obtained through subtracting the sulfur content in sulfate from the total sulfur analyses. Analyses of total sulfur and sulfate of the tailings samples were carried out at the Sección Analítica del Centro de Investigación y Desarrollo Tecnológico de Servicios Industriales Peñoles, S.A. de C.V. (ISO 9001–200 accredited). Total sulfur and total carbon were determined by sulfur/carbon analyzer Leco SC–244. Three reference samples were used in the analysis with a final S recovery of 98%. Sulfate was analyzed by the gravimetric conventional method using 10% BaSO4.
To estimate the NP, the modified method of Sobek (Morin and Hutt, 1997) was used on the samples before and after the leaching procedure described below. The first part of the Sobek method consists in adding a few drops of 25% HCl to 2 g of pulverized sample on a watch glass to observe the degree of reaction and to assign a fizz rating as "none, slight, moderate, or strong fizz". This test is just qualitative (to observe the degree of reaction), not quantitative, thus standard solutions were not required. Thereafter, approximately 2 g of pulverized sample were weighed in a 250 mL conical flask, and approximately 90 mL of distillated water were added. At the beginning of the test (time = 0), a volume of 1.0 N HCl (previously standardized with NaOH 1 N) was added according to the fizz rating (Table 1). The flask was placed on a shaking apparatus and maintained at room temperature. After two hours, the second 1.0 N HCl quantity indicated in Table 1 was added. After 24 hours, distilled water was added to the flask to make a volume of approximately 125 mL. The pH was measured and recorded, making sure that it is in the required range of 2 to 2.5. Finally, the content was titrated to a pH of 8.3 with standardized 0.5 N NaOH. Acid consumed in the digestion of the neutralizing species was used to calculate the NP according to:
NP (kg CaCO3/t) = [((N × vol (ml) of HC1) – (N × vol (ml) NaOH)) × 50] / [sample weight (g)]
where N is the normality of HCl or NaOH used in the digestion and titration, respectively, and vol is the volume of each solution used.

Finally, the NP/AP ratio (known as the neutralization potential ratio: NPR), was used as the criterion to evaluate the capacity of the material to generate acid drainage (Price et al., 1997). From this criterion, samples classified as potentially ARD generators were submitted to the kinetic studies described below.
The kinetic tests followed the procedure of the American Society for Testing and Materials (ASTM, 1996; Morin and Hutt, 1997) that uses humidity cells (HC) to simulate the natural oxidation of primary mineral samples. The leaching apparatus consisted of cylindrical cells (200 mm in diameter) containing 1 kg of mineral. An air inlet was located at the center of the cell top and a drain fitting in the bottom of the cell. The cell was connected to a regulated source of compressed air (~5 L/min) through a ½–inch diameter Tygon tubing. Humidified air was generated in a 5–gallon glass carboy, which was half–filled with deionized water. The sample was leached once a week for 30 weeks with 0.5 L of simulated rainwater (distilled water with pH adjusted to 5.5 by the addition of purified CO2). The procedure consisted of one day of leaching, followed by three days of exposure to humidity conditions. Compressed air was fed directly to the HC (dry–air cycle for three days) or pumped first to the carboy and then routed to the HC (humidified–air cycle for four days). After each humidified–air cycle, the sample was treated with 0.5 L of the simulated rain water and allowed to soak for 3 hours. The leachate (representing the water that flowed through the mine tailings material) was collected in glass beakers, filtered through 0.45 µm micropore membranes, acidified to pH=2, and stored at 4 °C until analysis. All the glassware and plasticware were cleaned with detergent, soaked in 0.12 M HC1, and rinsed with deionized water (Morin and Hutt, 1997). Humidity cells experiments were carried out by duplicate for both sample types.
The pH of the leached solutions was measured with a Beckman Φ320 pH–meter. The elemental analyses of solid samples and leachates were carried out with a Varian Spectra AA 220 Atomic Absorption Spectrometer with flame, while arsenic was analyzed with an AAS Perkin Elmer 2380 coupled with a hydride generator MHS–10. Analysis of a primary standard reference material (NIST–1643d) in each run were conducted as an internal quality control for the As analyses, achieving recoveries of 97%. Sulfate in the solutions was analyzed by the turbidimetric method in an UV–Visible Beckman DU–650 spectrophotometer, following the method 4500–SO42– E. Detection limits for leachates analyses were not estimated, although this should be done in future using the methodology and criterions recently proposed by Verma and Santoyo (2005).
To compare the chemical composition of the leachates obtained from samples of recent and historic tailings on an objective statistical basis, the F–ratio (two sides test) and the Student t–test (Verma, 2005) were applied. Common statistical parameters (number of data, mean, standard deviation) for chemical analyses were computed; no outliers were detected when the criteria of Verma and Quiroz–Ruiz (2006a, 2006b) were applied. The F–ratio test was applied for a chemical SO4 accumulative amount (SO4 is one of the most important products of tailings alteration) for each group of recent and historic tailings to find out which of the two hypotheses (H0 or H1) is true at 99% confidence level. The null hypotheses (H0) is "the variances for recent and historic tailings are statistically equal", whereas the alternative hypotheses (H1) is "the two variances are statistically different" thereby implying a two sided version of the F–test. If for a given SO4 accumulative, H0 was true, i.e., if there was no significant differences at 99% confidence level between the two variances (for recent and historic tailings), these two variances were combined to estimate the common variance, and the appropriate Student t–test was applied to find out if the two means were statistically equal or significantly different. Otherwise, if H1 was true, i.e., if the two variances differed significantly at 99% confidence level, these two variances could not be combined and the t–test was applied also at 99% confidence level using the test statistic proposed for this purpose by Miller and Miller (2000). For more information about these methodologies see Verma (1997, 2005).
RESULTS AND DISCUSSION
The chemical composition of samples from recent and historic tailings before the leaching tests is reported in Table 2. Noteworthy are the As concentrations of 0.88 and 1.55 wt.%, and the high concentrations of total sulfur (acidifying agent) and carbonates (neutralizing agent).

With respect to the mineralogical characterization, pyrite (FeS2) is the most abundant sulfide phase and the most important mineral in promoting ARD–LM generation. Minor amounts of pyrrhotite (Fe1–×S) and arsenopyrite (FeAsS) were identified by SEM–EDAX. In spite of the low concentrations of arsenopyrite, its dissolution releases considerable amounts of arsenic, which is very important from the environmental point of view. The presence of carbonates is controlled by calcite (CaCO3) and lesser amounts of ankerite [Ca(Fe,Mg)(CO3)2]. In addition, smaller concentrations of quartz (SiO2) and feldspar (KAlSi3O8) were identified. In the SEM images of mineral particles before leaching (Figures 1a and 1b), no oxidation evidences were observed in samples from both recent and historic tailings (clean surfaces). The size distribution analyses indicated that 80% (P80) of the particles in historic and recent tailings have sizes less than 225 and 134 µm, respectively. The particle size influence the permeability in the tailings: larger particle sizes are associated with higher permeability and vice versa.

The results of the ABA analyses of the samples before and after the leaching tests are presented in Table 3. This table also includes the screening criterion for the identification of potentially acid generating materials (Price et al., 1997). Recent and historic tailings samples had a NPR <1 before the leaching test, suggesting that those materials could potentially generate ARD–LM. To confirm the capacity of ARD–LM generation of materials with a NPR <1, the ABA test needs to be complemented with further kinetic tests (Price et al., 1997). After 30 weeks of leaching in humidity cells, recent and historic tailings samples maintained a NPR < 1. In both sample types, AP and NP decreased but not very significantly. The kinetic test confirms that those materials are ARD–ML generators.

Figure 2 shows the chemical evolution for pH and total dissolved Fe, Pb, Zn, Cu and As in the leachates from the recent and historic tailings samples obtained from the kinetic tests (alteration in humidity cells). Results showed here are the media values of the duplicated samples; data are reported in Table 4 and 5 with the standard deviation [the data were rounded according to the criterion proposed by Bevington (1969), Bevington and Robinson (2003) and Verma (2005)]. In both tailings, the pH values of the leachates (4.5–7.9) are not indicative of ARD–ML generation. The ARD–ML was probably produced, but the generated acid may have been neutralized by neutralizing agents like carbonates. This behavior could be associated mainly to a preferential oxidation of the pyrrhotite prior than pyrite. Both minerals are semi–conductors (galvanic pairs) (Cruz et al., 2000). Pyrrhotite has a lower rest potential and a higher tendency to oxidation, whereas pyrite is galvanically more protected (lower tendency to oxidation) (Abramov y Avdohin, 1997). The circum–neutral pH values of the leachates do not indicate the generation of acidity (oxidation processes), however, the heavy metals contents in solution indicate that dissolution processes (oxidation) have taken place. Thus, it can be considered that oxidation processes ocurred, but any acidity generated was neutralized by the high content of neutralizing elements in the system.
The historic tailings sample generated higher metal concentrations (Fe, Pb, Zn and Cu) in solution than the recent tailings sample because its larger particle size allowed more water infiltration in the sample and thus more ARD–LM generation and metal dissolution.
In the first week of leaching, the solutions had increased contents of some metals, which can be explained by the presence in the samples of soluble salts generated during the drying and treatment of the sample (Cruz et al., 2000). The highest Fe concentration in the leachates was 0.32 mg/L, but most samples had Fe contents of around 0.10 mg/L. In the recent tailings, the Pb concentration in solution decreased until reaching a stable level in the tenth week of leaching, maintaining a similar behavior for both samples in the last 12 weeks. The Zn concentration in solution increased during the first week of leaching for both tailings samples. Although leached Zn gradually decreased, it was the heavy metal found in higher concentrations in the leachates (up to 40 mg/L). Nevertheless, Zn concentrations in the leachates were relatively low, considering the Zn content in the samples (1.2 to 1.5 wt %). The Cu concentration in the leachates of the recent tailings was lower than 0.07 mg/L, whereas the leachates of historic tailings had higher concentrations of this element (0.1 mg/L).
The relatively low concentrations of metals in the leachates (except for Zn) could be related to processes of metal adsorption onto Fe oxy–hydroxides like ferryhidrite [Fe(OH)3], and to the pH values (4.5–7.9) that favored their precipitation as metal oxides or hydroxides. In both recent and historic tailings, the As was dissolved to reach concentrations of 0.0012 to 0.07 mg/L. Arsenic concentrations higher than 0.04 mg/L must be considered with special care, because this element is highly toxic and represents an environmental risk for natural waters. The current Mexican drinking water quality guideline for As is 0.03 mg/L (SA, 1996). Besides, it should be considered that arsenic can stay in solution as HAsO42– at the neutral pH values measured in the leachates (Cruz et al., 2000).
Figure 3 shows the accumulative mass of sulfates (SO4) measured in leachates plotted versus the accumulative amount of calcium and magnesium (Ca + Mg) in mg/kg for both samples. In this plot, sulfate represents the main oxidation products and the term (Ca + Mg) represents the main carbonate dissolution products. The obtained curves (called oxidation–neutralization curves) reflect the geochemical evolution of the acidic and neutralization potentials during the kinetic tests (Benzaazoua et al., 2001). The linear relation between the neutralizing and acidification agents in the system suggests that sulfate production exceeds the neutralizing capacity in both samples (almost at a 2:1 ratio). Thus, the oxidation capacity in both samples is greater than the neutralizing capacity, with no significant differences between both systems. The most striking difference is that, in the recent tailings, the accumulative concentration of Ca and Mg increases significantly in the last three sampling points, reaching an apparent balance equivalent to the total neutralization of generated acid. This indicates that neutralization of recent tailings becomes greater than that of historic tailings at the end of the kinetic test.
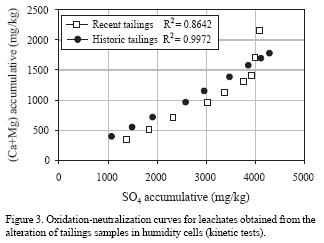
Ca and Mg concentrations demonstrate the dissolution of neutralizing agents and the high sulfate concentrations are characteristic of the ARD–ML process. Results in Figure 3 indicate that sulfide oxidation is more important in historic than in recent tailings, even though Fe concentration in historic tailings (15.06 %) is less than that of recent tailings (22.58%). This result is attributed to a higher permeability related to the larger particle sizes in historic tailings; this allowed more diffusion of oxygen and water, the main agents that promote ARD–LM.
In order to better understand the alteration effects, the chemical compositions of leachates from recent tailings were statistically compared to those of historic tailings. In both cases n = 9 (the samples analyzed for SO4). Results of the tests are presented in Table 6 and 7, which include the values for accumulative amount of SO4 and their mean, standard deviation, variances, number of samples and the parameters calculated for F–ratio test and Student–t test. In both tests, H0 is rejected and H1 is accepted, which means that "the two variances are statistically different" to a 99% confidence level. This can be mainly related to the difference in the content of SO4 between both solid samples (0.27% in recent tailings and 0.23% in historic tailings), although the other factors mentioned before can play an important role in this differences (particle size, content of chemical neutralizing components).


Our results confirmed the generation of ARD–ML in the mine residues analyzed, even though pyrite oxidation was not evident by the techniques used. They also demonstrated that ARD–ML generation, and the oxidation of the less reactive sulfide minerals (pyrrhotite, galena, sphalerite and arsenopyrite) promoted metal dissolution (Fe, Pb, Zn, Cu and As). The low metal concentrations in solution could be explained by the adsorption or precipitation of metal oxides or hydroxides at the circum–neutral pH of leachates. These conditions favor a continuous dissolution of arsenic, likely to cause environmental problems. The leaching of arsenic from this kind of residues could promote pollution of natural waters even in semiarid conditions as those existing in some historic sites in central and north Mexico where Pb–Zn–As skarn deposits were and are still mined (Buchanan et al., 1997; Castro et al., 1997).
ACKNOWLEDGEMENTS
This work was supported by the Consejo Nacional de Ciencia y Tecnología (CONACyT) and Servicios Industriales Peñoles, S.A. de C.V. First author is grateful to the CONACyT and COPOCyT for the M.Sc. fellowship. We also thank to Dr. Oscar Talavera and to an anonymous reviewer for their constructive comments to improve the manuscript. Special thanks go to Dr. Surendra P. Verma for his helpful comments for improving this work.
REFERENCES
Abramov, A.A., Avdohin, V.M., 1997, Oxidation of sulfide minerals in beneficiation processes: Amsterdam, The Netherlands, Gordon and Breach Science Publishers, 321 p. [ Links ]
American Society for Testing and Materials, 1996, ASTM Designation: D5744–96 – Standard test method for accelerated weathering of solid materials using modified humidity cell: West Conshocken, PA, American Society for Testing and Materials, 13 p. [ Links ]
Benzaazoua, M., Bussiere, B,, Dagenais, A.M., 2001, Comparison of kinetic test for sulfide mine tailings. tailings and mine waste, in Proceedings of the Sixth International Conference on Tailings and Mine Waste, January 24–27, 1999, Fort Collins, Colorado: Rotterdam, A.A. Balkema, Volume 1, p. 35–43. [ Links ]
Bevington , P.R., 1969, Data reduction and error analyses for the physical sciences: New York, Mc–Graw Hill, 336 p. [ Links ]
Bevington, P.R., Robinson, D.K., 2003, Data reduction and error analyses for the physical sciences: New York, Mc–Graw Hill, Third edition, 320 p [ Links ]
Buchanan, N., Mango, H., Lini, A., Abbott, M., 1997, Using oxygen isotopes to constrain the sources of arsenic contaminated ground water in Zimapán, Mexico: Lewiston, ME, Bates College, Department of Geology, Study report, 8 p. [ Links ]
Castro, L.J., Kramar, U., Puchelt, H., 1997, 200 years of mining activities at La Paz/San Luis Potosí/México–consequences for environment and geochemical exploration: Journal of Geochemical Exploration, 58, 81–91 p. [ Links ]
Cruz, G.R., Bertrand, V., González, M.I., Monroy, F.M., 2000, An electrochemical approach to study the reactivity of sulfide minerals: Application to the acid rock drainage generation, in Proceedings from the Fifth International Conference on Acid Rock Drainage (ICARD 2000), May 21–24, 2000, Denver, CO: Littleton, CO, Society for Mining, Metallurgy, and Exploration, Inc., 61–72 p. [ Links ]
Lawrence, R.W., Wang, Y., 1997, Determination of neutralization potential of acid rock drainage, in Proceedings from the Fourth International Conference on Acid Rock Drainage, May 31–June 6, 1997, Vancouver, B.C.: Vancouver, B.C., American Society of Surface Mining and Reclamation, 449–464 p. [ Links ]
Miller, J.N., Miller, J.C., 2000, Statistic and chemometrics for analytical chemistry: Harlow, England, Prentice Hall Pearson Education, 271p. [ Links ]
Morin, K.A., Hutt, N.M., 1997, Environmental geochemistry of minesite drainage: Practical and case studies: Vancouver, B.C., Minesite Drainage Assessment Group (MDAG) Publishing, 333 p. [ Links ]
Price, W.A., Morin, K., Hutt, N., 1997, Guidelines for the prediction of acid rock drainage, part I, in Proceedings from the Fourth International Conference on Acid Rock Drainage, May 31–June 6, 1997, Vancouver, B.C.: Vancouver, B.C., American Society of Surface Mining and Reclamation, vol. I:15–30 p. [ Links ]
Secretaria de Salud (SA), 1996, Norma Oficial Mexicana NOM–127, SSA, Salud ambiental, agua para uso y consumo humano –límites permisibles y tratamientos a que debe someterse el agua para su potabilidad: México D.F., Diario Oficial, 1er sec. 40–50. [ Links ]
Skousen, J.G., 1995, Acid mine drainage, in Skousen J.G, Ziemkiewicz, P. (eds.), Acid Mine Drainage Control and Treatment: Morgantown, West Virginia, West Virginia University and the National Mine Land Reclamation Center, 91–12 p. [ Links ]
Verma, S.P., 1997, Sixteen statistical tests for outlier detection and rejection in evaluation of international geochemical reference materials: example of microgabro PM–S: Geostandards Newsletter: The Journal of Geostandards and Geoanalysis, 21, 327–338 p. [ Links ]
Verma, S.P., 2005, Estadística básica para el manejo de datos experimentales; Aplicación en la geoquímica (Geoquimiometría): México, D. F., Universidad Nacional Autónoma de México, 186 p. [ Links ]
Verma, S.P., Quiroz–Ruiz, A., 2006a, Critical values for six Dixon tests for outliers in normal samples up to sizes 100, and applications in science and engineering: Revista Mexicana de Ciencias Geológicas, 23(2), 133–161. [ Links ]
Verma, S.P., Quiroz–Ruiz, A., 2006b, Critical values for 22 discordancy test variants for outliers in normal samples up to sizes 100, and applications in science and engineering: Revista Mexicana de Ciencias Geológicas, 23(3), 302–319. [ Links ]
Verma, S.P., Santoyo, E., 2005, Is odd–even effect reflected in detection limits?: Accreditation and Quality Assurance, 10 (4), 144–148. [ Links ]














