Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Sociedad Química de México
Print version ISSN 0583-7693
Rev. Soc. Quím. Méx vol.47 n.2 Ciudad de México Apr./Jun. 2003
Investigación
Mechanism of Glutamate Neurochemistry: Electron Transfer and Reactive Oxygen Species
Peter Kovacic1, Ratnasamy Somanathan2* and Michelle Inzunza1
1 Department of Chemistry, San Diego State University, San Diego, CA 92182-1030, USA.
2 Centro de Graduados e Investigación del Instituto Tecnológico de Tijuana, Apdo Postal 1166, 22000 Tijuana, B.C. México. E-mail: somanatha@sundown.sdsu.edu
Recibido el 8 de abril del 2003.
Aceptado el 3 de junio del 2003.
Dedicated to Professor Alfonso Romo de Vivar.
Abstract
Glutamate (Glu) undergoes metabolism to an imine derivative. We propose involvement of the conjugated α-iminocarboxylic acid in neurotoxicity and possibly in neurotransmission. Electrochemistry, captodative effect and bioactivity of related cyclic α-imino acids are relevant. There is also consistency with background literature of Glu indicating participation of oxidative stress, reactive oxygen species, and electron transfer. Alternatively, metal chelates of Glu and Glu imine may play a role. Various analogs of Glu imine were synthesized, namely, oxime and a cyclic model derived from cyclization of the intermediate hydrazone.
Keywords: Glutamate, neurotransmission, toxicity, imine, electron transfer, reactive oxygen species.
Resumen
El glutamato (Glu) se metaboliza a un derivado de imina. Se propone la inclusión del ácido iminocarboxílico conjugado en la neurotoxocidad y posiblemente en la neurotransmisión. La electroquímica, el efecto captodativo y la bioactividad del los ácidos α-iminocíclicos son relevantes. Los hallazgos son consistentes con los antecedentes de la literatura que indican la participación de Glu en el estrés oxidativo, en la química de las especies reactivas de oxígeno, y en la transferencia electrónica. Alternativamente, los quelatos metálicos de Glu y de iminas Glu, juegan un papel importante. Varios análogos de imina Glu fueron sintetizados, en particular la oxima, y un modelo cíclico derivado de la ciclización de la hidrazona intermediaria.
Palabras clave: Glutamato, neurotransmisión, toxicidad, iminas, transferencia electrónica, especies reactivas de oxígeno.
Introduction
During the last score of years extensive evidence has accumulated in support of involvement of oxidative stress (OS) with both endogenous and exogenous agents, including anti-infective drugs [1], anticancer agents [2], carcinogens [3], reproductive toxins [4], nephrotoxins [5], hepatotoxins [6], and various others [7a].
The most common reactive oxygen species (ROS) are superoxide, hydrogen peroxide, peroxyl radicals, and the important hydroxyl radical, that are generated by electron transfer (ET). ET functionalities comprise quinones (or precursors), metal complexes (or chelators), aromatic nitro compounds (or reduced products), and imines (or iminiums), which on redox cycling with oxygen give rise to ROS. The present article will focus on the conjugated imine category. In some cases, ET occurs without oxygen participation. Very many bioactive substances or their metabolites incorporate ET groups. OS can produce beneficial results, as with drugs, or unwanted side effects in toxicity. The mode of action is usually complex and probably multifaceted in many instances.
This report deals with the mode of action of glutamate (Glu), both in neurotransmission and in toxicity. We propose that the α-imino metabolite may play a role as an ET agent in these processes. Glu metabolism, generation of ROS and prior literature on bioactivity of related α-iminocarboxylic acids lend support to the thesis. Several synthetic analogs, both acyclic and cyclic, of the α-imino metabolite were synthesized.
Metabolism
In relation to mechanism, Glu metabolism has attracted scant attention even though metabolites often play an important role in physiological activity. It is significant that Glu can serve as substrate for an enzyme that effects conversion to the imine derivative (1a), particularly since Glu dehydrogenase plays a central role in amino acid deamination because in most organisms Glu is the only amino acid that has an active dehydrogenase [8].
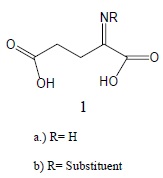
The labile product (1a) can undergo several subsequent conversions. Nucleophilic attack by a basic primary amino entity, such as protein lysine, with elimination of ammonia, gives rise to the more stable imine 1b possessing a substituent on nitrogen. Alternatively, 1a could undergo hydrolysis to the α-keto acid which also serves as precursor of (1b) by reaction with priamine [8]. We will explore possible ramifications relative to mode of action resulting from generation of conjugated imine (1b). Several common routes are generally available for imine and iminium synthesis in biological systems, including oxidation of aliphatic amines and nonenzymatic condensation of carbonyl compounds with the priamino moiety of basic amino acids in protein. Hence, these transformations are applicable to Glu, making for relevance to our report. Regarding the iminium types, they can also be derived simply by alkylation or protonation of imine.
Synthesis
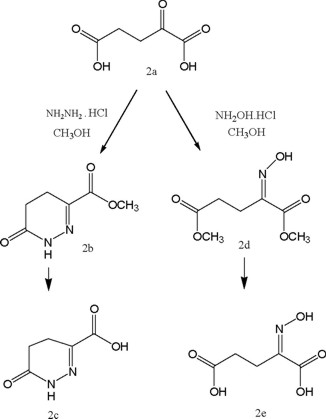
Cyclic iminocarboxylates were synthesized from α-ketoglutaric acid (2a), by condensing with hydrazine in methanol to yield cyclic ester 2 which on hydrolysis gave the cyclic iminocarboxylic acid (2c) in good yield. Similarly, acyclic types were prepared by condensing 2a with hydroxylamine in methanol to provide the corresponding methyl iminocarboxylate 2d and the carboxylic acid 2e, which were separated by preparative thin layer chromatography (Scheme-1).
Neurotoxicity
Even before a decade ago, substantial data had built up pointing to OS as a factor in neuropathology by excitory amino acids, with Glu as the major effector [9].
Prominent evidence includes generation of ROS and subsequent oxidative damage; radical scavengers and inhibitors prevent neuronal degradation. Other suspected sources of OS are nitric oxide, peroxynitrite, calcium, iron, and activated ROS-generating enzymes.
Two broad mechanisms were offered to account for cell vulnerability, based on OS and excessive activation of Glu receptors. In one case, a sequential process pertains, whereas the other, of particular interest, entails interaction of the two. Four years later, increasing evidence supported the claim that excitotoxicity and oxidative stress play important roles in pathogenesis of both acute and chronic neurologic diseases [10]. The view was reiterated that the two effects may cooperate to induce neuronal degeneration. Hydroxyl radical levels and the volume of lesions were attenuated by spin trapping, pointing to radical scavenging. From our standpoint, the cooperative effect consists of ET entailing the receptor bound species. On the other hand, ET by imine might occur at another site in keeping with the sequential scheme. The literature provides little discussion of specific, detailed pathways for OS generation from Glu, partly due to lack of working hypotheses. We suggest that the α-imine metabolite may play a role in redox cycling to produce ROS, at least in toxicity. This approach is in harmony with various observations, "Although the activation of Glu receptors is a key step in the sequence of events leading to neuronal degeneration, it is by no means all that is necessary... thus delayed neurotoxicity has been effectively dissociated from neuronal excitation..." [9]. A goodly number of other investigations document involvement of OS, of which several will be cited, including generation of ROS [11-13], induction of lipid peroxidation [13a], DNA fragmentation [13b] and protection by antioxidants [13-15]. In addition, involving OS from Glu imine in neurotoxocity represents yet another example in the widespread documentation of ROS from conjugated imine or iminium in toxic manifestations [3-7a].
Neurotransmission
Glu and related amino acids appear to be the major excitatory neurotransmitters in the brain with involvement in 40 % of the synapses [9]. The importance of Glu is reflected by its presence in the CNS in relatively large quantities. An hypothetical role for ET by Glu imine might also be invoked in this category. Although ROS at high concentrations induce adverse effects, OS at low levels may contribute in the transmission process. By analogy, ROS have both beneficial and damaging effects, depending on various factors, on sperm in the fertilization process [16].
There is general consensus that neurotransmission occurs by ionic pathways [17]. It should be recognized that ET is not necessarily incompatible with polar processes. Movement of negative electrons (ET) induces an electric field which could affect the migration of negative and positive ions, e.g. Cl, Ca, Na and K, in the vicinity. Electrons have been shown to migrate over substantial distances [18]. Another analogy might comprise movement of electrons in a conducting copper wire. The ET framework may also be applicable to other neurotransmitters, such as, nitric oxide [19] and catacholamines [7b].
Metal complexes
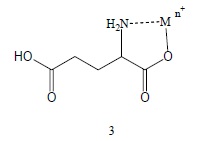
Another plausible scenario for ET by Glu involves the corresponding metal complexes. This general class of ET agents is omnipresent in the major drug and toxin categories [1-7]. There is the favorable feature generally of quite positive reduction potentials which energetically favor ET in vivo. Since the α-aminoacid moiety is a facile chelator, metal derivatives (3) are well documented [20].
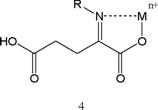
Similarly metal chelates (4) of the α-imino metabolites should also be considered. Hence, it is conceivable that such complexes 3 and 4 might participate in toxicity and/or transmission. Possible involvement of cyclic analogs in physiological activity is discussed in the subsequent section.
Cyclic α-iminocarboxylic acids
Prior literature contains various reports on participation of these species in physiological activity from the ET viewpoint. ß-Lactams inactivate bacterial cell wall enzyme by covalent binding. Little attention has been given to the fact that a-iminocarboxylic acids, e.g., 5 from cephalosporins, arise in the process [21].
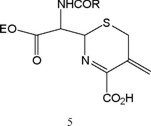
Model compounds, such as 6 and 7, displayed favorable reduction potentials, raising the possibility of ET participation by 5 in vivo subsequent to site binding, both in toxicity and in antibacterial action. The values increased with decreasing pH in line with formation of conjugated iminium which conceivably might be an actor in the physiological manifestations.

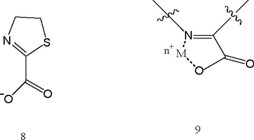
In relation to bioactivity of Δ2-thiazoline-2-carboxylate (8), this compound in the category has been the object of most attention [22]. Hypotheses have been advanced that it is an intracellular messenger for insulin, and an effector of diverse metabolic activities, such as diuretic response and cell growth [23]. Also, it is a potent inhibitor of dopamine-ß-hydroxylase [24], a metalloenzyme responsible for producing norepinephrine.
Compound 8 possessed electron affinity compatible with ET in vivo [21]. Alternatively, all ligands (6-8) in this category are expected to be avid chelators of metal ions in the biological milieu, forming complexes of type (9). Cu and Fe chelates with (6) and (8) exhibit quite positive reduction potentials [22]. The role of ETOS in eliciting a variety of physiological responses from metal complexes is discussed elsewhere [1-7a].
A related chelator is pyridine-2-carboxylic acid [25], whose anticancer activity has been attributed to redox cycling entailing OS by a derived metal complex [26].
Conformational Restriction and Bioactivity
The relatively high activities of kainic (10) [27] and domoic (11) [28] acids in neurotransmission have been attributed to conformational restriction [29] imposed by the cyclic structures. Just how this property translates into improved activity is unknown.
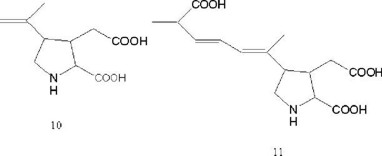
It is indicative that thiazolidine-4-carboxylic acid undergoes dehydrogenation in vivo to the corresponding α-imino acid (7) [22]. By analogy, 10 and 11 would be converted into derivatives of 6 which has favorable properties for ET in bio-systems.
Although the synthetic counterpart (2c) fits into the cyclic imino acid category, it is deficient in lacking an N-alkyl containing substituent and a second carboxyl group.
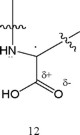
The favorable influence shown by 10 and 11 may result from assistance in coplanarity for the radical anion formed from the proposed imine metabolite on electron uptake, thus promoting resonance stabilization. There is widespread presence of a γ-carboxyl group in the various neurotransmitters, whose role has not been delineated, perhaps as a site binder. In the acyclic structures, the γ-carboxyl may decrease adverse steric interaction with the other carboxyl by association with iminium nitrogen, as depicted in 12, but not as effectively as for the covalent cyclic category.
Captodative effect
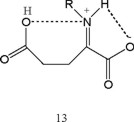
Various reports have shown that carbon radicals benefit from enhanced stabilization when attached to both an electron with-drawing and electron donating substituent [30]. As a result, the combined influence is synergistic. Investigators predicted that this effect would find application in vivo [30]. The following examples can be cited: paraquat, flavins and quinones [22]. The captodative radical formed from one- electron reduction of α-iminium carboxylic acid is depicted in 13.
Future studies with model compounds 2c and 2e might provide further insight into mechanistic aspects. The unifying theme of ET and ROS in neurochemistry is further elaborated in a review [31] dealing with nitric oxide, catecholamines, and Glu.
Experimental
Synthesis of 1,4,5,6-tetrahydro-6-oxo-3-pyridazine carboxylic acid methyl ester (2b). α-Ketoglutaric acid (1.61g, 11 mmol) and hydrazine dihydrochloride (1.16g, 11 mmol) were stirred together in methanol at room temperature overnight. Following removal of solvent under reduced pressure, the residue was dissolved in dichloromethane and washed with water. After the organic phase was dried over anhydrous sodium sulfate, removal of solvent gave a colorless solid (1.37 g, 80 % yield). mp. 120-125 °C; IR (KBr) 3138, 2938, 1714, 1622, 1435, 1287, 1206, 1000 cm−1; 1H NMR (200 MHz, CDCl3) δ 3.91( s, 3H), 2.86 ( t, 3H, J=8.0Hz), 2.60 (t, 3H, J=8.0Hz) ppm; EIMs: 156 (100 %), 124.
1,4,5,6-Tetrahydro-6-oxo-3-pyradazinecarboxylic acid (2c). The above ester (2b) (0.5g) was hydrolyzed with 10 % methanolic sodium hydroxide and neutralized with DOWEX cation exchange resin to give the acid (2c) in quantitative yield. mp. 194-196 °C; IR ( KBr) 3382, 1720; 1H NMR ( 200 MHz, CDCl3) δ 10.85 (s, 1H), 2.84 (t, 2H, J= 8.0 Hz), 2.48 ( t, 2H, J=8 Hz) ppm. Prior synthesis: α-Ketoglutaric acid and hydrazine [32,33], lit. [34] mp.194 °C for 2c.
Synthesis of 2-(Hydroxyimino)pentanedioic acid dimethyl ester (2d) and 2-(Hydroxyimino)pentanedioic acid (2e) [35]. α-Ketoglutaric acid (1.61 g, 11 mmol) and hydroxylamine hydrochloride (0.77 g, 11 mmol) were dissolved in methanol (20 mL) and stirred overnight at room temperature. After solvent was removed under reduced pressure, the residue was dissolved in dichloromethane and the organic phase was washed with water. The organic phase was dried over anhydrous sodium sulfate and removal of solvent gave a viscous liquid which on triturating with diethyl ether gave a crystalline solid (1.76g, 85 % yield). The crude solid was separated on Chromatotron using dichloromethane: methanol (3 %) as eluting solvent to give (2d) and (2e) in 30 and 45 % yields, respectively, which were characterized as follows:
2-(Hydroxyimino)pentanedioic acid dimethyl ester (2d). [35]. Colorless solid (30 %); mp 113-115 °C; IR(KBr) 3382, 2956, 1733, 1442, 1127, 1035, 801 cm−1; 1H NMR (200 MHz, CDCl3) δ 3.70 (s, 3H ), 3.60 (s, 3H), 2.82 ( t, 2H, J= 8.0 Hz), 2.52 ( t, 2H, J= 8.0 Hz) ppm; 13C (50 MHz, CDCl3) δ 172.92, 163.75, 150.87, 52.40, 51.58, 29,59, 20.03 ppm; MS (EI) 189, 156 (100 %).
2-(Hydroxyimino)pentanedioic acid (2e) [36]. Colorless solid (45 %); mp. 152-153 °C; lit. [36] mp. 152 °C. IR (KBr) 3422, 1734, 1666, 1448, 1029, 635 cm−1; 1H NMR (200 MHz, CDCl3) δ 12.16 ( 2H), 2.83 (t, 3H J= 8.00 Hz), 2.52 ( t, 2H, J= 8.0 Hz) ppm.
References
1. Kovacic, P.; Becvar, L.E. Curr. Pharmaceut. Des. 2000, 6, 143-167. [ Links ]
2. Kovacic, P.; Osuna, J.A. Curr. Pharmaceut. Des. 2000, 6, 277-309. [ Links ]
3. Kovacic, P.; Jacintho, J.D. Curr. Med. Chem. 2001, 8, 773-796. [ Links ]
4. Kovacic, K.; Jacintho, J.D. Curr. Med. Chem. 2001, 8, 863-892. [ Links ]
5. Kovacic, P.; Sacman, A.; Wu-Weis, M. Curr. Med. Chem. 2002, 9, 823-847. [ Links ]
6. Poli, G.; Cheeseman, K.H.; Bianzani, M.U.; Slater, T.F. Eds. Free Radicals in the Pathogenesis of Liver Injury. Pergamon Press, New York. 1989. [ Links ]
7. Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine. Oxford University Press, New York, 1999, (a) pp. 1-859. (b) p. 570. [ Links ]
8. Lehninger, A. Biochemistry. Worth, New York, 1975, pp. 565-566. [ Links ]
9. Coyle, J.T.; Puttfarcken, P. Science 1993, 262, 689-695. [ Links ]
10. Lancelot, E.; Revaud, M.L.; Boulu, R.G.; Plokine, M.; Callebert, J. Free Rad. Biol. Med. 1997, 23, 1031-1034. [ Links ]
11. Said, S.I.; Pakbaz, H.; Berisha, H.I.; Raza, S. Free Rad. Biol. Med. 2000, 28, 1300-1302. [ Links ]
12. Loikkanen, J.J.; Naarala, J.; Savolainen, K.M. Free. Rad. Biol. Med. 1998, 24, 377-384. [ Links ]
13. Yasui, Y.; Mawatari, K.; Higuchi, Y.; Tanu, H.; Kato, S. In: Free Radicals in Brain Physiology and Disorders, Packer, L.; Hiramatsu, M.; Yoshikawa, T. Eds., Academic Press, New York, 1996, (a) p.58, (b) pp. 60, 6. [ Links ]
14. Kobayashi, M.S.; Han, D.; Packer, L. Free Rad. Res. 2000, 32, 115-124. [ Links ]
15. Hampson, A.J.; Grimaldi, M.; Lolic, M.; Wink, D.; Rosenthal, R.; Axelrod, J.; Ann. N.Y. Acad. Sci. 2000, 899, 274-282. [ Links ]
16. Sikka, S.C. Curr. Med. Chem. 2001, 8, 851-862. [ Links ]
17. Voet, D.; Voet, J.G. Biochemistry, Wiley, New York. 1995, Sect. 34C. [ Links ]
18. McLendon, G. Acc. Chem. Res. 1988, 21,160-167. [ Links ]
19. Kovacic, P. Bioelectrochem. Bioenerg. 1996, 39, 155-159. [ Links ]
20. Lipschitz, J.; Schouteden, F.L.M. Rec. Trav. Chim. 1939, 58, 411-422. [ Links ]
21. Kovacic, P.; Jawdosiuk, M.; Ames, J.R.; Ryan, M.D. Bioorg. Chem. 1987, 15, 423. [ Links ]
22. Kovacic, P.; Popp, W.J.; Timberlake, J.W.; Ryan, M.D. Chem. Biol. Interact. 1989, 69, 235- 244 and references therein. [ Links ]
23. Hamilton, G.A. Adv. Enzymol. 1985, 57, 85-178. [ Links ]
24. Naber, N.; Venkatesan, P.P.; Hamilton, G.A. Biochem. Biophys. Res. Commun. 1982, 107, 374-380. [ Links ]
25. Schneider, F.; Schaeg, W. Z. Physiol. Chem. 1962, 327, 74. [ Links ]
26. Kovacic, P.; Ames, J.R.; Lumme, P.; Elo, H.; Cox, O.; Jackson, H.; Rivera, L.A.; Ramirez, L.; Ryan, M.D. Anti- Canc. Drug. Des. 1986, 1, 197-214. [ Links ]
27. Cooper, J.R.; Bloom, F.E.; Roth, R.H. The Biochemical Basis of Neuropharmacology, Oxford University Press, New York, 1996, p. 174. [ Links ]
28. June, D.E.; Hoo, K.; Kamboj.; Deversill, M.; Blackman, D.; Mandelzys, A.J. Med. Chem. 1997, 40, 3645-3650. [ Links ]
29. Guroff, G. Molecular Neurobiology, Marcel Dekker, New York, 1980, p. 430. [ Links ]
30. Viehe, H.G.; Janousek, Z.; Merenyi, R.; Stella, L. Acc. Chem. Res. 1985, 18, 148-154. [ Links ]
31. Jacintho, J. D.; Kovacic, P. Curr. Med. Chem. in press. [ Links ]
32. Lagna, W.M.; Callery, P.S. J. Labelled Compd. Radiopharm. 1984, 21, 337. [ Links ]
33. Kline, G.B.; Cox, S.H. J. Org. Chem. 1961, 26, 1854-1856. [ Links ]
34. Kaupp, G.; Schmeyer, J. J. Phys. Org. Chem. 2000, 13, 388. [ Links ]
35. Hoffman, C.; Tanke, R.S.; Miller, M. J. Org. Chem. 1989, 54, 3750-3751. [ Links ]
36. Ahmad, A.; Spenser, I. D. Can. J. Chem. 1961, 39, 1340-1359. [ Links ]














