Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Sociedad Química de México
Print version ISSN 0583-7693
Rev. Soc. Quím. Méx vol.45 n.2 Ciudad de México Apr./Jun. 2001
Investigación
Gibberellins in apple seeds and the transport of [3H]-GA4
Homero Ramírez,*1 Gordon V. Hoad,2 Adalberto Benavides1 and Eloy Rangel1
1 Departamento de Horticultura, Universidad Autónoma Agraria Antonio Narro, Buenavista, Saltillo, Coahuila, Tel.: (01) 8 4173-022. E-mail: homeror@infosel.net.mx
2 AFRC Institute of Arable Crops Research, University of Bristol, Long Ashton Research Station, Bristol BS18 9AF, England.
Recibido el 9 de enero del 2001
Aceptado el 4 de mayo del 2001.
Abstract
With the purpose of increasing the knowledge on the presence of endogenous gibberellins in apple tissue, immature seeds were collected during summer from developing fruits of Golden Delicious trees and analyzed using the gas chromatography-mass spectrometry technique. Gibberellins A4, iso-A7, A9, A12 and A20 were identified. When [3H]-GA4 was applied to the seeds of fruits, higher radioactivity was found in the bourse bud. These results are related to flower bud inhibition in apple trees.
Keywords: Gibberellins, apple seeds, [3H]-GA4.
Resumen
Con el propósito de ampliar el conocimiento sobre la presencia de giberelinas endógenas en manzano, semillas inmaduras de frutos "Golden Delicious", fueron muestreadas durante el verano y analizadas mediante la técnica de cromatografía de gases acoplada a espectrometría de masas. Se identificaron las giberelinas A4, iso-A7, A9, A12 y A20. Cuando [3H]-GA4 fue inyectada a semillas de frutos, se localizó mayor radioactividad en la yema del dardo de manzano. Los resultados se relacionan con la inhibición de la formación de yema floral.
Palabras clave: Giberelinas, semillas de manzana, [3H]-GA4.
Introduction
The presence of fruits on apple trees is a decisive factor in subsequent flower bud formation. The general evidence is that seeded fruit, exert some inhibitory effect on the formation of flower buds [1]. This inhibitory effect is highly dependent on the amount of crop present on the tree. Williams [2] has demonstrated that in many fruit trees, flower formation tends to be reduced when the tree carries a heavy crop. Consequently, the next year crop is light and a cycle of biennial or periodic bearing may be induced.
It has been shown that exogenous applications of GA3 reduces the formation of flowers in apple and other deciduous fruit species [3].
Immature apple seeds are exceptionally rich sources of gibberellins A4 and A7. They reflect their maximum level about 8-10 weeks after full bloom in some cultivars [4]. However, since it has been reported by Ramírez [5] that the inhibitory effect on flower formation by gibberellins could be more qualitative than quantitative, it is necessary to learn more about the presence of different gibberellins in apple seeds. Therefore, the purpose of this work was to find out the existence of endogenous gibberellins other than GA4 and GA7 in immature Golden Delicious apple seeds, to study the movement of applied [3H]-GA4 out of the fruits, and to determine its location within the spur tissue.
Results and discussion
The gibberellins detected by GC-MS spectral analysis in immature Golden Delicious seeds were identified as GA4, iso-GA7, GA9, GA12 and GA20. The structure of these hormones as their elution behaviour in EtOAc-n-hexane is shown in figure 1.
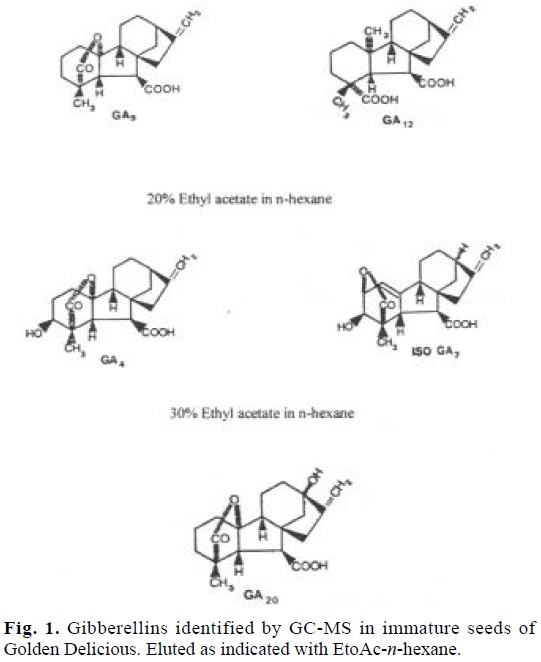
Table 1 shows the amount of radioactivity present in different spur tissues of Golden Delicious when [3H]-GA4 was applied to seeds within the fruit. Radioactivity of this gibberellin previously confirmed with TLC analysis was present in all the tissues which were examined. However, the highest activity was detected in the bourse-bud samples.

If endogenous gibberellins produced in apple seeds are the main factors of flowering inhibition, it may be expected that differences in production of these hormones may be found. This proposal has been supported by Hoad [6], who found in biennial apple cultivars more gibberellins moving out of the fruit than in more regular cropping ones. However, this effect is not seen in other cultivars such as Golden Delicious [7]. These reports suggest that the qualitative seed gibberellin factor may play an important role in the inhibition of flower bud.
The identification in this work of gibberellins A4, A9, A12, A20 and iso-GA7 (Fig. 1), suggests that the inhibition of flowering by gibberellins could be more complicated than has previously been supposed. On these basis, it is possible that specific gibberellins found in seeds may exert a direct effect on the inhibition process.
No evidence as to the identity of the endogenous gibberellins moving out of fruits has been obtained by using diffusates [8, 9], because not enough gibberellins come out of fruits for GC-MS analysis. However, on the basis of results obtained in the present work and previous data [10, 11], it is possible to speculate as to which gibberellins are involved in the inhibition of fruit-bud initiation. Feeding labelled gibberellins to intact fruit has shown that some degree of hydroxylation is necessary for movement. GA9 is immobile [12], but GA3 [13], and GA4 (Table 1) both move from the fruit into spur tissue. Therefore, it is likely that gibberellins A9 and A12 are immobilized due to their lack of hydroxylation.
Also of interest is that [3H]-GA4 moved out of fruits into the bourse-bud without being further hydroxylated (Table 1), indicating that metabolism of applied gibberellins in the tissues is not extensive.
The situation is different when the compound is applied directly to the spur as it has been shown by Looney [14], thus when [3H]-GA4 were fed to spurs of Golden Delicious trees, there can be considerable metabolism of GA, and the more extensively this occurs the greater is the tendency for flower initiation to proceed. Thus on the basis of these results it seems that the more highly hydroxylated gibberellins such as GA1, 15-βOH-GA4 and 15-βOH-GA7 [15] are not so important for inhibiting flowering as those with one hydroxyl group (Fig. 1).
The presence of a range of gibberellins in seed extracts opens further possibilities for explaining the way in which they exert their effect on the inhibition of flower bud in apple.
Conclusions
1. The hypothesis of flower bud inhibition in apple by gibberellins appears to be related to a qualitative effect of these hormones.
2. Gibberellins A4, isoA7, A9, A12 and A20 should play an important role in fruit and flower bud physiology.
3. Labelled gibberellin A4 move out of the fruit into the spur tissue, particularly to the bourse bud.
4. It is possible that specific gibberellins emanating from developing fruitlets may be involved in the inhibition of flowering and that the degree of hydroxylation of gibberellins is an important factor in the action of these hormones.
Experimental section
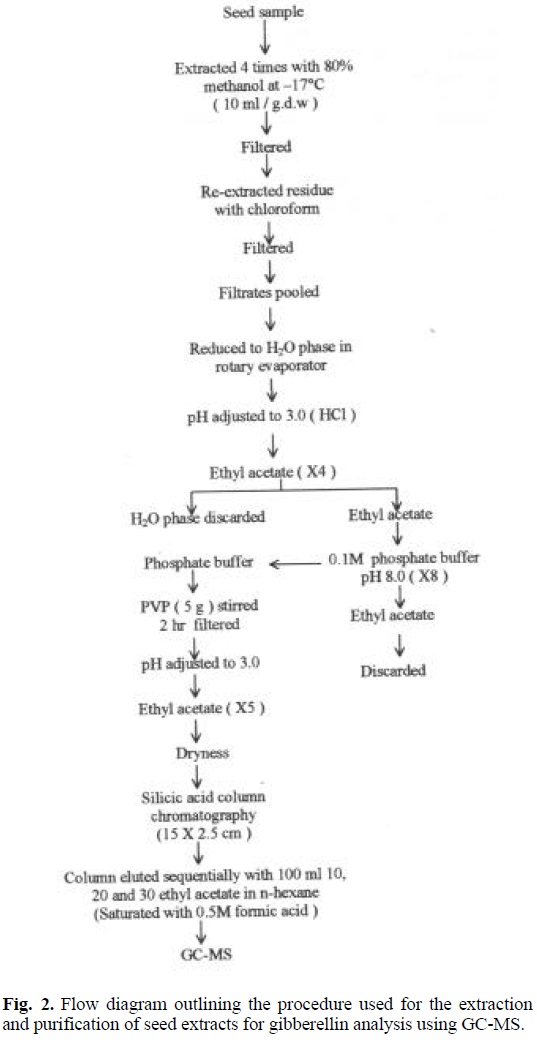
Seed samples (100 g dry weight) were collected on July 12, 1999 from developing fruits of 15 years old Golden Delicious / MM106 apple trees grown in a conventional plantation system and used for the identification of gibberellins by gas chromatography-mass spectrometry (GC-MS). The seeds were collected at the time when the highest levels of gibberellins activity occurred, i.e. about 10 weeks after full bloom. Removed seeds were frozen, freeze-dried and grounded. The extraction and purification procedure prior to GC-MS analysis is illustrated in figure 2. Purified extracts of immature apple seeds were dissolved in a few drops of methanol and methylated with diazomethane. A portion of the methylated extract was dissolved in pyridine and treated with trimethylchlorosilane and hexamethyldisilazane. Aliquots were examined using a Pye 104 GLC coupled through a silicone membrane separator to an AEI MS30 dual beam mass spectrometer. Silanized glass columns (213 × 0.2 cm) were packed with 2 % SE-33 on 80-100 Gas Chrom Q. The He-flow rate was 25 mL / min and the column temperature was programmed from 180 °C to 280 °C at 2° / min. The MS were determined at 24eV at a source temperature of 210 °C and a separator temperature of 190 °C with a scan speed of 6.5 s per mass decade. The spectra were recorded by a DEC Linc 8 computer. Identification of gibberellins was conducted by comparison of KRI and MS spectra of their methyl ester trimethylsilyl ether derivatives with those of authentic samples [15].
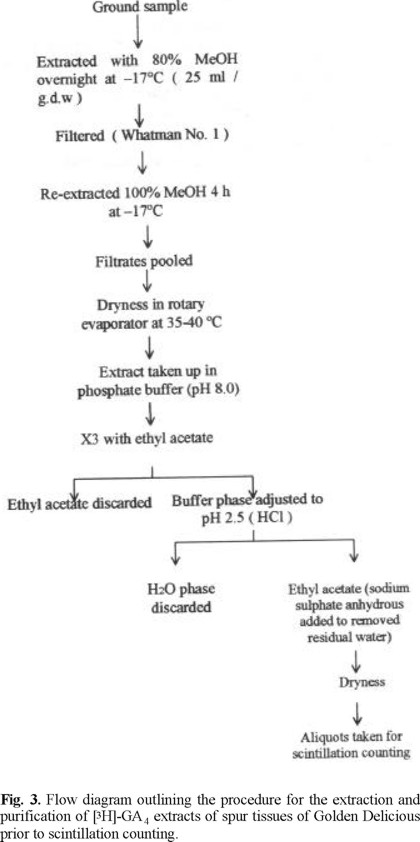
For the study of [3H]-GA4 transport, three fruits carrying spurs from selected Golden Delicious / MM106 apple trees grown on the same plantation were used. The fruits were sliced across the stylar end just above the seeds. Thus the intact seeds, partly immersed in the flesh, became visible. A 5 µL drop of 50 % ethanolic solution containing 0.5 µCi of [3H]-GA4, was applied to the seeds of each fruit using a microsy-ringe; the top of the fruit was replaced and sealed in position using adhesive tape. The operation was performed on July 19, 1999 e.g. about the time when the peak of endogenous gibberellin activity in the seeds occurred. After a 48 h period, the spurs with the treated fruits were collected from the trees and the fruits removed from the spur. The spur was divided into leaves, bourse-bud and remaining spur tissue. Each tissue sample was freeze-dried and ground. The extraction and purification procedure for [3H]-GA4, is illustrated in figure 3. Aliquots of extracts were pipetted into glass vials and 10 mL of PPO-POPOP scintillation solution was added to each one. The radioactivity of the original compound present was measured in an LS150 automatic liquid scintillation counter and dpm values worked out with the aid of a correlation curve [15].
References
1. Greene, D. W. J. Hort. Sci. 1993, 68, 171-176. [ Links ]
2. Williams, M. W. J. Amer. Soc. Hort. Sci. 1972, 97, 210-212. [ Links ]
3. Dennis, G. F., in: Tree Fruit Physiology, Maib, K. M., Ed., Good Fruit Grower, Washington DC, 1997, 107-116. [ Links ]
4. Ramírez, H.; Hoad, G. V. Acta Hort. 1981, 120, 131-136. [ Links ]
5. Ramírez, H. Acta Hort. 1995, 394, 101-103. [ Links ]
6. Hoad, G. V. Acta Hort. 1981, 80, 93-103. [ Links ]
7. Ramírez, H. Acta Hort. 1993, 329, 95-97. [ Links ]
8. Looney, N. E.; Pharis, R. P.; Noma, M. Planta 1985, 165, 292-294. [ Links ]
9. Tromp, J. J. Hort. Sci. 1992, 57, 277-282. [ Links ]
10. Hoad, G. V.; Monselise, S. P. Scientia Hort. 1976, 4, 41-47. [ Links ]
11. Looney, N. E., in: Manipulation of Fruiting, Wright, C. J., Ed., Butterworths, London, 1990, 39-50. [ Links ]
12. Sharma, N.; Jindal, K. K. Acta Hort. 1994, 395, 113-121. [ Links ]
13. Looney, N. E.; Lidster, P. D. J. Amer. Soc. Hort. Sci. 1980, 105, 130-134. [ Links ]
14. Looney, N. E., in: Tree Fruit Physiology, Maib, K. M., Ed., Good Fruit Grower, Washington DC, 1997, 31-40. [ Links ]
15. Pharis, R. P.; King, R. W. Ann. Rev. Plant Physiol. 1985, 36, 517-568. [ Links ]














