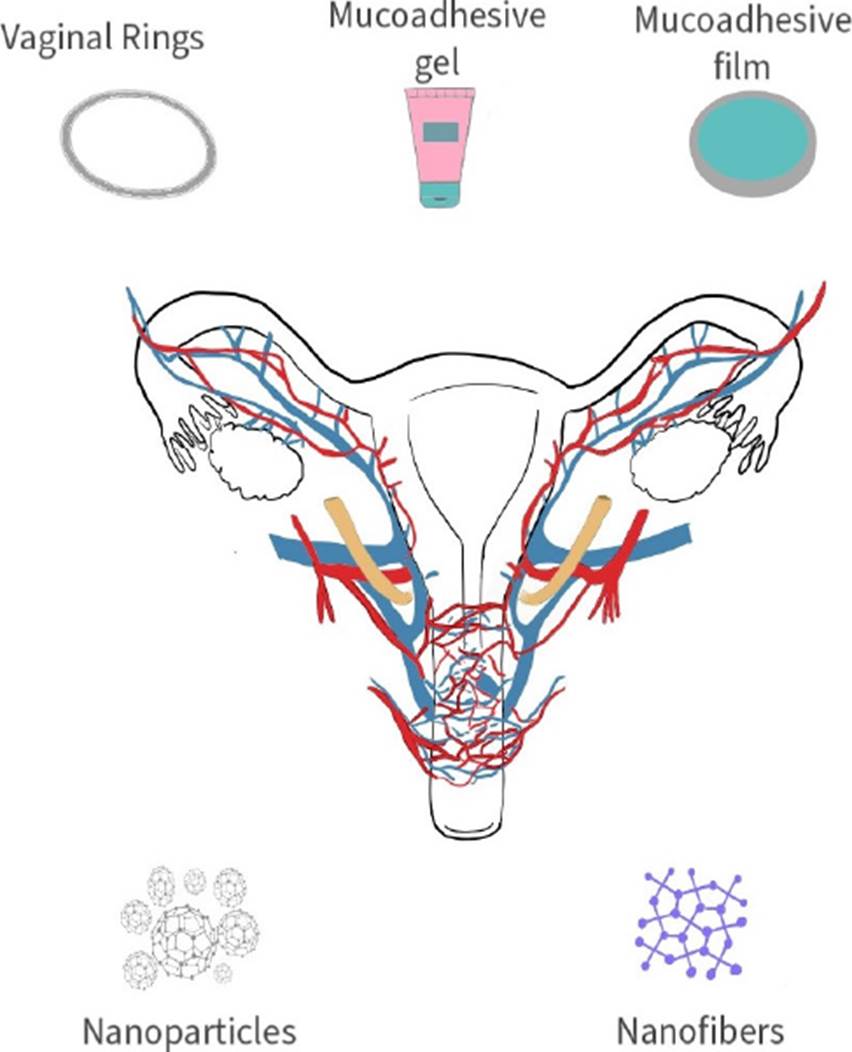
INTRODUCTION
The modern history of intravaginal drug administration began around 1918, when Dr. David I. Macht published his research called “On the Absorption of Drugs and Poisons Through the Vagina” in which through this route morphine, atropine and other drugs, like contraceptives, were provided to female patients that were unable to take their medications orally due to vomiting or any other stomach-related illnesses [1][2][3]. This was followed by the research “Absorption from the Vagina” done by Robinson in 1925, in which he focused on the absorption of different molecules such as insulin, potassium iodide, sodium salicylate and others, obtaining that not all molecules were absorbed, such as methylene blue [2]. These are not the first attempts to provide treatment vaginally. Before common era (BCE), the internal part of female reproductive system was already used to apply remedies passed from generation to generation without really knowing where it all began. The first ever record found dates back to 1850 BCE in ancient Egypt, showing that gynecology is an important part of our medicine with more than 4,000 years of practice [3].
The female genital track provides local effect on what was considered “woman affairs” using products such as douches, tampons, suppositories, “uterine wafers”, among others, to administer natural or more sophisticated remedies [1][4]. Back in common era, since the pioneering work done by Dr. Macht and Robinson, multiple products have been synthesized to carry different types of drugs with the purpose of treating multiple diseases from chronical, infectious, and even hormonal imbalances which can be attended with local and systemic effects through this route using ovules, creams, gels, rings, films, tablets, nanoparticles, and the like [5][6][7] (Figure 1).
Even though gynecology is an ancient medical practice, the use of this route for drug administration has slowly but steadily developed fighting against stereotypes and taboos that have interfered with its growth, but science has made its way through, and the technique has become for some ailments even more reliable than oral and intramuscular route [7]. The local and systemic effect achievable with vaginal drug delivery systems has many advantages and can be used for many purposes. It does not require a first pass effect and does not involve the circulatory system for local effects [8]. It can carry drugs to prevent and treat different illnesses even during pregnancy and lactation and can interact with mucus and skin [9][10]. That is why every day more groups are focusing their research on active molecules that can be administered through the vagina [11][12][13][14][15].
Many biomaterials can be used to administer drugs through different routes but for the intravaginal administration specifically, polymers are preferred. Their mechanical properties, biocompatibility and overall performance has made them the number one choice for this application. Hydroxypropyl methylcellulose (HPMC) [11] Chitosan [12], sodium alginate [12], Carbopol [13], Polyethylene glycol (PEG) [11] and different types of gums [14] are only some of the more commonly used polymers that can be synthetized in forms of tablets, gels, rings, nano structures, films, patches, and so on (Figure 1). Furthermore, polymers also have the ability of fixing with the mucosa layer, becoming bioadhesive thus gaining various advantages like reduction in doses and in frequency of application due to increased residence time, also a prolonged and controlled release, higher bioavailability with lower concentration of bioactive molecules, no first-pass metabolism, specific targeting, and lower or nonenzymatic degradation [15].
MATERIALS AND METHODS
The present investigation was performed based on an extensive bibliographical review, consulting the Science Direct, SciELO, and PubMed Central databases, with a search strategy designed to obtain publications related to vaginal mucoadhesive drug delivery systems. The inclusion and exclusion criteria used in the bibliographic search are found in Table 1. Results included 76 articles, all in English, in which mucoadhesive drug delivery systems were made with biodegradable polymeric matrices.
Table 1 Inclusion and exclusion criteria used in the bibliographic review.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Publications between the years 2004 - 2022. | Publications prior to 2004, except for transcendental references that are an inherent part of the history of these devices. |
| English language publications. | Publications in a language other than English |
| Publications in vaginal drug delivery adhesive systems. | Publications related to drug delivery systems of other organs and tissues. |
RESULTS AND DISCUSSION
Mucoadhesive systems to delivery drugs
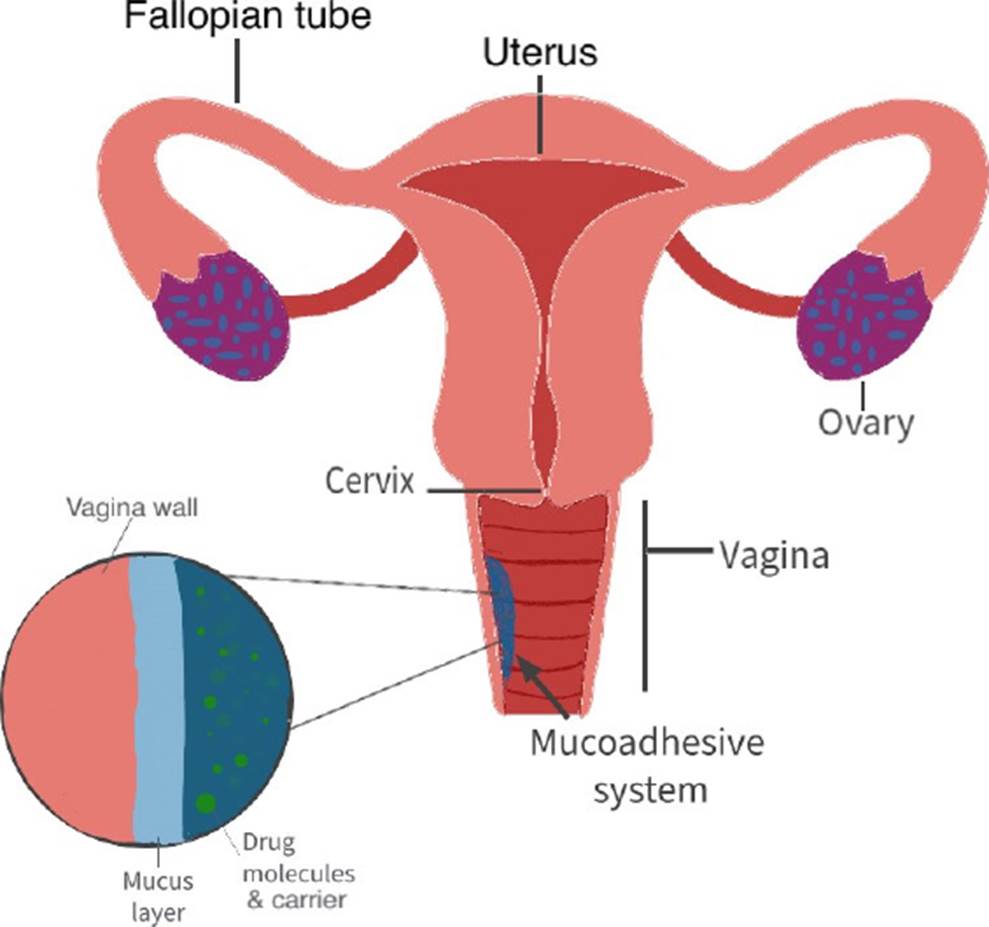
The cervix is covered by a semipermeable mucus which is mostly mucin. This makes a protector barrier and becomes an obstacle for the absorption of drugs within the medium (Figure 2). In the last decade, systems for intravaginal applications have been made with different mucoadhesive polymers such as PEG 400, HPMC, Chitosan, Carbopol, and the like to promote the absorption of the drugs liberated in the vagina [16] [1]. These systems present advantages over conventional treatments by having big contact surfaces allowing a deeper mucus penetration and avoiding as much waste as possible. This leads to a better absorption that can be as slow or fast as wanted depending on the materials used; also, allows to a better penetration of the metabolic and physiological barriers leading to bigger biodisposition of the drugs and lower risk of unwanted side effects [1][2].
In the synthesis of controlled release systems, many different polymers have been used both natural and synthetic because it has been seen that the adhesion between mucus and materials occur because hydrogen links are formed. Hydrophobic interactions can also happen or electrostatic, even macromolecule entanglements. This can produce mucoadhesion because the contact between the mucus and the system allows the humectation and expansion of the material leading to diffusion of the carrier lessening the chances of interactions between the drugs and mucus [16].
Choosing the right polymer depends mostly on the application, the mucoadhesiveness that is needed, the release time and the drug that is going to carry. As an example, HPMC and PEG 400 helps penetrate the mucus barrier and allows the release closer to the tissue. This system has characteristics such as biocompatibility, biodegradation and does not produce an immune response. It also allows hydrogen links to form which facilitate the interaction with the mucus barrier; through FTIR analysis, it was shown that hydrogen bonds and Van Der Waals forces are present [1][2][15][16].
Mucoadhesive systems to delivery anesthetics
From our current time, the first medical report of vaginal administration of drugs was done by Dr. Macht in 1918. In it, he developed research and provided pain medication called morphine to female patients that couldn't take it orally. In his methodology he introduced a pill that was first synthetized to be taken orally and administered through the vaginal canal and waited for the signs of the medication to take effect [3][6]. At this time oral purpose pills were not administered through this female cavity, but several presentations to provide drug delivery intravaginally had been developed from diaphragms, capsules, creams, ovules, suppositories, etc. Today drug administration focuses on bioadhesive forms that produce less leakage such as films [11], soft gel capsules [17], rings [18], hydrogels [19], foams [20], and others [3][6][21]. Since Macht’s study several trials [16][22][23] [24] have been made to provide pain medication from different sources to women with or without capacity to take the active molecules through other rout. from ketorolac to morphine. Compounds are used in these studies to alleviate the pain these people are suffering due to different causes, either chronic or acute, from surgeries, diseases, or hormonal imbalances [25][26][27].
In 2011, Perioli and their team tried alleviating the pain from a group of women using Benzydamine, which is a Non-Steroidal Anti-Inflammatory Drug also known as NSAID, that reduces pain and inflammation and can be contained in different presentations including mucoadhesive systems. In this work they synthesized vaginal tablets using polymers such as HPMC and/or Carbopol with prolonged release periods of over 40 hours obtaining promising results, especially on the release and residence time of the drugs [28].
Sometime later in 2017, Sanz et al. utilized Doxepin (which is a drug typically used to treat depressive disorders and other mental disorders, but it has shown good reactions when used in the treatment of pain) to relieve pain after a gynecological surgery using a mucoadhesive system for the delivery with orafix as the bioadhesive platform, menthol and transcutol [29]. Another example of VDDS is the work published by Pinheiro de Queiroz et al., which administered 5 % lidocaine for the treatment of localized pain after a cesarean and obtained results even after 36 h. That’s why it was considered ideal for drug delivery for long periods of time [30].
Viral infections such as Human Papilloma Virus (HPV) can be manifested through painful symptoms, erosions, ulcers, and scars are usually part of these processes [31]. To treat these infections, antivirals are accompanied by anti-inflammatory and analgesic drugs (Table 2), like Ketorolac. This compound has properties like opioids and can be used to treat from moderate to severe pain, but with no addictiveness potential or sedative properties. It has a similar yet less intense effect to morphine but without the side effects [32].
For all these characteristics El Moussaoui S. and her work group used a mucoadhesive Alginate-based hydrogel, using sodium alginate to deliver ketorolac vaginally to treat Condyloma acuminata, a disease caused by HPV. In this study they concluded that up to 73 % of the ketorolac was released in the first 6 h after administered, a faster permeation compared to through human skin and higher retention time up to 24 h [16]. Another drug often used in vaginal administration is diazepam, this molecule part of the benzodiazepines is often administered through suppositories and have the capacity of relaxing the pelvic floor and relieve pelvic pain locally because it controls the painful spasms and stiffness that causes them [23][33].
To treat Sexually Transmitted Infections
According to the World Health Organization (WHO) 2021, more than 1 million people acquire STIs daily. Also 500 million have a genital infection with herpes simplex. There are more than 30 different parasites, viruses, and bacteria that cause these infections; 8 of those being most common, 4 curable: syphilis, gonorrhea, chlamydia and trichomoniasis, and 4 incurables: Hepatitis B, Herpes simplex (HSV), Human Immunodeficiency Virus (HIV), HPV [34].
Candidiasis is the second most common vaginal infection worldwide with 1.4 million cases every year only in the US. It is considered one of the main causes of inflammation of the vagina, also known as vaginitis. Several approaches have been tested in order to treat and prevent this yeast infection (Table 3).
Table 3 Diseases (STIs) treated with mucoadhesive delivery systems.
| Disease | Materials | Active molecule | Reference |
|---|---|---|---|
| Candidiasis | Chitosan/HPMC | Tioconazole | [13] |
| Na-CMC, Carbopol, Chitosan | Probiotics | [14] | |
| HPMC, sodium alginate | Clotrimazole | [15] | |
| PVA-SA | [16] | ||
| Chitosan | Econazole nitrate, miconazole nitrate | [17] | |
| Gelatin | Econazole nitrate | [18], [19] | |
| Poly acrylic acid | Nystatin | [20] | |
| HIV/AIDS | HPMC, Carbomer, PEG400 | cabotegravir | [21] |
| HPMC, chitosan, guar gum and Eudragit | Tenofovir | [22] | |
| Gums and Eudragit L100 | [23] | ||
| Ethylcellulose and Xanthan gum | [24] | ||
| Microbial infections | chitosan | Metronidazole | [25] |
| Gellan gum, AMENA | [26] | ||
| Silicon | Ciprofloxacin | [27] | |
| Herpes | β-CD-CHO | Acyclovir | [28] |
| HPMC and polymethacrylate Eudragit S100 | [29] | ||
| polyvinyl pyrrolidone- Eudragit | [51] |
Vaginal drug administration is not the exception, vaginal films made of polymers such as HPMC, Chitosan, and such have been developed to administer the necessary drugs to eradicate this fungus [35][36]. Worldwide many research groups work to provide options for better treatment of STIs. In 2021, one group worked with a mucoadhesive films to deliver tioconazole to treat vaginal candidiasis [35]. In 2017 another one prefers to treat the fungus infection by administering probiotics [13] while in 2014 another group administered clotrimazole through a hydroxypropyl cellulose mucoadhesive film [36] and in 2014 another research group used a chitosan bioadhesive gel to administer econazole nitrate and miconazole nitrate [37]. Hombach on the other hand, opted for delivering Nystatin and compared the advantages between mucoadhesive gels and mucoadhesive tablets made out of Polyacrylic Acid (PAA) obtaining good results on both, depending on how the release wanted to be. For example, if it needs a prolonged release time, the tablet would be a better option, but if the rheology needs to be more stable, then gel is the option. Gel interacted better with water, but the interaction of the PAA and nystatin seem better on the tablet, therefore, both presentations are good realizing this drug [38]. In general, all research cited showed promising results due to the high levels of absorption of the active molecule compared to the oral alternatives. The residence time of all applications were less than 20 minutes with no secondary effects reported caused by the carrier materials.
Candidiasis and many other infections can be a consequence of a weaker immune system resulting in one of the most feared diseases, HIV/ acquired immunodeficiency syndrome (AIDS). According to WHO, HIV continues to be a public health issue with an estimated 37.7 million people living with the disease. It is mostly acquired through body fluids from infected people, especially from high-risk sexual behavior [39]. In order to treat this STI, several polymeric systems have been created, such as Enggi et al., who developed a mucoadhesive gel to administer with cabotegravir, registering great permeation results and residence time lapse. Better than the regular oral and injection route, making it a possible future replacement. Cazorla-Luna and his team preferred a bilayer film made of ethylcellulose and other biopolymers, obtaining good mechanical properties and adherence to the vaginal mucosa with presence of the drug even after a week allowing a biweekly administration [40][41]. Nematpour et al. administered clotrimazole using nanofibers of polyvinyl alcohol (PVA) and sodium alginate (SA) obtained from electrospinning generating properties suitable for a drug delivery system [42]. Stella Dolci and her team used the advantages of vaginal films, gelatin and polymers to deliver econazole. In both of their studies (2018 and 2020), they obtained good results improving in 2020 the release time frame with Gelucire, a combination of glycerides that can also work as crosslinker [43][44].
For microbial infections, several approaches have been made, but attacking directly and with a prolonged drug release has demonstrated better results for antimicrobial activity. Similarly Lupo reported using metronidazole with a chitosan mucoadhesive system, as well as Sousa who bet for a chitosan-based system to treat common vulvovaginal infections like trichomoniasis, candidiasis and bacterial vaginosis with a Drug Delivery System mucoadhesive to increase the retention time of the drug [45][46]. Campaña-Seoane M. and her team studied the pharmacokinetics and residence time of vaginal mucoadhesive silicone emulsions with ciprofloxacin, which is an effective antibiotic often used to treat STIs, showing that even after 24 hours of administration the system still had a significant concentration of the drug making possible its future in vivo trials with bigger species [47]. In 2019 Jalil and their team synthetized vaginal films made of gellan gum and a combination of other polymers, named by this team AMENA, to administer metronidazole, concluding that the film has all the properties needed to perform as an antimicrobial system [12]. Herpes viruses have infected approximately 4 billion people globally with either HSV-2 or HSV-1 or both. This virus can be transmitted very easily and be present even without symptoms, therefore, many people have been infected and don’t even know [48]. In order to fight this unwanted disease Ijaz M. and collaborators developed a mucoadhesive delivery system for Acyclovir using thiolate polymers showing good results for the vaginal mucoadhesion on porcine vaginal adhesion [49]. Edisson-Mauricio and collaborators worked on a polymeric film made of HPMC and polymethacrylate Eudragit S100 for the release of acyclovir to protect against herpes [50]. As for Ramyadevi et al., the mucoadhesive gel and nanoparticles with acyclovir allowed them to reduce the normal dosage ten times, showing promising results for further applications [51].
To prevent Sexually Transmitted Infections
As previously stated, according to WHO some STIs are incurable, therefore, their prevention is a key element of selfcare. VPH, Hepatitis B and HIV are a few of the most feared STIs because once infected there's no cure, only treatment to lessen the symptoms. In the case of VPH and Hepatitis B, several injections have been released to the market to prevent the spreading of these infections and teenagers are immunized before they are sexually active, thus preventing future infections [34][52]. Throughout history different active molecules have been administered using the most common medication routes, like oral, parental and intravenous to prevent and/or treat STIs, specifically HIV, with good results. Unfortunately, continued administration can affect different organs like the liver and gastrointestinal system due to the toxic nature of the molecules. Therefore, other approaches must be studied to provide these patients with a more effective treatment to better their life expectancy and general health. Also, to try to lessen the number of new cases by preventing the spreading with more information sites and providing the convenient drugs to community sectors at risk. For women, the intravaginal route is a promising way to prevent, treat and alleviate local and systemic infections that threatens their wellbeing [6][53].
HIV is one of the most feared diseases worldwide, existing since late 1800s in Africa, but truly being acknowledged in mid-1970s when it came to America. According to the Centers for Disease Control and Prevention (CDC) [54] it is considered one of the biggest epidemics and one of the most dangerous viruses that attacks the body’s immune system and if not treated can lead to AIDS. In order to prevent the transmission of this vicious virus, barrier methods are required. Specifically male condoms, but it still represents a risk, therefore, new methods have been developed in order to try to reduce new infections. Notario-Perez’s and his team worked with mucoadhesive vaginal tables made of HPMC, Chitosan, guar gum and Eudragit to prevent sexual transmission of HIV releasing tenofovir [55]; and later on, switch from using a vaginal table to a vaginal film based on HPMC administrating the same drug [56]. Martins-Illana and her team used several types of gums and Eudragit L100 to administer Tenofovir to prevent the transmission of this virus [14]. The same drug that Cazorla R. and his team administered through a bilayer film based on biopolymers such as ethylcellulose and xanthan gum, which is a natural polymer that can be plasticized with glycerol. Some of the obtained results showed a longer release period than expected, approximately 15 days with zero toxicity and optimal mechanical conditions [41].
Hormonal treatment and birth control
From the start of human history vaginal related medical assistance has evolved. Contraception, abortion and pregnancy practices were the gynecologic beginnings of women sexual care. Even the Egyptians (1850 BCE) considered the vaginal route one of the five routes of drug administration, but religion beliefs and “good morals” have gotten in the way of its development. The importance of women’s sexuality wasn’t considered until 1885 CE, when the first contraceptive drug was commercialized, based on quinine [20]. Nowadays women health care, specifically sex related, is looked after and freely spoken opening the possibility of vaginal drug administration [5][57].
Several infections within the pelvic area related to hormones have been treated using the vaginal route, from endometriosis, problems related with menopause, to abortions. All can be handled with medication administered intravaginally [58]. Several presentations have been used in order to carry these drugs and mucoadhesive polymeric systems are not the exception as shown in Table 4[51][55].
Table 4 Mucoadhesive drug delivery systems for Hormonal treatment and birth control.
| Presentation | Materials | Drug | Reference |
|---|---|---|---|
| Mucoadhesive ring | NE | Progestogen and estrogen | [30] |
| Gel | NE | globulin, estradiol, and progesterone | [31] |
| Ring | medroxyprogesterone acetate | progestin, progesterone, etonogestrel, levonorgestrel/ norgestrel, megestrol, nestorone, norgestrien estrogen, and progestin combinations | [32] |
| Bio adhesive tablet | HPMC, Carbopol 934 and PEG 6000 | Salbutamol | [8] |
For endometriosis several approaches have been applied throughout the years. Surgical to hormonal treatments are administered to reduce the symptoms of this chronic disease that affects roughly 190 million women in the reproductive age worldwide. Hormones like progestogen and estrogen or combinations of both are used to treat the pain and/or infertility associated with this disease [59]. Normally, oral route is the approach used to administer these drugs, but recently vaginal methods are being used to reduce the side effects associated with taking hormones. Vercellini et al. compared a combination of progestogen and estrogen carried by a vaginal ring and a patch obtaining better results with the vaginal mucoadhesive ring because the mucoadhesive system showed a longer residence time and a steadier release pattern [27].
Menopause is a natural process that all females go through, where hormones are involved. It marks the end of the menstrual cycles and finishes the carrying capacity of new life for women meaning big changes in the life of the person going through it. This stage of life has big repercussions physically and mentally speaking, thus in order to lessen the symptoms present within this process, several drugs have been developed, from natural hormones to processed molecules with different forms of administration including oral and injections. In 1988 Carlstrom and collaborators suggested the vaginal administration of folliclestimulating hormones, globulin, estradiol and progesterone. In this study they used a mucoadhesive gel inserted intravaginally with a special applicator, obtaining higher concentration levels of the hormones on the blood system even though a lower dosage than normal was administered. In addition to reporting a lower liver and kidney activity than when oral administration was used. In this study in general, the new intravaginal administration of hormones was an “overall success” [60]. Later, Ballagh reported the use of a vaginal ring made of medroxyprogesterone acetate for hormone delivery for contraception and menopause purposes. It contained progestin, progesterone, etonogestrel, levongestrel/ norgestrel, megestrol, nestorone, norgestrien, and in general estrogen and progestin combinations. The hormone release rate was higher in the first 48 hours, but effects could be seen even after a week of the administration proving to be a great alternative to oral administration [18]. On the other hand, are the traditional remedies, many communities have used Curcuma comosa and its extracts to treat hormonal imbalances because it contains phytoestrogens. Estrogen replacement therapy can help the cognitive decline women experiment postmenopausal, that is the reason Tunpanich P. and team synthesized a system made of polycarbophil (PCP), HPMC and silica, for Curcuma comosa controlled release [17] [61].
Preterm labor is one of the major causes of infant illnesses and deaths around the world. It can affect the new life and the mother's health, hence, should be avoided as much as possible and treated with caution. Salbutamol is a drug used to arrest preterm labor, usually administered intravenously, but Abu El-Enin and his team showed that it can also be administered using a vaginal bioadhesive tablet made of HPMC, Carbopol 934 and PEG 6000 obtaining higher concentrations within time than the leader drug Ventolin [8]. Another example and application of this administration route is shown in El-Refaey's work, published in 1994, were they administered a combination of oral mifepristone and intravaginal misoprostol for an early induction of abortion; showing good results with fewer side effects compared to other techniques [26]. This last example is also a great concern in the overall public health, because many women suffer from bad practices due to the illegalness of abortion, thus early alternatives that care for the health of patients are very important [6].
Mucoadhesive systems used for Cancer treatment
Women’s reproductive system, as the rest of the body, is capable of growing cancer cells and tumors. When it happens, it is called gynecologic cancer; it can affect the cervix, ovaries, uterus, vagina, vulva, and even the fallopian tubes (Figure 3). According to the CDC this is one of the main causes of women deaths around the globe [62]. In any cancer treatment the whole body is attacked with drugs to try to kill as many cancer cells as possible, but in the process many healthy cells are destroyed, since most active molecules used for chemotherapy are highly toxic. Therefore, localized systems are required to minimize the side effects, result of the shatter cells [63] [64] [65].
In an effort to apply the active molecules as close to the tumor as possible, many research groups have used the vaginal route to treat gynecologic cancers (Table 5). Zong et al. showed in their study, that carrying cisplatin through a polymeric film and gel can help to shrink tumors considerably. They obtained better residence results with the HPMC film than the HPMC gel because it was more mucoadhesive and it had less leakage [66]. Cisplatin, since its discovery in 1972, has been one of the most used anticancer drugs for its mechanism capable of taking the cells to apoptosis [67]. That is the reason Mohammad Reza et al., delivered this molecule using PAA hydrogels, reducing the regular doses of cisplatin but maintaining the efficiency [68]. Later on, Aggarwal’s team developed poly-caprolactone and chitosan nanofibers to vehicle cisplatin to treat cervical cancer. Because poly-caprolactone has an adhesive nature, the studies showed that it can transport and stay in the cervix for an extended period with high bioavailability [69].
Table 5 Mucoadhesive drug delivery systems for cancer treatment.
| Presentation | Materials | Drug | Reference |
|---|---|---|---|
| Film | HPMC | Cisplatin | [33] |
| Hydrogel | |||
| PAA | [34] | ||
| Nanofibers | poly-caprolactone and chitosan | [35] | |
| Sponge | Collagen | Retinol | [36] |
| Cervical patch | Carbopol / glycerin | 5- Fluorouracil | [37], [38] |
| Mucoadhesive nanoparticles | phenylboronic acid | Mucin responsive drugs | [39] |
| Hydrogel | Poloxamer 407 and acetate gossypol (AG) | [40] | |
| Mucoadhesive polymeric liquid | polyethyleneimine (FPC) and chitosan | Curcumin | [41] |
Another group working on treating cancer is Meyskens F. L. et al., with a retinol like molecule vehicle with a collagen sponge, which changed daily and helped reduce the tumor size, but had high toxic levels [22][24]. Woolfson D. and team preferred a bio adhesive cervical patch of Carbopol and glycerin to deliver 5-Fluorouracil directly to cervical tissue with sufficient drug release to obtain a good clinical effect [70][71].
Mucins are highly glycosylated proteins used for protection and control of the local molecular microenvironment of organs and tumors during cancer process and metastasis. Because of this, they can be used as markers in cancer as a therapeutic target [72]. Chunyan Li et al. worked on a mucoadhesive phenylboronic acid-rich nanoparticles to vehicle and release a mucin-responsive drug to treat cervical carcinoma, with release up to 72 h. [73]. Similarly, Liqian Ci synthetized a system amino-functionalized hydrogel with Poloxamer 407 and acetate gossypol (AG) to carry different drugs showing mucin sensitivity. This is why it is considered an important biomarker for tumor targeting, because it covers most tumors; that is one of the many reasons Liqian’s work is considered ideal to be used on chemotherapy [74].
As for natural medicine, curcumin is in the spotlight for its anti-cancer effects. That is why Damiani and his team decided to deliver it through a mucoadhesive polymeric liquid made of polyethyleneimine (FPC) and chitosan, showing that the system has an affinity for Hela cells [75]. Berginc and team opted for mucoadhesive liposomes coated with chitosan and Carbopol to deliver curcumin. In their study they compared liposomes coated and not coated, evaluating the tissue retention and permeability and obtaining a better residence time, release and mucoadhesion with the coated group [76].
CONCLUSIONS
Presently, intravaginal delivery systems are a more common way to administer drugs. Its growth has been slow but steady, showing satisfactory results locally and around the system. This form of administration has broken stereotypes and taboos about women’s health and care, and even though their existence is long before current era, it has flourished in the last decades. On the other hand, mucoadhesive systems have evolved throughout the years alongside this administration route, covering the necessity of long release periods. Due to their capacity of working for many hours, days and even weeks, reducing the loss of active molecules through discharge and delivering drugs for several purposes from STDs to hormonal and cancer treatments with overall good results. These mechanisms allow lower dosages with steadier residence times and permit the avoidance of the first pass effect. Mucoadhesive VDDS are mostly manufactured with biocompatible and bioinert polymers to ensure a secure interaction with the active molecules and the mucus layer. This allows the penetration of the molecules into the vagina wall thus enhancing drug absorption.











 text new page (beta)
text new page (beta)