INTRODUCTION
The peridinioid family Podolampadaceae Er. Lindem., 1928, characterized by the absence of a transversal furrow, cingular lists and a depressed sulcus bordered by the lists from both sides and therefore easily recognizable, includes eight genera: Podolampas F. Stein, 1883, BlepharocystaEhrenb., 1873, GaarderiaCarbonell-Moore, 1994, HeterobractumCarbonell-Moore, 1994, LessardiaSaldarriaga et Taylor, 2003, Lissodinium Matzenauer, 1933 emend. Carbonell-Moore, 1991, MysticellaCarbonell-Moore, 1994, and Roscoffia Balech, 1956. The morphological differences between the genera are, to a greater extent, in the structure of the apical pore complex (APC) including the cover plate (Carbonell-Moore, 1994a); the genera Gaarderia, Heterobractum and Mysticella were described based on the differences in the APC structure, cell compression and cell bilateral asymmetry. Only recently, based on molecular data, Gómez et al. (2010) proved that Roscoffia and Lessardia are also from the podolampadacean (also called podolampacean) clade, although Carbonell-Moore (2004) considers the latter as belonging to the family Lessardiaceae Carbonell-Moore, 2004, due to the difference in plate formula from the rest of the podolampadaceans.
Of them, Podolampas and Blepharocysta are the most common genera constituting plankton communities in both tropical and temperate waters. Six well-separated Podolampas species are known, without considering a poorly described P. curvatus Schiller from the Adriatic Sea (Schiller, 1937: 476, fig. 549), also illustrated by Wood (1968: 119, fig. 363) from the Caribbean Sea, and P. antarctica Balech (Balech & El-Sayed, 1965: 121, pl. 4, fig. 56-64) described from the Weddell Sea and also pictured in Taylor (1976: 170, pl. 27, fig. 283) from the southwestern Indian Ocean. For the genus Blepharocysta, six species names were known by the beginning of the 1960s: B. splendor-maris Ehrenb., 1859, B. striataSchütt, 1895, B. paulseniiSchiller, 1937, B. denticulataNie, 1939, B. compressaGaarder, 1954, and B. matzenaueriGaarder, 1954 (Balech, 1963). At present, five species names are accepted taxonomically: B. splendor-maris, B. denticulata, B. paulsenii, B. hermosillae Carbonell-Moore, 1992, and B. okamuraeAbé, 1966 (Guiry & Guiry, 2022); however, the validity of B. paulsenii described from the Adriatic Sea is considered doubtful and to be synonymous to B. splendor-maris (Nie, 1939). Balech (1988) accepted B. paulsenii, noting that this species has a lower epitheca compared to others; in addition, he considered B. okamurae a doubtful species, at the same recognizing the rather wide morphological variability of B. splendor-maris. Furthermore, Balech (1988) stressed two peculiar features in B. denticulata: a shorter sulcus and the sulcal membranes projecting completely to the ventral side of the cell. More differences in detail of this species from others in the genus are given in Balech (1963). Recently, based on light microscopy and scanning electron microscopy observations, Hernández-Becerril & Arce-Rocha (2021) recognized all five aforementioned species, with a special emphasis on B. paulsenii and B. splendor-maris; they also reviewed the other authors’ opinions on the synonymy of Blepharocysta species. Finally, based on Blepharocysta-like species, Mertens et al. (2023) described two new podolampadacean genera, Sphaeralata Nézan, Carbonell-Moore, K. N. Mertens et Chomérat and Pseudosphaeralata Nézan, Carbonell-Moore, K. N. Mertens et Chomérat, using both morphological and molecular criteria.
Although the podolampadaceans have been known since the end of the nineteenth century, and Kofoid (1909) described the theca of Podolampas in detail, their morphology had been not well determined until the mid-twentieth century (Nie, 1939, 1942; Balech, 1954, 1963). In addition to the aforementioned literature, studies dedicated especially to the Podolampadaceae and Podolampas in particular were published by Rampi (1941), Andreis & Andreoli (1975), Carbonell-Moore (1994a, b, 2004), Saldarriaga et al. (2003) and Gómez et al. (2010). The monograph by Balech (1988) on the dinoflagellates of the South Atlantic also contains detailed information on the morphology of thecae of the podolampadaceans.
In the Mexican Pacific, five Podolampas and two Blepharocysta species have been reported since the early 1940s (Gilbert & Allen, 1943; Barreiro-Güemes, 1967; González-Villalobos, 1971; Okolodkov & Gárate-Lizárraga, 2006). Occasionally, their records were documented with illustrations (Licea et al., 1995; Hernández-Becerril, 1988a, b; Gárate-Lizárraga et al., 2007; Esqueda-Lara & Hernández-Becerril, 2010; Hernández-Becerril & Arce-Rocha, 2021), with P. reticulata and P. spinifera illustrated only twice (Hernández-Becerril, 1988a, b; Esqueda-Lara & Hernández-Becerril, 2010). The objective of the present study was to document the presence of the Podolampas and Blepharocysta species in the Mexican Pacific and Atlantic.
MATERIAL AND METHODS
As a part of an ongoing toxic and noxious microalgal monitoring program, phytoplankton bottle samples were collected monthly at two fixed sampling stations in the Bahía de La Paz, southern Gulf of California, Mexican Pacific. The first sampling station was located above the shallow basin at the southernmost end of the bay (24°21’N, 110°31’W; see Gárate-Lizárraga & González-Armas, 2015) with samples collected from October 2010 through September 2016, and the second one was in Alfonso Basin (24º39’N, 110º36’W), from which samples were taken from June 2016 through December 2018 (see Silverberg et al., 2006). Phytoplankton samples were collected in plastic flasks of 250 ml capacity, fixed with an acid Lugol’s solution, and later preserved with 37% formalin to a final concentration of 4%. Surface horizontal tows were taken with a 20 µm mesh net. Sea surface temperature was measured with a bucket thermometer. A sub-sample was taken for live phytoplankton observations. Examination and identification of Pacific podolampadacean species was made under a Carl Zeiss phase-contrast microscope. A digital Konus camera (8.1 MP) was used to record the images.
Atlantic samples were taken from the coastal waters of the State of Veracruz, southwestern Gulf of Mexico, at 27 stations located within the National Park Sistema Arrecifal Veracruzano. Approximately 700 samples were taken by hand with a 20 µm or 30 µm mesh phytoplankton net during 5 min. horizontal tows at a boat speed of ca. 2.5 knots to sample the uppermost 30-cm layer. Collections were made almost every week during the period from May 2005 through April 2008 as a part of the monitoring program of the Aquarium of Veracruz (AVM) and during two monthly monitoring programs by ICIMAP-UV from September 2006 through September 2007 (CEP-I) and from April 2007 through May 2008 (CEP-II). Site depths ranged from 1.5 m to 34 m. The samples were fixed with a stock formaldehyde solution to a final concentration of 4% and stored in 100-ml plastic bottles. Some samples were taken sporadically from the northern Yucatan coastal waters in the southeastern Gulf of Mexico from 2008-2019.
In the laboratory, a 0.2% Trypan Blue solution was added to water mounts (Lebour, 1925). This stain has been used to better distinguish sutures between thecal plates, allowing examination of the shape of individual plates and their connections with the adjacent ones and the tabulation pattern in general. A Nikon TS100 and an Olympus CKX41 inverted phase-contrast microscope were used in combination with a Sedgwick-Rafter 1-ml chamber and an Olympus BX51 compound microscope equipped with phase-contrast objectives, and a digital Olympus C7070 Wide Zoom camera (5.1 MP) was used for water mounts and microphotography. Some samples were examined primarily in a JEOL JSM-7600F scanning electron microscope (SEM) at a working distance of 15 to 21 mm and a voltage of 1.2 to 5.0 kV after a preliminary wash in distilled water, followed by dehydration in a series of ethanol solutions of increasing concentration (30, 50, 70, 90 and 100%), air drying on 0.5” aluminum mounts and sputter coating with gold-palladium using a Polaron SC7640 High Resolution Sputter Coater (Quorum Technologies, Newhaven, East Sussex, U. K.). Occasionally, an environmental SEM Philips XL30 was used at a working distance of 9.9-10.0 mm and a voltage of 25.0 kV. Species were identified using exclusively SEM images (Blepharocysta) or light microscopy (Podolampas); in the latter case, SEM images were not critical for species identification and provided additional information about the dinoflagellate thecae.
After each description given below, only references to publications with illustrations are included, and they are marked with asterisks: an asterisk (*) indicates line drawings, two asterisks (**) indicate light micrographs and three asterisks (***) indicate scanning electron micrographs. Abbreviations of authors of scientific names are used according to Brummitt & Powell (1992) unless they were not listed in the book.
RESULTS
Family Podolampadaceae Er. Lindem., 1928 (for synonymy, see Fensome et al., 1993: 141-143).
Diagnosis: “Peridiniineans in which the cingulum is not readily apparent but in which a series of three plates occur posterior to the equator of the cell” (Fensome et al., 1993: 143). The thecal formula: Po, 3’, 1a, 5”, 3c, 3-2”’ (two precingulars is an exceptional case), 3”” (as interpreted by Fensome et al., 1993); the sulcus is formed by four main plates and, in some cases, another one or two (Balech, 1988). There are neither longitudinal nor latitudinal furrows, characteristic for most dinoflagellates: the zone that corresponds to the cingulum has no membranes, and the sulcus is marked with well-developed lists; these lists are nearly absent only in B. denticulata (Balech, 1963). Interpretation of plates, and thus the thecal formula, differs with the authors. According to Balech (1963, 1988), the cingular plates are very large, forming a band that is higher than the hypotheca so that the epitheca, the cingulum and the hypotheca form a continuous surface uninterrupted by grooves, membranes or ridges. Theca smooth or weakly reticulated, with sparsely scattered round or elliptical pores. Blepharocysta splendor-maris, Podolampas bipes and P. palmipes are non-photosynthetic (Hallegraeff & Jeffrey, 1984). Kleptochloroplasts present in two Podolampas species (Schweikert & Elbrächter, 2004). Resting cysts unknown.
Genus BlepharocystaEhrenb., 1873
Cell shape widely elliptical to slightly oval along the longitudinal axis, not compressed dorsoventrally, without neck and antapical spines. The apical area is only just marked with a slight concavity rounded with almost indiscernible ridges. Two antapical-ventral lists are located very close to each other, almost parallel to the longitudinal axis of the cell and protrude backward. Plates 2’ and 3’ are very small, embracing the Po plate. The 1a plate is small and rectangular; it appears to be connected to the Po, but it is separated by thin prolongations of the 2’ and 3’ plates. Cingular groove is absent. Sulcus is narrow and very shallow at the posterior end located between the two membranes mentioned above. The apical pore complex is button-like, rather large. The pores are more or less dense in the precingulars and apicals, and denser in the postcingulars, where they do not form a double row as in most Podolampas species; the cingulars bear finer and sparser pores (Balech, 1988). Nucleus is large, with condensed chromosomes as striae easily seen under a light microscope. Chloroplasts absent. Resting cysts unknown. The thecal formula: Po, Pt, x, 3’, 1a, 5”, 3c, 4s, 4-5”’, 1”” (Okolodkov, 2011).
Blepharocysta denticulataNie, 1939: 32, pl. 2, fig. 20-25. (Fig. 2a)
Cell globose or subglobose, with two membranes located ventrally, almost parallel to the longitudinal axis of the cell, closer to the antapex, but more ventrally and shorter than in B. splendor-maris and B. okamurae. Theca is coarsely areolated, with densely situated pores, each of them located in rather deep depressions. The cingular plates are shorter in relation to the longitudinal axis of the cell than in the mentioned two species. Cell length 46 μm, width 45 μm.
Morphological note: According to the original description of Nie (1939), the sulcal area of the species is broader and shorter than in B. splendor-maris; theca is without markings, sutures are zigzags, the 1a plate is quadrangular (in B. splendor-maris it is rectangular); of the postcingular plates, the 3”’ plate is the largest (in B. splendor-maris the 2”’ is the largest); there is a differentiation between transversal series of plates as to the pore types.
Blepharocysta okamurae T. Abé, 1966: 144, fig. 33-38. (Fig. 2b-e)
Cell ovoid, with two membranes located ventrally, almost parallel to the longitudinal axis of the cell, closer to the antapex, longer than in B. denticulata. Theca is less coarsely areolated than in B. denticulata, with densely situated pores, each of them located in shallow depressions. The cingular plates longer than in the latter. Cell length 46 μm, width 41 μm.
Morphological note: As for the cell shape, according to Abé (1966), this species is more rounded than B. splendor-maris and more similar to B. paulsenii; the sulcal lists are located more anteriorly and distinctly areolated; the precingular and postcingular plates are shorter; and the 1 a plate is rectangular.
Blepharocysta paulsenii J. Schiller, 1937: 478, fig. 552a-i. (Fig. 2f)
Cell globose. Theca is smooth, densely perforated with pores. The sulcal lists are situated ventrally and not seen in dorsal view. Cell width 61 μm.
Morphological note: According to the description of Schiller (1937), the cells of the species are rounded; however, mistakenly, the 1a plate was not distinguished, and the apical pore was described as surrounded by a collar situated on the pentagonal apical plate orientated anterior-posteriorly narrowing towards the antapex (presently, this complex of plates is known as the apical pore complex (APC), the canal platelet located between the APC and the narrow 1’ plate, the 2’ and 3’ plates attached to the APC laterally, and the 1a plate situated dorsally). The sulcal lists are pictured as located ventrally (Schiller, 1937: fig. 552a, b, d, g, h) rather than ventrally-posteriorly as in B. splendor-maris).
Blepharocysta splendor-maris (Ehrenb.) Ehrenb., 1873: 4. (Fig. 2 g-h, 4a-d)
Basionym: Peridinium splendor-maris Ehrenb., 1860.
Description. Cell ovoid, with two membranes located ventrally, closer to the antapex, parallel to the longitudinal axis of the cell, emerging posteriorly out of the cell body, which is visible in ventral view. Theca is smooth, sparsely perforated with pores. Cell length 56 μm, width 52 μm.
Literature:Stein, 1883*: pl. 7, fig. 17-19, pl. 8, fig. 3-5; Schütt, 1895*: 162, pl. 20, fig. 61; Okamura, 1907*: pl. 5, fig. 34a-d; Paulsen, 1908*: 93, fig. 126; Lebour, 1925*: 160, fig. 52c; Schiller, 1937*: 477, fig. 550; Nie, 1939*: 31, pl.1, fig. 1-16, pl. 2, fig. 17-19, text-fig. 1, 2 (after Schiller, 1937); 1945*: fig. 12-14 (after Nie, 1939); Rampi, 1941*: 148, fig. 8, 9; Balech, 1963* (Bol. Inst. Biol. Mar., 2): 16, pl. 3, fig. 34-44; Abé, 1966*: 141, fig. 21-32 (as Blephalocysta splendor-maris); Wood, 1968*: 22, fig. 35; Steidinger, 1972*: pl. 5, map 5; Pesantes-Santana, 1978*: 6, pl. 2, fig. 6; Dodge, 1982*: 254, fig. 33H; Sournia, 1986*: fig. 190 (after Abé, 1966), 191a, b (after Rampi, 1941); Balech, 1988*: 125, pl. 52, fig. 16-19 partim; Carbonell-Moore, 1994a***: pl. 1, fig. 1; Steidinger & Tangen, 1996*: 533, pl. 7 (in figure legend as Blepharocysta sp.), 49; Konovalova, 1998*: 168, fig. 35, 36 (6a, b); Al-Kandari et al., 2006**: 187, 336, pl. 39, fig. O; Gárate-Lizárraga et al., 2009**: 25, fig. 58; Omura et al., 2012** ***: 127, fig. a-e; Almazán-Becerril et al., 2016**: 84, fig. 201; Al-Yamani & Saburova, 2019**: 288, pl. 157, fig. a-i; Hernández-Becerril & Arce-Rocha, 2021** ***: 3, fig. 1-12.
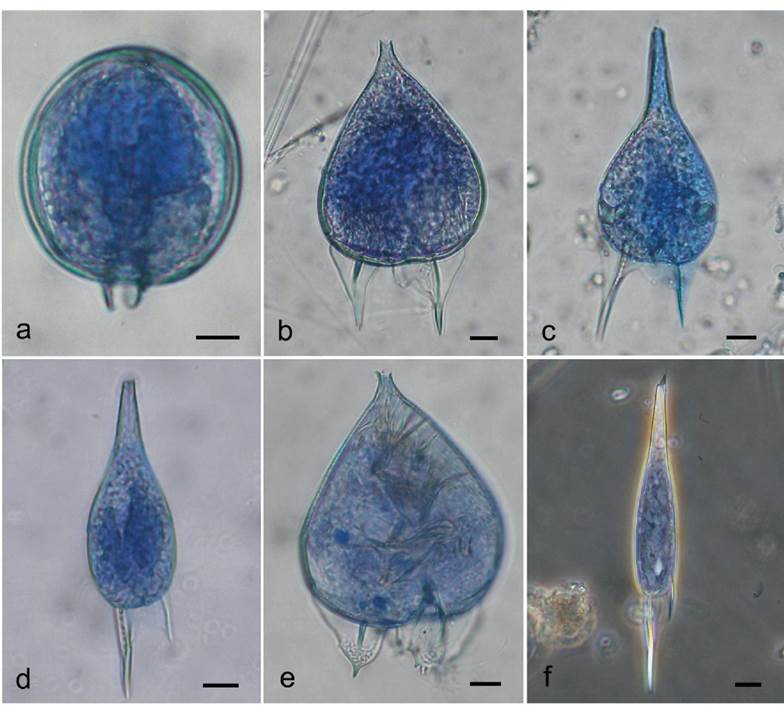
Figure 1 Light microphotographs of the Podolampadaceae species from the southern Gulf of Mexico (State of Veracruz): a - Blepharocysta sp. (ventral view), b - Podolampas bipes (ventral view), c - P. elegans (ventral view), d - P. palmipes (dorsal view), e - P. reticulatum (ventral view), f - P. spinifera (ventral view). Thecae were stained with Trypan Blue; a-e - bright field images, f - phase contrast image. Scale bar: 10 μm.
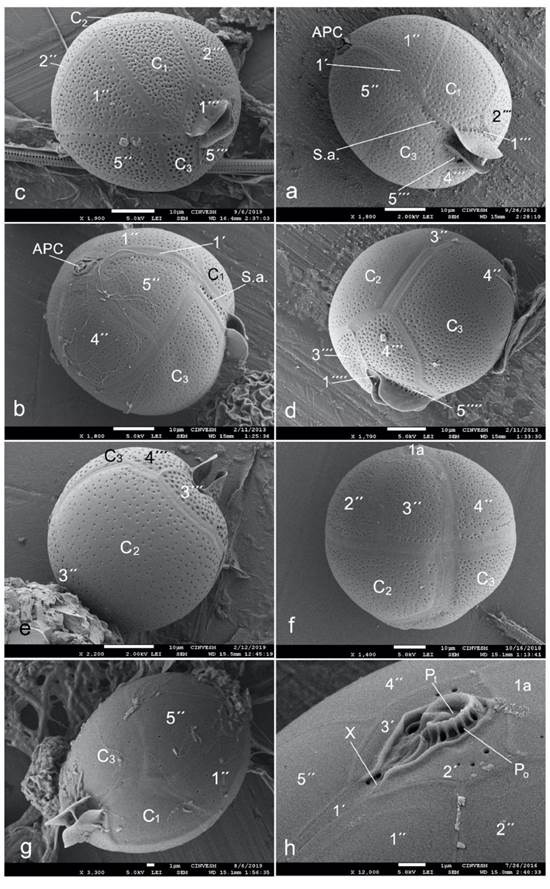
Figure 2 Scanning electron micrographs of Blepharocysta species from the southern Gulf of Mexico (states of Veracruz and Yucatan): a - B. denticulata in left-side-ventral view, b-e - B. okamurae (b - ventral view, c - ventral-right-side-apical view, d - right-side-dorsal-antapical view, e - dorsal view), f - B. paulsenii in dorsal view, g - B. splendor-maris in ventral view, h - the apical pore complex and adjacent epithecal plates in B. splendor-maris. Symbols of the Kofoidean tabulation system: APC - apical pore complex, Po - pore plate, Pt - cover platelet, X - canal platelet, 1´-3´ - apical plates, 1a - intercalary plate, 1´´-5´´ - precingular plates, C1-C3 - cingular plates, S.a. - sulcal anterior plate, 1´´´-5´´´ - postcingular plates, 1´´´´ - antapical plate. Scale bars: 10 μm in a-f; 1 μm in g and h.

Figure 3 Scanning electron micrographs of the Podolampas species from the southern Gulf of Mexico (states of Veracruz and Yucatan): a - P. bipes (ventral-apical view), b-e - P. palmipes (b - ventral view, c - left-side view, d - posterior half of the cell, e - fragment of the posterior part of the cell in right-side-ventral view), f-h - P. reticulata (f - ventral view, g - dorsal view, h - posterior end of the cell in dorsal view). Symbols of the Kofoidean tabulation system: 1´-3´ - apical plates, 1a - intercalary plate, 1´´-5´´ - precingular plates, C1-C3 - cingular plates, S.a. - sulcal anterior plate, S.d. - sulcal right plate, 1´´´-5´´´ - postcingular plates, 1´´´´ - antapical plate. Scale bars: 10 μm in a-d, g and h; 1 μm in e; 20 μm in f.
Genus Podolampas F. Stein, 1883
Syn.: ParroceliaGourret, 1883: 81, pl. 3, fig. 48, 48a.
Cells widely or narrowly pear-shaped, terminated anteriorly with a neck, short or long, and posteriorly with antapical spines (1 to 3). The hypotheca is shorter than the epitheca. The cingulum is somewhat descendant. Each of the postcingular plates has a double row of densely arranged well visible pores. The antapicals bear spines, each of them bordered with membranes (Balech, 1963, 1988). The thecal formula: Po, Pt, x, 3’, 1a, 5”, 3c, 4-5s, 5”’, 1”” (Okolodkov, 2011). Kleptochloroplasts present in P. bipes and P. reticulata (Schweikert & Elbrächter, 2004).
Podolampas bipes F. Stein, 1883, pl. 8, fig. 6-8. (Fig. 1b, 3a, 4f-i)
Syn.: Parrocelia ovataGourret, 1883: 82, pl. 3, fig. 48, 48a.
Description. Cell widely pear-shaped, somewhat compressed dorsoventrally, with a short, well separated apical horn, about 1.3-1.4 times longer than wide, with two long, slightly curved antapical spines, almost equal in length and bearing broad lists with smooth margins. Cell length 78-81 μm (102-105 μm with the antapical spines), width 43-58 μm. Two types of kleptochloroplasts are present (Fig. 4g, i).

Figure 4 Light microphotographs of the Podolampadaceae species from Bahía de La Paz, including Alfonso Basin, southern Gulf of California: a-d - Blepharocysta splendor-maris (a, b - lateral view, c - ventral view, showing precingular and cingular plates, d - left-side view), e - Blepharocysta sp. in right-side view, f-i - Podolampas bipes (f, g, i - ventral view, h - dorsal view), j-m - P. elegans (j-l - ventral view, m - dorsal view), n-q - P. palmipes (n, o, q - ventral view, p - dorsal view, q - hyaline cyst), r-u - P. reticulata (r - ventral view, s-u - dorsal view), v-y - P. spinifera (v-y - ventral view). N - nucleus; V - vacuole; yellow arrows indicate kleptochloroplasts. c, f, p - empty thecae. e, l - fixed with Lugol; the rest are living cells.
Literature:Bütschli, 1885*: pl. 55, fig. 9a; Schütt, 1895*: pl. 19, fig. 56; Paulsen, 1908*: 92, fig. 125; Okamura, 1912*: 16, pl. 2, fig. 37; Lebour, 1925*: 160, fig. 52b; Schiller, 1937*: 474, fig. 544a, b (after Stein, 1883); Rampi, 1941*: 146, fig. 2, 5; Nie, 1942*: 56, pl. 1, fig. 1-14; Kiselev, 1950*: 250, fig. 434 (after Stein, 1883); Trégouboff, 1957*: 119, pl. 27, fig. 16; Abé, 1966*: 150**, fig. 55-68; Yamaji, 1966*: 107, pl. 51, fig. 19; Steidinger et al., 1967**: pl. 4, fig. a; Wood, 1968*: 119, fig. 362; Steidinger & Williams, 1970**: 60, pl. 35, fig. 125; Andreis & Andreoli, 1975** ***: 388, fig. 3, 9, 9A; Taylor, 1976* ***: 171, pl. 27, fig. 288, pl. 45, fig. 524; Dodge, 1985***: 117; Sournia, 1986*: fig. 193 (after Balech, 1963); Balech, 1988*: 123, pl. 52, fig. 20, pl. 53, fig. 1, 2; Gárate-Lizárraga, 1988**: pl. 6, fig. 8; Hernández-Becerril, 1988a*** (Inv. Pesq. 52): 529, fig. 33, 34; Delgado & Fortuño, 1991* ***: 9, fig. 5U, pl. 25, fig. b; Carbonell-Moore, 1994a* ***: fig. 4I, 6I, 8H, pl. 1, fig. 9; Carbonell-Moore, 2004*: fig. 20, 29 (after Carbonell-Moore, 1994a); Licea et al., 1995* **: 77, pl. 8, fig. 11, pl. 22, fig. 3; Steidinger & Tangen, 1996***: 534, pl. 7; Konovalova, 1998*: 166, fig. 36 (3a, b); Dodge & Lee, 2000***: fig.55; Schweikert & Elbrächter, 2004**: 615, fig. 1-6; Ojeda, 2005* **: 159, lám. 31, fig. 1, lám. 57, fig. 4; Al-Kandari et al., 2006**: 189, 336, pl. 39, fig. R; Esqueda-Lara & Hernández-Becerril, 2010**: 133, fig. 126a-c; Gómez et al., 2010**: 214, fig. 1; Omura et al., 2012** ***: 128, fig. a-f, non g-k; Al-Yamani & Saburova, 2019**: 290, pl. 158, fig. a-h; Yovera-Galvez et al., 2020**: 167, fig. 228.
Podolampas elegans F. Schütt, 1895: pl. 18, fig. 57. (Fig. 1c, 4j-m)
Description. Cell narrowly pear-shaped, not compressed dorsoventrally, about 1.93 times longer than wide, with the epitheca much longer than the hypotheca. Epitheca is drawn into a long, not well separated apical horn. Hypotheca with two subequal antapical spines, the right one slightly longer. Cell length 81 μm (110 μm with the antapical spines), width 42 μm. Kleptochloroplasts are present (Fig. 4 j, k, m).
Literature:Kofoid, 1909*: 48, pl. 3, fig. 1-7; Lebour, 1925*: 160, fig. 53; Schiller, 1937*: 475, fig. 546; Rampi, 1941*: 146, fig. 1, 4; Kiselev, 1950*: 262, fig. 435b (as P. palmipes; after Schütt, 1895); Gaarder, 1954*: 55, fig. 73a-e (after Kofoid, 1909); Trégouboff, 1957*: 119, pl. 27, fig. 17; Curl, 1959*: 306, fig. 125; Balech, 1963*: 6, pl. 1, fig. 1-7; 1988*: 124, pl. 53, fig. 7, 8, 12; Wood, 1963*: 50, fig. 186; Abé, 1966*: 149, fig. 52-54; Wood, 1968*: 119, fig. 364; Steidinger & Williams, 1970**: 60, pl. 36, fig. 127; Taylor, 1976*: 171, pl. 27, fig. 290, 281; Dodge, 1985***: 118; Sournia, 1986*: fig. 196 (after Balech, 1963); Gárate-Lizárraga, 1988**: pl. 6, fig. 11; Hernández-Becerril, 1988b** (Bot. Mar. 31): 433, fig. 33; Delgado & Fortuño, 1991***: 9, pl. 25, fig. a; Ojeda, 2005* **: 160, lám. 31, fig. 2; Gómez et al., 2010**: 214, fig. 2, 3; Omura et al., 2012**: 128, fig. a, b.
Podolampas palmipes F. Stein, 1883, pl. 8, fig. 9-11. (Fig. 1d, 3b-e, 4n-q)
Description. Cell narrowly pear-shaped, not compressed dorsoventrally, about 1.53-2.60 times longer than wide, with the epitheca much longer than the hypotheca. Epitheca is drawn into a long, not well separated apical horn (sometimes called neck in the literature). Hypotheca is very low, obtusely rounded posteriorly, with two long, broadly winged unequal spines, parallel or slightly divergent, the left spine being about twice as long as than the right one (a characteristic feature). Cell length 46-63 μm (88-95 μm with the antapical spines), width 24-30 μm. Hyaline cysts are observed for the first time (Fig. 4 q). Kleptochloroplasts are present (Fig. 4 o, q).
Literature:Bütschli, 1885*: pl. 55, fig. 96; Schütt, 1895*: pl. 18, fig. 58; Entz, 1905*: fig. 61-63; Paulsen, 1908*: 92, fig. 24; Okamura, 1912*: 16, pl. 2, fig. 36; Lebour, 1925*: 159, fig. 52a; Schiller, 1937*: 475, fig. 547a, b; Rampi, 1941*: 147, fig. 3, 6; Nie, 1942*: 57, pl. 1, fig. 15, 16; Margalef, 1948*: 50, fig. 3d; Kiselev, 1950*: 262, fig. 435a (after Stein, 1883), non b; Gaarder, 1954*: 57, fig.74a, b; Wood, 1954*: 317, fig. 352a, b; Trégouboff, 1957: 119, pl. 27, fig. 19; Balech, 1963*: 12, pl. 2, fig. 20-27; Abé, 1966*: 147, fig. 45-51; Yamaji, 1966*: 18, pl. 51, fig. 18; Wood, 1968*: 119, fig. 365; Steidinger & Williams, 1970**: 60, pl. 35, fig. 128a, b; Andreis & Andreoli, 1975** ***: 388, fig. 1, 4; Taylor, 1976*: 171, pl. 27, fig. 278, 279, (286?); Dodge, 1982*: 254, fig. 33I; Sournia, 1986*: fig. 194 (after Balech, 1963); Balech, 1988*: 124, pl. 52, fig. 21, pl. 53, fig. 3, 4; Delgado & Fortuño, 1991*: fig. 5V (after Margalef, 1967); Carbonell-Moore, 1994a***: pl. 1, fig. 8; Licea et al., 1995**: 77, pl. 9, fig. 1; Steidinger & Tangen, 1996*: 534, pl. 50; Balech, 1988*: 124, pl. 52, fig. 21, pl. 53, fig. 3, 4; Gárate-Lizárraga, 1988**: pl. 6, fig. 5; Konovalova, 1998*: 166, fig. 36 (5a, b); Avancini et al., 2006* **: 375, fig. A, B (after Balech, 1980); Ojeda, 2005*: 161, lám. 32, fig. 1; Al-Kandari et al. 2006**: 188, 336, pl. 39, fig. P-Q; Gómez et al., 2010**: 214, fig. 4; Esqueda-Lara & Hernández-Becerril, 2010**: 134, fig. 127a, b; Omura et al., 2012** ***: 128, fig. a-f; Almazán-Becerril et al., 2016**: 84, fig. 202; Al-Yamani & Saburova, 2019** ***: 290, pl. 159, fig. a-e; Yovera-Galvez et al., 2020**: 167, fig. 229.
Podolampas reticulataKof., 1907: 187, pl. 2, fig. 11. (Fig. 1e, 3f-h, 4r-u)
Syn.: Podolampas bipes f. reticulata (Kof.) J. Schiller, 1937: 474, fig. 545; Podolampas bipes var. reticulataTaylor, 1976: 171, pl. 27, fig. 287.
Description. Cell widely pear-shaped, somewhat compressed dorsoventrally, with a short neck, about 1.2-1.3 times longer than wide, with two long, slightly curved antapical spines, almost equal in length and bearing broad lists with serrated margins. Cell length 83-85 μm (102-105 μm with the antapical spines), width 68-70 μm. Kleptochloroplasts present (Figs. 4 r-q).
Literature:Schiller, 1937*: 474, fig. 545 (after Kofoid, 1907; as P. bipes f. reticulata); Wood, 1954*: 317, fig. 251b (as P. bipes f. reticulata); Balech, 1963*: 11, pl. 2, fig. 15-19; Abé, 1966*: 150, fig. 60-62 (as P. bipes of reticulata-type or reticulata-form); Steidinger & Williams, 1970**: 60, pl. 36, fig. 126a, b; Balech, 1988*: 124, pl. 53, fig. 5, 6, 11; Hernández-Becerril, 1988a*** (Inv. Pesq. 52): 530, fig. 35 (misspelled as P. reticulada); Carbonell-Moore, 1994a***: pl. 1, fig. 10; 2004***: fig. 4; Esqueda-Lara & Hernández-Becerril, 2010**: 135, fig. 128a-c; Omura et al., 2012** ***: 128, fig. g-k (as P. bipes); Yovera-Galvez et al., 2020**: 168, fig. 230.
Podolampas spiniferaOkamura, 1912: 17, pl. 2, fig. 35. (Fig. 1f, 4v-y)
Description. Cell very narrowly drop-shaped, not compressed dorsoventrally, about 4.5-6 times longer than wide. Epitheca is drawn into a long, not separated apical horn, bearing a noticeable spine (a characteristic feature), Hypotheca with one narrowly winged, long (37-45 μm long), straight spine (another characteristic feature of the species). Cell length 77-102 μm (114-147 μm with the antapical spines), width 68-70 μm.
Morphological note. Unlike other Podolampas species that have one left-ventral and two dorsal postcingulars, P. spinifera has two lateral and one dorsal postcingular (Balech, 1963).
Literature:Pavillard, 1916*: 41, pl. 2, fig. 6, 7; Schiller, 1937*: 476, fig. 548 (after Pavillard, 1916); Rampi, 1939*: 468, fig. 17; 1941*: 148, fig. 10; Trégouboff, 1957*: 119, pl. 27, fig. 18; Wood, 1963*: 50, fig. 187; Balech, 1963*: 14, pl. 2, fig. 28-33; Abé, 1966*: 145, fig. 39-44; Yamaji, 1966*: 107, pl. 51, fig. 17; Steidinger et al., 1967**: pl. 4, fig. b; Wood, 1968*: 120, fig. 366; Steidinger & Williams, 1970**: 60, pl. 36, fig. 129; Andreis & Andreoli, 1975** ***: 388, fig. 2, 7, 8; Taylor, 1976*: 172, pl. 27, fig. 284, 285; Sournia, 1986*: fig. 195 (after Balech, 1963); Balech, 1988*: 125, pl. 52, fig. 22, pl. 53, fig. 9, 10, 13; Hernández-Becerril, 1988b** (Bot. Mar. 31): 433, fig. 32; Delgado & Fortuño, 1991*: fig. 5W (after Margalef, 1967); Carbonell-Moore, 1994a***: pl. 1, fig. 6; Konovalova, 1998*: 166, fig. 36 (4a, b); Ojeda, 2005* **: 161, lám. 32, fig. 2, lám. 57, fig. 3; Esqueda-Lara & Hernández-Becerril, 2010**: 136, fig. 129a, b; Gómez et al., 2010**: fig. 5, 6; Omura et al., 2012** ***: 128, fig. a-d. Occasionally, in the literature the species name is misspelled as Podolampas spinifer.
DISCUSSION
The present study represents the most complete report of the podolampadaceans sampled from Mexico. The podolampadaceans found in Mexican coastal waters are known from other tropical regions. However, few of them exclusively from the Mexican Pacific have been documented with micrographs (Licea et al., 1995; Hernández-Becerril, 1988a, b; Esqueda-Lara & Hernández-Becerril, 2010; Almazán-Becerril et al., 2016; Hernández-Becerril & Arce-Rocha, 2021). Podolampas antarctica is probably the only exception in the genus; eight cells of this species were found in the Weddell Sea, the Antarctic Ocean (Balech & El-Sayed, 1965). The podolampadaceans, in general, should no longer be regarded as exclusively warm-water species; however, the maximum species richness has been reported from the tropics (Carbonell-Moore, 1994b). The Podolampas species examined in the present study from the literature (four publications) have been found mainly between 10.17oC and 28.60oC (Carbonell-Moore, 1994b); the minimum temperature (2.44oC) was registered for P. palmipes (Balech, 1988). In Bahía de la Paz, the Podolampas and Blepharocysta species occurred at temperatures of 16 to 30oC. Four species of Podolampas and two Blepharocysta species were identified at the two sampling stations from Bahía de La Paz (Fig. 4a-y).
We found only two genera, Podolampas and Blepharocysta, and the rest of the Podolampadaceae appear to be characteristic of oceanic waters. Until the present, Podolampas species have not been problematic in their identification (although sometimes P. elegans and P. palmipes are not well distinguished based only on cell shape). Among Blepharocysta species, only B. splendor-maris is widely known and has been reported from Mexican waters. There are several Blepharocysta species as yet unidentified. For example, apart from B. okamurae and B. splendor-maris, Omura et al. (2012) report four unidentified species of this genus from the Western Pacific. Morphological differences between Blepharocysta species are not as pronounced as between Podolampas species. Our identification of Blepharocysta species are based exclusively on SEM observations and should be considered tentative due to rare cells not examined in all views, which resulted in some limitations, such as the impossibility of characterizing the 1a plate and the sulcal lists. The structure of thecae, including the relative number of pores and their arrangement, does not appear to be a reliable feature because it is known to vary depending on the cell age. Detailed analysis of the thecae allow us to differentiate between B. splendor-maris, B. striata and B. okamurae as had been done by Abé (1966). Furthermore, Trypan Blue did not allow us to distinguish plates in most examined species such as in the genus Protoperidinium Bergh. Apart from this, in general, the original descriptions of Blepharocysta species are incomplete and deficient to such an extent that it is difficult to compare their morphology.
Based on the number of the antapical spines and their relation to the posterior sulcal plate and the antapical plates, Abé (1966) considered it reasonable to subdivide the genus Podolampas into two groups, spinifera (includes only P. spinifera) and bipes (includes the remainder of the Podolampas species), and excluded the possibility of dividing the genus into two. Regarding the separation between P. bipes and P. reticulata, we followed Kofoid (1907) and Balech (1988), although the latter author separated them with some doubt. Balech (1988) stressed that the main differences between the species are in the morphology of the antapical spines, sulcal and postcingular plates, and they are constant. The poorly described P. curvatulus is another monospiny species that might be grouped with P. spinifera.
Various taxonomic groups of non-photosynthetic organisms possess plastids, and dinoflagellates are among them (Fast et al., 2001; Yoon et al., 2002). Two species of Blepharocysta and one of Podolampas have been regarded as heterotrophs (Steidinger & Williams, 1970; Carbonell-Moore, 2004; Schweikert & Elbrächter, 2004; Gárate-Lizárraga et al., 2009). In this study, we found several cells of B. splendor-maris with an attached pigment mass, probably indicating the first stages of extracellular digestion. Kleptoplasty has been hypothesized to represent either a mechanism permitting functional flexibility or perhaps an early evolutionary stage in the permanent acquisition of chloroplasts (Gast et al., 2007). These authors mention that the nature of the relationship between the dinoflagellate and its plastids appears to be more than kleptoplasty, but not yet an endosymbiosis.
Based on transmission electron microscopic studies, the presence of kleptochloroplasts, also known as kleptoplasts, have been previously proven only for P. bipes and P. reticulata (Schweikert & Elbrächter, 2004). Dinoflagellate chloroplasts observed in previous investigations were shown to be autofluorescent endocytobionts from the class Dictyochophyceae, most probably from the order Pedinellales. Due to methodological limitations, detection of kleptochloroplasts in all podolampadacean species in this study is tentative (Fig. 4 a, b, g-k, m, o, q-u, w, x); however, our observations of living cells from the Gulf of California allowed us to suggest the presence of plastids as small bodies distributed irregularly around the nucleus. This implies that at least the Blepharocysta and Podolampas species should be functionally considered as phytoplankton sensu stricto.
In the Central Equatorial Pacific, Carbonell-Moore (1994b) found the highest abundances of podolampadacean cells between 100 m and 150 m depth. To obtain cells of other podolampadacean genera than Podolampas and Blepharocysta, offshore sampling during oceanographic cruises is necessary. Moreover, other factors should be considered. According to Carbonell-Moore (1994b), the apparent paucity of podolampadaceans in the literature is due to inadequate sampling procedures: mesh size larger than the cell size of most podolampadaceans, insufficient filtration volumes, and/or inappropriate sampling depths (most historical collections are based on surface tows).
Before the mid-1950s, Rampi (1941) had examined the theca of Podolampas in the most detail; he distinguished 19 thecal plates, while other authors could distinguish only 16. However, he misinterpreted some plates, considering that Podolampas species have no cingular plates (probably due to the absence of a transverse equatorial or subequatorial furrow); therefore, the thecal formula he suggested was 2’, 1a, 6”, 0c, 3s, 3”’, 4””. Balech (1954) found more plates (in total, 23), and proposed another interpretation of the thecal formula: 3’, 1a, 5”, 3c, 5s, 3”’, 3””, based on the thecal morphology of P. bipes, P. elegans and P. palmipes. Incidentally, long before this publication, Schütt (1895) considered a postmedian series of three plates as representing the girdle.
SSU rDNA phylogenies showed that podolampadaceans and the genus Roscoffia Balech with the only marine sand-dwelling species R. capitata Balech form a well-supported monophyletic group, composed of two subclades: (1) R. capitata and Blepharocysta sp., and (2) the four examined Podolampas species (Gómez et al., 2010). However, there have been no investigations of the podolampadaceans at the infraspecific, species and generic levels. The morphological diversity of Blepharocysta cells illustrated in the present study (Fig. 2 a-h) gives us serious doubts as to the correct species identification. The scarcity of SEM observations is another obstacle for interpreting variability in morphological features of the theca resulting from cell age or environmental factors. We expect that molecular techniques can also reveal the real species diversity within the genus Blepharocysta, confirming the validity of some doubtful species and the phylogenetic distance among Podolampas species, in particular, between the morphologically close P. elegans and P. palmipes and between P. bipes and P. reticulata, as well as between the aforementioned two intrageneric groups recognized by Abé (1966). From its morphology, P. spinifera appears to be more separated from the others and probably includes cryptic species.











 nueva página del texto (beta)
nueva página del texto (beta)



