Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Hidrobiológica
Print version ISSN 0188-8897
Hidrobiológica vol.10 n.1 Ciudad de México Jul. 2000
Artículos
Cadmium and lead removal from water by the duckweed. Lemna gibba L. (Lemnaceae)
Guadalupe Miranda1, Agustín Quiroz2, and Margarita Salazar1
1 Universidad Autónoma Metropolitana-Iztapalapa. Dptos. Hidrobiología & Biotecnología Apdo. Postal 55-535 C.P. 09340 México, D.F.
2 Instituto de Ecología UNAM. Apdo. Postal 70-275 C.P. 04510, México D.F.
Recibido: 5 de junio de 1999
Aceptado: 21 de octubre de 1999
Resumen
La macrofita acuática Lemna gibba fue expuesta durante siete días a cadmio (Cd) y plomo (Pb), en un rango de concentraciones de 50 a 300 mg/L, en invernadero con fotoperiodo y temperatura controladas. Las determinaciones de Cd y Pb en las plantas y en la solución nutritiva se llevaron a cabo usando un Espectómetro de emisión de plasma secuencial. No hubo correlación entre los contenidos de Cd y Pb en las plantas con los contenidos de los mismos elementos en las soluciones nutritivas, esto es indicativo del rápido estado de saturación y resultados de la combinación de los fenómenos de absorción y adsorción en las plantas. Los efectos del Cd y Pb sobre en crecimiento de Lemna gibba fueron examinados durante trece días bajo el mismo diseño experimental. En todos los grupos experimentales el máximo crecimiento relativo fue al tercer día, así los efectos de toxicidad fueron evaluados por los contenidos de clorofila a, b y total al tercer día de crecimiento. El contenido de clorofilas disminuyó en los grupos experimentales en más del 50% con relación al grupo control.
Palabras clave: Lemna gibba; lenteja de agua; cadmio; plomo; remoción, metales pesados.
Abstract
Duckweed was exposed during 7 days to cadmium, and lead, at concentrations ranging from 50 to 300 mg/L in a greenhouse with controled photoperiod and temperature. The Cd and Pb measurements were performed on plants and nutrient solution using Plasma emission spectrometer. There was not correlation between the Cd and Pb content in plants and in the nutrient solution, this is indicative of the fast attaiment of plants saturation state and the result of combination of absorption and adsorption phenomena. The effects of Cd and Pb on the growth of Lemna gibba were examinated during 13 days in the same experimental design. In all test the maximum growth were at the third day then, the toxicity effects were aproached by chlorophyll a, b and total content at the third day of growing. There was more than 50% decrease in chlorophyll content in all test in relation with control.
Key words: Duckweed; Lemna gibba; cadmium; lead; heavy metals removal.
Introduction
There is a large group of metallic elements which is present in living organisms in limited amounts, these metals are usually divided en two subclasses; the first includes Fe, Mg, Mn, Co, Zn, Cu which are "essential" for the correct funtioning of biochemical processes, Cd, Pb, Hg, Cr, and others belong to a second subclass which is made up of metals without any established biological function and includes the more important contaminants in aquatic environment (Viarego, 1985).
Cd is relatively rare element, and its concentration in the earth crust is lesser than Pb or Hg concentration. Cd pollution does not seem to be widespread, serious consequences have been encountered next to geographic areas used to industrial activity. Cd is a recongnized health hazard in relative low dosages in exposed industrial workers, most notably producers of alkaline batteries, plastic manufacturing, mining and metallurgical processes Because of the importance of cadmium as a pollutant, this metal was chosen to test the efficacy of a new treatment process for industrial wastewater and other contaminated waters. (Butter et al 1998). On the other hand Pb is widely distributed in the atmosphere, soil, ocean, and ground water; it is absorbed, and it can be identified in most plant and animals tissues to far greater extent than is known for Cd and Hg. Significant, isolated societies and the element is encountered in humans remains of eras predating the massive employment of Pb in industry (Valle and Ulmer, 1972).
The term "duckweed" commonly refers to a group of aquatic vascular angiosperms of the family Lemnaceae. Duckweed plants are divided into four genera, Spirodela, Lemna, Wolfiella and Wolffia. There are approximately 40 species worldwide, they are widely distributed in the world from the tropical to the temperate zones from freshwater to brackish estuaries, and throughout a wide range of trophic conditions (Hillman and Culley, 1978).
Duckweed plants are extremely fast growing. The plants form a thick mat, frecuently dominated by a single species, in a lake or a pond (Lewis amd Bender, 1961).
Wang (1990), reported the doubling time for Lemna gibba to be 0.7 days. The same author reported Lemna minor has been exposed with Cd and Pb during four days time in which it showed the 50% inhibition growth. Many others duckweed species have been used in toxicity test with metal and organic pollutants, the results described duckweed as tolerant to environment toxicity because of the plants may be highly adaptative.
This work is the first in the use of specie L. gibba in Mexico in order to remove Pb and Cd.
The capacity of aquatic plants to remove potentially toxic heavy metals from water is well documented (Rodgers et al 1978); Staves and Knaus, 1985) This study is focused on the capacity of Lemna gibba to remove Cd and Pb from water, besides the evaluation of toxicity effects are approached by the relative growth rate and the chlorophylls content.
Materials and methods
The test compounds were chosen to represent two different heavy metals Pb and Cd; neithr play any rol in life metabolism. Technical grade Pb(NO3)2 and CdCl2 (99% purity) were obtained from J.T. Baker S.A. C.V. Xalostoc, México. The test concentrations were: 50, 100, 200, 300 mg/L for Pb and Cd. Control test without heavy metals were performed.
The duckweed species used was Lemna gibba L. which was taken from the Xochimilco canals in south of Mexico City. Before inoculation the plants were rinsed in distilled water. Test solutions made up in Hoagland nutrient solution (Penningsfield and Kurzman, 1975), which was buffered to pH using 0.01 M NaOH. The plants (10 g fresh weight) were grown in metal free polypropylene containers with 1.5 L Hoagland solution. It was diluted to 1:40 (medium : distilled water) avoiding the Pb(NO3)2 precipitation according to Miranda e Ilangovan, 1996. Throughout the experiments, distiller rater was added as necessary to replace evaporated water. Three replicate containers were used for each test concentration. The rest containers were keep in a greenhouse under controled temperature (22.5±1.5 °C) and light (210μE/m2/s). Photoperior (12 h light and 12 h dark) were used.
For the measurament of Cd and Pb content in experimental plants and nutritious solution, the plants were dryed at 60°C during 72 h, grinded in a porcelain mortar; 0.2 g of this vegetal material and 10ml of nutrient solution were placed in teflon tubes with 5 ml of concentrated HNO3 and were digested in a microwave oven Floyd INC 544; for plants; 50% power during 5.5 min at 84°C; and for water: 100% power durign 11 min at 168°C. The digested solutions were examined usig a Perkin-Elmer 2380 inductively coupled plasma atomic emission spectrometer (ICPAES). Quality control blanks and standards with a detection limit of 0.02 μg/L were run before and after each set of analysis.
The Relative growth rate is given in g g-1day-1 units and was calculated according the formula R=In(Wt/W0)T; where W0 is the weight at time zero; Wt- is the wet weight at final time; T the time in days (Porath, 1979). Chlorophyll a, b and total content in the plants were evaluated at an experimental time of day 3 by colorimetric method according Arnon, 1949.
The results were analysed using ANOVA and CORRELATION tests in STATGRAPHICS Ver. 7.
Results and discussion
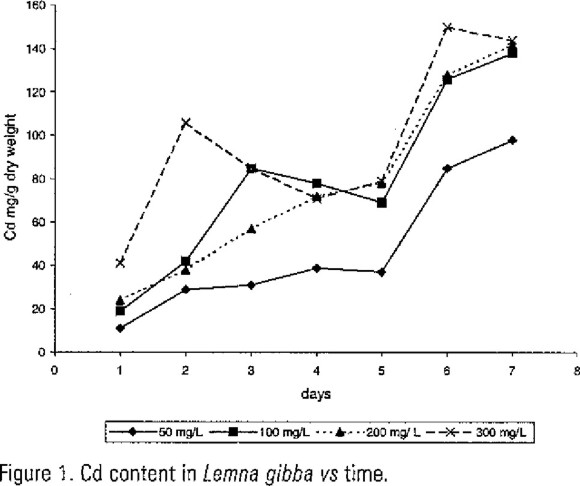
Data for Cd content in Lemna gibba (Figure 1) showed quantitative variation in relation to amount of Cd in media and time, there was increased only in 50 and 200 mg/L; nevertheless the ANOVA test grave significant variation responses (P=0.01) between the experimental Cd groups excepting in days three and four. The maximum Cd content was at seven day except in 300 mg/L.
Results for Pb content in Lemna gibba (Figure 2) showed similar behavior in all the experimental groups. There were significant variation responses (P=0.01) between all different Pb concentrations tests and the time. In 50 and 100 mg/L tests there were the maximum Pb content in seventh day while in 200 and 300 mg/L tests were in the sixth day.

In nutrient solution the results of Cd and Pb content are show in Figures 3 and 4. There were not correlation between these data with Cd and Pb content in the plants; the ANOVA test showed P>0.05. Nevertheless can be observed at the first day an important decrease value in relation with the initial. In Table 1 are shown the Cd and Pb percentage removal at experimental day one. We noticed the maximum values were in 50 mg/L test groups. This fact means duckweed absorbed Cd and Pb from the water as do with any essential microelements; but in the next days plants detected its toxicity showing a metabolic answer. Then the metals concentration in water was increased.

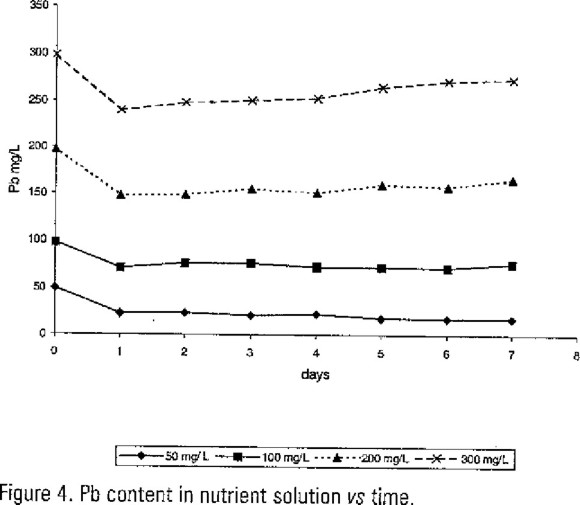

We can notice during the next days an increase in metals water concentrations, it could be due because of at the begining of the experiment the heavy metals were uptaked like an essential microelements which later were influenced by decreased metabolic activity as toxicity set in.
Plants roots absorb ions from a complex medium containing not only essential nutrients ions, but also nonessential ions. If severe imbalances arise in this supply, the plants may not able to take up nutrients efficiently, either because of direct effects of the toxic ions on the root metabolism or function, or simply by competition or other interaction with nutrients ions. As a result even essential ions can become toxic, and plants show great differences in the extent to which they can tolerate variation in ionic ratios. More specific effects, however, occur without actual membrane damage, where ions interfere directly which each others uptake. Generally such interactions may be competitive, where closely related ions compete for the same uptake sites, then for example, Cd competes with Zn; or non-competitive, where the toxic ion simply inactives the uptake mechanism. (Fitter and Hay, 1987).
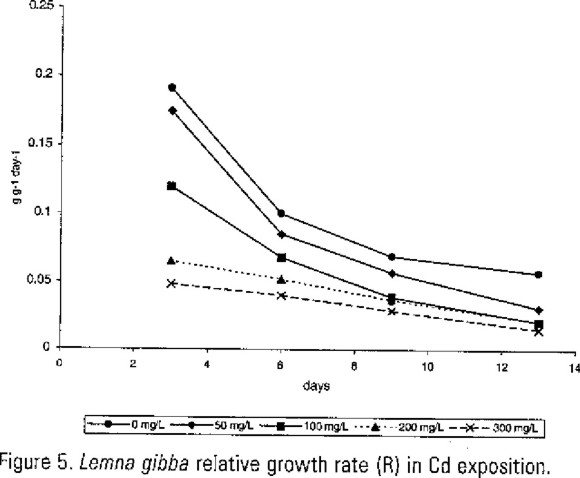
The results of relative growth rate (R) in Lemna gibba are show in Figure 5 for Cd exposition, and in Figure 6 for Pb exposition. In these data there were significant variation (P=0.01) between experimental tests and control tests without Cd and Pb. The R maximum value in both experiments were in the third day. These results showed there were not correlation between relative growth rate and the Cd and Pb content in plants.

The results of chlorophyll content can be observed in Figure 7 for Cd and Figure 8 for Pb. The concentration of heavy metals used in this study showed significant (P=0.01) chlorophylls content decrease in more than 50% in comparison to control tests without Cd and Pb. Therefore chlorosis symptons were detected since 100 mg/L test to higher concentrations.

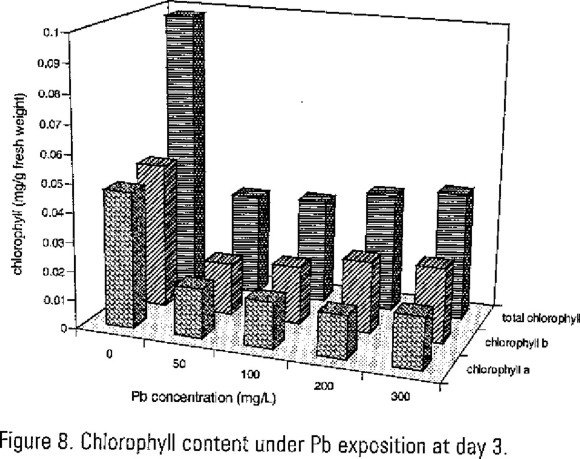
According Chawla et al. (1991), the influence of dose and time on the uptake of metal form water could be through the physicochemical aspects of cation transport as well as the physiological status of the plant age. It is known that Pb inhibits porphyrin synthesis which is very related to chlorophyll origin and inhibits most enzymes bearing a single functional –SH group (Crawford, 1989; Marschner, 1995). The effects of Cd, had been reported in the reduction in photosynthesis and interference with mitochondrial respiration lead to reduced growth, in addition when it replace Zn uptakes sites altering activity of many enzymes (Bazzaz et al. 1974).
Some papers it had been reported that heavy metals content in plants tended to incrase with increase in the initial concentration in the nutritious solutions and with the passage of time (Wolverton and McDonald, 1979, 1980; Chigbo et al 1982). In this work the total amount of Pb taken up during the multiple exposure study increases as this heavy metal concentrations in the nutritious solutions increases, but for Cd it did not observe clearly.
The concentrations of Cd and Pb remaining in the residual nutrient solutions treated with dukweed after the initial showed an decreased at first day and then remains almost constant during the next six days, this is indicative of the fast attainment of a saturation state. As soon as the saturation state is reached, it seems that it is not possible for these plants to further absorb Cd or Pb significantly. Similar results have also been reported by Muramoto and Oki, (1983) and Jain et al (1998) for water hyacinth; water velvet and dukweed respectively.
Nevertheless Cd and Pb produced toxic effects on chlorophylls content in Lemna gibba; the results presented here demonstrated that duckweed are able to remove Cd and Pb at the experimental concentrations used. Although the heavy metals did not have significant effects on plants growth in relation to control test, the survival potential reached was 10 days, after that, plants showed necrosis. The fact that Cd and Pb content in plants increased with the passage of time (6-7 days) (see Figs 1 and 2) could be the result of combination of absorption and adsorption phenomena.
As the potential dangers of heavy metal pollution in aquatic environments is well known, it is very important the treatment of wastewater, the costs and energy requirements of conventional treatments are in a continuous improvement then it might prove an economical alternative biological treatment techniques, such as the use of duckweeds for removal Cd and Pb from industrial wastewater. Effluents containing these metals may be treated by continuously passing them through a bed of these plants growing in pounds.
References
Arnon, D., 1949. Copper enzime in isolated chloroplast. Polyphenoloxidase in (Beta vulgaris). Plant Physiology 24: 1-15. [ Links ]
Bazzaz, F., G., Rolfe and R. Carlson, 1974. Effect of Cd on Photosynthesis and Transpiration of Excised Leaves of Corn and Sunflower. Physiologia Plantarum 32: 373-376. [ Links ]
Butter, T., L., Evison, I., Hancock, F., Holland, K., Matis, A., Philipson, A. Sheikh and A. Zuoboulis, 1998. The Removal and Recovery of Cadmium from Diluted Aqueous Solutions by Biosorption and Electrolysis at Laboratory Scale. Water Research 32 (2): 400-406. [ Links ]
Crawford, R., 1989 Studies in Plant Survival (Ecological case histories of plant adaptation to adversity). Blackwell series. Pub. Oxford. [ Links ]
Chawla, G., J., Singh and P. Viswanathan, 1991. Effect of pH and Temperature on the Uptake of Cadmium by Lemna minor L. Bulletin Environmental Contamination and Toxicology 47: 84-90. [ Links ]
Chigbo, F., R. Smith and F. Shorf, 1982. Uptake of arsenic, cadmium, lead and mercury from polluted waters by the wear hyacinth (Eichhornia crassipes) Environmental. Pollution Ser. (A) 27: 31-36. [ Links ]
Fitter, A. and R. Hay, 1987. Environmental Physiology of Plants. Academic Press Inc. San Diego, CA U.S.A. pp 225-257. [ Links ]
Hillman, W. and D. N. Culley, 1978. The uses of duckweed. American Science 66: 442-451. [ Links ]
Jain, S., P. Vasudevan and N. Jha, 1998. Removal of some heavy metals from polluted water by aquatic plants: Studies on duckweed and water velvet. Biological Wastes 28: 115-126. [ Links ]
Lewis, W. and M. Bender, 1961. Effect of a cover of duckweed and the alga Pithophora upon the dissolved oxygen and free carbon dioxide of small ponds. Ecology 42: 602-603. [ Links ]
Marschner, H., 1995. Mineral Nutrition of Hiher Plants. Academic Press. New York (2a). [ Links ]
Miranda, M. and K. Ilangovan, 1996. Uptake of lead by (Lemna gibba L.): Influence on specific growth rate and basic biochemical changes. Bulletin Environmental Contamination and Toxicology 56: 1000-1007. [ Links ]
Muramoto, S. and Y. Oki, 1983. Removal of some heavy metals from polluted water by water hyacinth. Bulletin Environmental Contamination and Toxicology 30: 170-177. [ Links ]
Penningsfeld, F. and P. Kurzman, 1975. Cultivos Hidropónicos y en Turba. Ediciones Mundi-Prensa, Madrid. pp 53. [ Links ]
Porath, D., B. Hepher and A. Koton, 1979. Duckweed as an aquatic crop. Evaluation of clones for aquaculture. Aquatic Botany 7: 273-278. [ Links ]
Rodgers, J., D. Cherry and R. Guthrie, 1978. Cycling of elements in duckweed (Lemna perpusilla) in an ash settings basin and swamp drainage system. Water Research 12: 765-770. [ Links ]
Staves, R., and R. Knaus, 1985. Chromium removal from water by three species of duckweeds. Aquatic Botany 23 (3): 261-273. [ Links ]
Vallee, B. and D. Ulmer, 1972. Biochemical effects of mercury, cadmium and lead. Annals Review of Biochemistry 41: 91-128. [ Links ]
Viarego, A., 1985. Biochemical effects of trace metals. Marine Pollution Bulletin 16 (4): 153-158. [ Links ]
Wang, W., 1990. Literature Review on Duckweed Toxicity Testing. Environmental Research 52: 7-22. [ Links ]
Wolverton, B. and R. McDonald, 1979. Upgrading Facultative Wastewater Lagoons with Vascular Aquatic Plants. Journal of Water Pollution Control. Federation 51: 305-313. [ Links ]
Wolverton, B and R. McDonald, 1980. Vascular plants for water pollution control and renewable sources of energy. NASA National Spice Technol. Lab Mississipi 39529. Paper presented at Bio-energy'80 Conference, Atlanta Georgia. 21-24 April. [ Links ]














