Introduction
The greater body development of an animal is associated to factors such as the quality of nutrients, water, health, and environmental factors, such as temperature and humidity, which allow an animal to express its genetic potential (Vázquez & Hernán, 2012). The nutrients are absorbed in the small intestine, which has modifications to increase the surface of absorption and secretion, as its height, folds, villi and microvilli (Banks, 1996; Gartner & Hiatt, 2008).
In physiology and morphology of the intestine the villi are functional structures that allow the digestion and absorption of nutrients, which are performed when there is functional integrity in the villus cells, both in the luminal membrane and in the lateral basal membrane (Ávila, Niño, Parra, Tarazona, Rodríguez & Torres, 2009; Vázquez & Hernán, 2012). In addition, the villi serve as a barrier against microorganisms present in the lumen and facilitate the transit of solid waste to the caudal portion of the digestive system for disposal (Miles, Butcher, Henry & Littell, 2006; Vázquez & Hernán, 2012). The longer villi are associated with a healthy digestive system and a high efficiency in the absorption of nutrients (García, Catala-Gregori, Hernández, Megias & Madrid, 2007), while the shorter villi and deep crypts have been associated with the presence of toxins or greater renewal of tissue (Miles et al., 2006).
The villi of the intestine vary in height and area, depending on the section and the animal species, being long and thin in carnivores and wide in herbivores (Dellman, 1994). The villi of the jejunum are narrower, shorter and sparser than in the duodenum, while those of the ileum are the fewest, shortest and narrowest of the three segments of the small intestine (Gartner & Hiatt, 2008). Several authors have concluded in their studies that animals with greater body weight have a greater height or area of the intestinal villi than those with lower weight gain (Chávez, López & Parra, 2016; García et al., 2007; Itza-Ortiz et al., 2018; Pan & Yu, 2014; Tzora et al., 2017); however, they are controlled studies.
Generally, the investigations carried out in the study of intestinal morphometry are associated with some type of additive or growth promoter that obeys to a greater body weight or animal health and not directly on the height or area of intestinal villi. Therefore, there is little information establishing any correlation between the body weight and the height or area of the intestinal villi outside the mentioned context of controlled studies or the use of additive or growth promoter.
The objective of the study was to calculate the correlations between body weight and the height or area of the villi in each of the three sections of the small intestine.
Materials and Methods
Ethical considerations
All procedures involving animals were conducted following guidelines approved in official techniques of animal care and health in México (Ley Federal de Sanidad Animal; Articles 19 to 22), NOM-051-ZOO-1995 (Diario Oficial de la Federación[DOF], 1998).: Humanitarian care of animals during mobilization; and NOM-033-ZOO-1995 (DOF, 1996): Humanitarian slaughter of domestic and wild animals, and the international guiding principles for biomedical research involving animals by the Council for International Organizations of Medical Sciences (CIOMS).
Experimental location and animals
The present study was carried out in the Department of Veterinary Sciences of the Universidad Autónoma de Ciudad Juárez. A random sample of 42 finished pigs of the Landrace and Yorkshire cross was taken with an average body weight of 73.61 kg ± 10.72 kg slaughtered in the municipal slaughtered house of Ciudad Juárez (Industrializadora Agropecuaria of Ciudad Juárez, Chihuahua, México) following the conventional procedures recommended. It is important to mention that the pigs come from local farms and correspond to 367 animals slaughtered.
Intestinal sampling
Immediately after the slaughtering and evisceration of the pigs, the gastrointestinal tract was identified to obtain transverse samples of approximately 5 cm in height (Wick, 2008). Three methods of sampling were used according to the methodology described by Itza-Ortiz et al. (2018) of the three segments of the small intestine: washed, knotted, and squeezed. Three samples were taken corresponding to each section of the small intestine. The first sample was taken from the portion of the descending loop of the duodenum, the second from the middle part of the jejunum, and the third from the ileum in the immediate area before the ileocecal valve. All samples were stored in 10% formalin until processed (Itza-Ortiz et al., 2018).
The tissues were processed by the conventional paraffin inclusion method, obtaining samples of 4 μ thick, staining with hematoxylin and eosin for later microscopic observation.
Measurement of the villi (height and area)
Slides were evaluated using a Leica DM3000 microscope connected to a processor with imaging software (LAS Interactive Measurement ES, LEICA license; Leica Microsystems AG, Wetzlar, Germany). Evaluations were made by measuring 100 intestinal villi per slide, obtaining the average of the following variables: villi height (μm), measured from the apex to the base of each villus, villus surface area (μm2).
Statistical analysis
A total of 378 samples from the different sections of the small intestine were analyzed using the Pearson's simple linear correlation, using PROC CORR from the SAS program version 9.4 (Statistical Analysis System [SAS], 2013), and it was considered an alpha of 0.05 as significant (Mendenhall, Beaver & Beaver, 1994).
Results
Figure 1 shows the distribution of the villus heights (μm). Between the weights 65 kg to 75 kg and 80 kg to 85 kg the heights were agglomerated, observing several peaks in the body weights of 55 kg to 85 kg in the height of the duodenum villi. For the jejunum and ileum, the peaks were not as high.
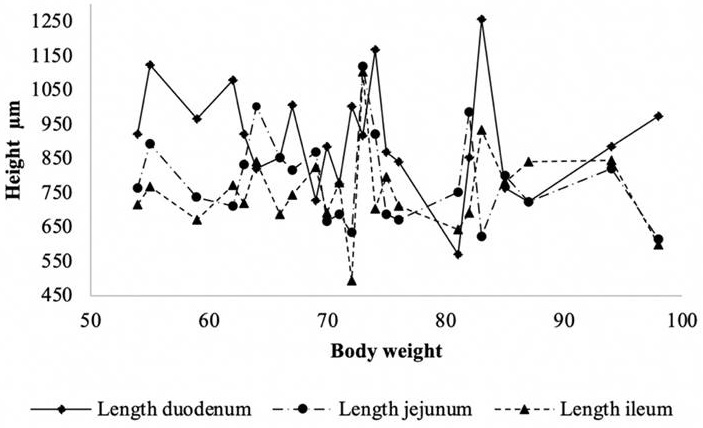
Source: Author's own elaboration
Figure 1 Distribution of intestinal villi height (μm) according to slaughter weight in pigs.
Figure 2 presents the distribution of the area of the villi (μm2). Between the body weights of 65 kg to 75 kg and 80 kg to 85 kg the areas agglomerated. In this case, the peaks were not as noticeable as in the heights; however, the area of the duodenum was larger with respect to the other sections of the intestine.
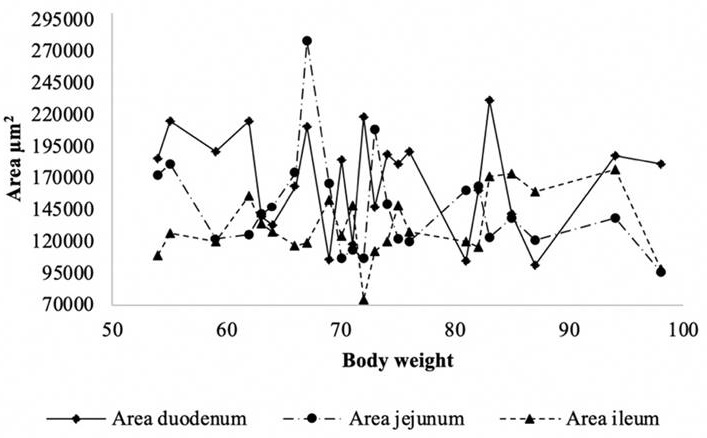
Source: Author's own elaboration
Figure 2 Distribution of intestinal villi area (μm2) according to body weight in pigs.
The correlations found according to the sampling methodology are presented in Table 1. It can be seen that, except for the correlations of the height and area of the villi of the ileum that are positive and of low correlation (r = 0.168; n = 42; p ≤ 0.05), the correlations of the height and area of the different bowel sections were negative and of low correlation (r = -0.140; n = 42; p > 0.01).
Table 1 Correlations between sample methods and villi height and area in finishing pig.
| Duodenum villis height (μm) |
Duodenum villis area (μm2) |
Jejunum villis height (μm) |
Jejunum villis area (μm2) |
Ileon villis height (μm) |
Ileum villis area (μm2) |
|
|---|---|---|---|---|---|---|
| Washed | -0.108* | -0.062 | -0.140** | -0.113* | 0.064 | 0.127* |
| Knotted | -0.112 | -0.111 | -0.160* | -0.092 | 0.048 | 0.062 |
| Squeezed | -0.173* | -0.028 | -0.092 | -0.182* | 0.006 | 0.168* |
* p = 0.05; ** p > 0.01.
Source: Author's own elaboration
Discussion
The animals sampled in the slaughtered house were not subject of any study, and their feeding, management, and health may have been multivariate. Most studies on the subject suggest that, through feeding, animals are exposed to foreign substances or agents; and it is the intestinal wall that acts as a physical barrier against the bacteria and toxic compounds present in the intestinal mucosa that could put at risk the intestinal health and, consequently, reduce the corporal weight (Chávez et al., 2016). The gastrointestinal tract of the pig contains 10x14 prokaryotic and eukaryotic microorganisms. This intestinal microbiota represents a complex system of interactions, and the most studied role is the protective effect of certain bacterial species such as lactobacilli and bifidobacteria against enteric infections and intestinal morphometry, which has been associated as a major factor in the performance of body weight (Soraci et al., 2010).
The main pathology found in pig farms are gastroenteric problems; and in growing pigs, this influences weight gain and feed conversion due to the high rate of passage of intestinal content that causes the absence of nutrients in the mucosa and generates a progressive and severe atrophy of the villi (Lu, Idris, Harmon, Hofarce & Maurer, 2003; Rodríguez, Eliecer & Sabrina, 2010). This pathology is considered multifactorial because it involves the management, the inputs, the host and its susceptibility to etiological agents such as bacteria or viruses, which may occur by themselves or in association (Frandson, 1988; Rodríguez et al., 2010).
The food stimulates the intestinal morphological development, so that a low density of the intestinal villi can produce a decrease in the height and width of the villus, which would reduce the digestive and intestinal absorption capacity and, therefore, affect the body weight (Uni, Ganot & Sklan, 1998; Yamauchi & Isshiki, 1991). On the other hand, Ávila et al. (2009) mention that in the morphology of the villi, if these increase in numbers, they decrease in height. However, the number and size of them depends on the number of cells that compose it. Thus, the greater the number of cells, the larger the villus size is and, consequently, the greater the area of nutrient absorption (Ávila et al., 2009).
Among the nutritional components of the food that can intervene in the performance of body weight is glutamine, an amino acid that intervenes in the composition of proteins and mainly maintains the morphology and intestinal function (Cabrera et al., 2013; Yi et al., 2005).
The results of the literature do not suggest a positive association between the height of the intestinal villi in one of the sections of greater absorption of nutrients such as the jejunum (Ávila et al., 2009). On the other hand, the low positive correlation recorded in the ileum (height and area) was not relevant and does not support body weight alone.
Rosero, Odle, Moeser, Dean & Van Heugten (2016) suggest that lipid peroxidation reduces animal growth, reduced digestion, and absorption of nutrients. This is due to the modification of the villi morphology of the small intestine due to alterations in the mechanism of the oxidation-reduction reaction that takes place in the intestinal environment, as well as strong oxidative stress that can alter cell integrity and activate proinflammatory transcription factors.
Conclusions
No association was found between the body weight of finished pigs and the height or area of the intestinal villi. In general, the correlations were negative and of low correlation. Only positive and low correlations were observed in the ileum. It is likely that other factors may affect the height or area and not only be associated with the absorption of nutrients or nutrition.











 text new page (beta)
text new page (beta)


