Introduction
Turnera diffusa (damiana) grows in arid and semi-arid areas in the northwestern region of Mexico (Alcaraz-Meléndez, Real-Cosio & Rodríguez-Álvarez, 2011). In Baja California Sur (BCS), it grows wild, and it is also micro propagated and cultivated in the Plant Biotechnology Laboratory at Centro de Investigaciones Biológicas del Noroeste (CIBNOR) in La Paz, BCS, (Alcaraz-Meléndez, Real-Cosio & Bashan, 1994). Essential oils are commercially employed for the preparation of liquors, cosmetic products, and food flavoring (Alcaraz-Meléndez, Delgado-Rodríguez & Real-Cosío, 2004). Its chemical composition includes flavonoids, phenolic compounds, terpenoids, among others (Zhao, Pawar, Ali & Khan, 2007), most of them known as antioxidants. Two varieties have been identified in La Paz, BCS region: T. diffusa Willd var. diffusa and T. diffusa Willd var. aphrodisiaca (Ward) Urb (damiana var. diffusa and damiana var. aphrodisiaca) (Tropicos.org, 2016).
Traditionally, infusions from the aerial parts of damiana var. aphrodisiaca are used as medicinal due to their effects as well as stimulant and aphrodisiac (Estrada-Reyes, Carro-Juárez & Martínez-Mota, 2013), and it is used as a diuretic to treat diabetes mellitus (DM) (Paul, Ezcurra & Roberts, 2012).
DM is a multifactorial disease characterized by chronic hyperglycemia by defects in insulin secretion, action or both (American Diabetes Association [ADA], 2010), affecting 8.5% of adults aged 18 and older worldwide (World Health Organization [WHO], 2016). The last Mexican National Health Survey revealed that ~9.17% of the adult population was diagnosed with diabetes; in BCS the prevalence was between 8.2%-9.2% (Gutiérrez et al., 2012). According with International Diabetes Federation (2015), it is a fact that Mexico is within the top six countries with DM worldwide. Early interventions to prevent its development have shown an increase in life expectancy and quality; additionally, when DM is controlled, it delays the onset of its complications, including diabetic nephropathy (DN) (ADA, 2013), which is serious and progresses towards end-stage renal failure (Zheng, Powell, Zheng, Kantharidis & Gnudi, 2016).
Glucose overload may damage the cells through oxidative stress generated mainly in the mitochondria, the most important site for reactive oxygen/nitrogen species production responsible for the complications in DM (Ceriello, Testa & Genovese, 2016). Suppression of oxidative stress by natural products improves overall health, including DM and its complications.
The use of medicinal plants has been maintained by humanity throughout history. Herbal medicine is still the mainstay of about 80% of the whole population for primary health care because of its acceptability and fewer side effects. Some of them are rich in flavonoids and phenolic compounds, as well as in alkaloids, glycosides, and others that usually show positive effects (de Sousa-Araújo, de Melo, Júnior & Albuquerque, 2016). The main aim of this study was to evaluate the beneficial effects of water ethanol extract (WEE) from damiana var. diffusa and var. aphrodisiaca on blood glucose (BG), body weight (BW), urea, creatinine, uric acid, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, total lipids, total protein, and Cell Blood Counts (CBC) in a type 1 DM rat model. Also, nitric oxide (NO•), thiobarbituric acid reactive species (TBARS) and mitochondrial superoxide dismutase (mSOD) were measured in kidney mitochondrion when damiana var. diffusa was administered to observe its antioxidant potential.
Materials and methods
Plants and extracts
Plant materials
Samples of wild damiana var. diffusa and cultivated damiana var. aphrodisiaca were used in this study. Damiana var. diffusa was collected in June 2015 at El Saltito region in BCS (24°140'13.5'' N 110°12'10.2'' W), and damiana var. aphrodisiaca was provided by Alcaraz-Meléndez (CIBNOR). Damiana was identified by Sergio Real-Cosío (CIBNOR), and vouchers with accession numbers HCIB30319 for T. diffusa var. diffusa and HCIB30069 for T. diffusa Willd var. aphrodisiaca Ward were deposited in CIBNOR herbarium.
Extract preparation
Aerial parts of the plants were dried, pulverized, and stored at room temperature in a dry place and protected from light until use. One g of plant material was extracted with 10 ml of water-ethanol (1:1) by maceration (five days, 25 °C) and filtered and concentrated (IKA®RV05basic, Staufen, Germany) under vacuum (Vacuum System™2026 Welch, Niles, IL, USA) at 40 °C. WEE were stored at 4 °C until its use.
Hypoglycemic potential
Experimental animals and type 1 diabetes induction
Male Wistar normoglycemic rats weighing 120 g - 180 g were obtained from the animal house of the FES-Iztacala-UNAM. Rats were fed with rodent chow by Purina™ and tap water ad libitum. Animals were housed under standard laboratory conditions at room temperature and a normal photoperiod of 12 h light-darkness cycle during the whole experiment. Rats were previously submitted to fasting for 24 h, and experimental DM was induced by a single intraperitoneal (IP) injection of streptozotocin (STZ) by Sigma Aldrich™ in a dose of 65 mg/kg of BW (Ganda, Rossini & Like, 1976). Control rats were injected with citrate buffer (pH 4.5). Five days after STZ injection, the animals were fasted overnight, and BG levels were determined using an accucheck-performa system by Roche™. Animals exhibiting BG levels were considered as those whose basal glycemia was ≥ 140 mg/dl. All animal procedures were conducted in accordance with the federal regulations for animal experimentation and care (de Aluja, 2002) and protocols were approved by the Institutional Ethics Committee of CIBNOR for the use and care of animals (March 10, 2017).
Experimental protocol
The animals were set into two groups, damiana var. diffusa and damiana var. aphrodisiaca. Each group was separated into four groups (n = 4-6); control (C); control + WEE (C+E); diabetic (DM); and diabetic + WEE (DM+E). Treated groups received 150 mg/kg BW of the WEE and the untreated group received water as a vehicle by oral gavage for 8 weeks after DM induction. BG levels were recorded every week previous to, at least, an eight-hour fasting. Animal weight was recorded at the same time to adjust the dose using a digital scale.
Oxidative stress assays
Mitochondria isolation
Mitochondria were isolated by standard differential centrifugation as described previously (Saavedra-Molina & Devlin, 1997). Briefly, the kidneys were removed and gently homogenized. After differential centrifugation, the mitochondrial pellet was suspended in a medium containing 220 mM mannitol, 70 mM sucrose, and 10 mM MOPS (pH 7.4). All centrifugations were carried out at 4 °C. Additionally, the mitochondria was purified in a Percoll gradient. Protein concentration was determined by Bradford reagent by Sigma using bovine serum albumin (BSA) as standard in a Thermo scientific Multiskan Spectrum, Vanta, Finland.
Mitochondrial lipid peroxidation
Lipid peroxidation levels in kidney mitochondria were assessed by quantifying the product formed by the reaction of malondialdehyde (MDA) and thiobarbituric acid (TBA) forming an adduct that strongly absorbs light at a wavelength of 535 nm. The method reported previously by Buege & Aust (1978) was used with a slight modification; one mg of protein was used to determine the TBARS levels.
Mitochondrial nitric oxide
Mitochondrial NO• metabolite content (NOx) was determined by using the Griess colorimetric reaction (Green et al., 1982). Aliquots containing one mg of protein were used to determine the NO• levels.
Mitochondrial SOD activity
Kidney mSOD (EC1.15.1.1) activity was done spectrophotometrically using the method described previously (Suzuki, 2000).
Clinical studies
Blood chemistry parameters
Serums from blood samples were isolated and analyzed for glucose, urea, creatinine, uric acid, total cholesterol, triglycerides, and total lipids using the Randox™ kit reagents; HDL-cholesterol and LDL-cholesterol using the QCA™ kit reagents; and total protein using the BSA method by Sigma Aldrich™ in the Physiological Biochemistry Laboratory (CIBNOR). In all cases the recommendations of the manufacturer were followed to obtain the colorimetric products.
Blood sampling and CBC
Blood samples were collected using the terminal procedure technique in tubes with EDTA (Becton-Dickinson) from posterior vena cava in all rats involved in protocols (Parasuraman, Raveendran & Kesavan, 2010). Lymphocytes were counted with a standard manual method, involving blood cells (WBC) by Bright-line Neubauer hemocytometer.
Statistical analysis
The results were expressed as the mean ± SEM of at least four independent experiments. Statistical significances (p ≤ 0.05) were determined with Student’s t-test using Sigma Plot 14.0. Moreover, in all cases the Analysis of variance (Anova) with post-hoc Tukey- HSD test for unbalance observations was performed.
Results
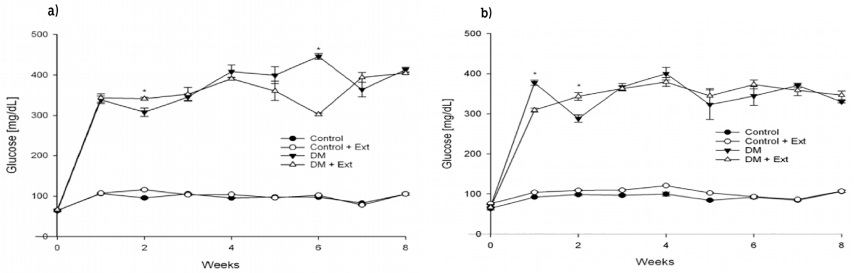
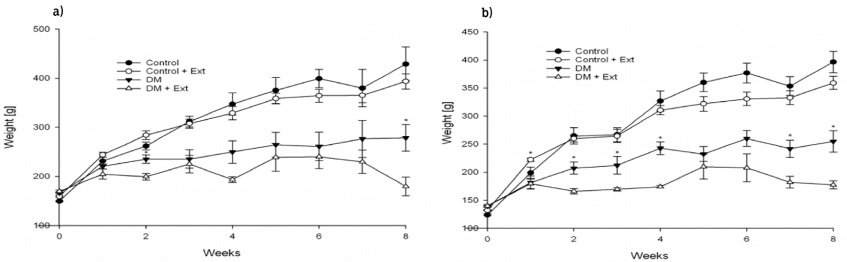
Intraperitoneal administration of STZ at a dose of 65 mg/kg BW caused an increase on BG (339 mg/dL ± 9.1 mg/dL in damiana var. diffusa and 377 mg/dL ± 6.3 mg/dL in damiana var. aphrodisiaca groups) as compared to normoglycemic rats (106.5 mg/dL ± 1.2 mg/dL in damiana var. diffusa and 92.5 mg/dL ± 0.2 mg/dL in damiana var. aphrodisiaca groups) in an overnight fasting. The WEE (150 mg/kg/8 weeks) from damiana var. diffusa and var. aphrodisiaca did not produce any significant diminution of BG levels in all treated groups (Figure 1a, 1b). We observed that IP injection of STZ caused a decrease in BW (35%-36%) as compared to the control groups. However, as a result of the treatment with WEE control animals tended to lose BW (8%-9%) as well as DM + WEE (30%-35%) compared with untreated groups (Figure 2a, 2b). A special observation was made in treated DM groups; the animals entered in the protocols with an average of 154 g ± 1.7 g of BW and ended at 178 g ± 13.2 g of BW in both groups, which means they tend to keep their BW because the DM + WEE from damiana var. diffusa group only gains 6% and DM + WEE from damiana var. aphrodisiaca group 21%, a beneficial effect in this pathology.
Source: Author's own elaboration.
Figure 1 Effects of Turnera diffusa on blood glucose levels. a) T. diffusa var. diffusa and b) T. diffusa var. aphrodisiaca. Values were obtained weekly after Wistar male rats previously fasted for at least eight hours; they represent the mean ± SEM of n = 4-6 rats. *Significant differences were found between untreated versus treated rats (t test p ≤ 0.05).
Source: Author's own elaboration.
Figure 2 Effects of Turnera diffusa on body weight. a) T. diffusa var. diffusa and b) T. diffusa var. aphrodisiaca. Values were obtained weekly after Wistar male rats previously fasted for at least eight hours; they represent the mean ± SEM of n = 4-6 rats. *Significant differences were found between untreated versus treated rats (Student’s t-test p ≤ 0.05).
To investigate the implication of WEE from damiana var. diffusa in oxidative stress generated in DM, the mitochondria was firstly assessed for TBARS as an indirect measure of TBARS, NO• and mSOD. DM-induced lipid peroxidation was significantly reduced by WEE administration at the end of the eight weeks of treatment. As expected, chronic WEE treatment significantly decreased (p ≤ 0.05) the TBARS levels when compared to untreated DM versus treated DM groups (Table 1). Controls or treated control groups remained unchanged. To evaluate part of the nitrosative stress generated in kidney mitochondria, the NO• levels were measured. No differences in kidney mitochondrial nitric oxide levels between treatments were observed (Data not shown).
Tabla 1 Effects of Turnera diffusa var. diffusa on kidney mitochondrial lipid peroxidation (TBARS)
| Experimental groups | C | C + E | DM | DM + E |
|---|---|---|---|---|
| 8 weeks | ||||
| TBARS (nMole/mg prot.) | 2.99 ± 0.14 | 3.15 ± 0.06 | 3.44 ± 0.08 | 3.00 ± 0.06* |
Values were obtained at the end of the eight weeks of treatment from fresh mitochondria isolated, immediately after animal sacrifice; results represent the mean of at least n = 4 ± SEM. C = Control groups; C + E = Control group plus 150 mg/kg BW of WEE. DM = Diabetes mellitus groups; DM + E = Diabetes mellitus groups plus 150 mg/kg BW of WEE. *Significant statistical differences (Student’s t-test p ≤ 0.05).
Source: Author's own elaboration.
Continuing with the implication of damiana var. diffusa in oxidative stress, the renal protective effects of WEE in treated animals were evaluated as a function of SOD activity in kidney mitochondria. As anticipated, because of the induction of DM in experimental animals, it was expected to see a diminution on mSOD activity; nonetheless, this observation was not possible at mitochondrial level. De facto, there were no statistically significant differences when all groups were compared. Notwithstanding this finding, it was possible to observe that mSOD activity tended to increase in C and DM treated groups (Data not shown).
To evaluate the clinical implication of damiana in DM, after sacrificing the animals we rapidly collected the blood samples and serum was separated. Table 2 depicts the results obtained for blood chemistry. During the experiments, BG was measured using the glucose oxidase method, but at the end of the eight weeks, glucose concentration was measured using the GOD/PAP method in serum. The results were similar to those observed using whole blood. With both varieties of damiana, the results indicated that T. diffusa had no hypoglycemic effect because no changes were observed in glucose levels after treatment: Damiana var. diffusa groups showed C = 73.22 mg/dL ± 6.60 mg/dL; C + WEE = 72.04 mg/dL ± 2.77 mg/dL; DM = 293.68 mg/dL ± 11.65 mg/dL; and DM + WEE = 276.22 mg/dL ± 21.65 mg/dL of serum glucose. Damiana var. aphrodisiaca groups showed C = 64.52 mg/dL ± 2.14 mg/dL; C + WEE = 66.27 mg/dL ± 1.93 mg/dL; DM = 245.97 mg/dL ± 34.14 mg/dL; and DM + WEE = 285.10 mg/dL ± 44.78 mg/dL of serum glucose. No changes in urea, creatinine, and uric acid values were observed in both groups assayed because values behaved as the control. Serum lipid levels as total cholesterol, HDL-cholesterol, LDL-cholesterol, total lipids, and triglycerides were measured. Our results have shown that groups treated with either variety of damiana did not have the capacity to modify total cholesterol and did not alter LDL-cholesterol. However, HDL-cholesterol tended to increase in control groups (33.49 mg/dL ± 1.35mg/dL to 40.88 mg/dL ± 1.75 mg/dL) treated with damiana var. diffusa and in DM groups (29.57 mg/dL ± 4.80 mg/dL to 37.76 mg/dL ± 2.56 mg/dL) treated with damiana var. aphrodisiaca. A decrease in total lipids was observed in the treated control group (151.18 mg/dL ± 5.95 mg/dL to 116.47 mg/dL ± 4.02 mg/dL) although no changes were observed in treated DM versus untreated DM groups when damiana var. diffusa was provided.
Table 2 Effects of Turnera diffusa var. diffusa and Turnera diffusa var. aphrodisiaca on blood chemistry
| Concentration | T. difusa Var. diffusa | T. difusa Var. aphrodisiaca | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | C + WEE | DM | DM + WEE | C | C + WEE | DM | DM + WEE | ||
| Gluc | mg/dL | 73.22 ± 6.60 | 72.04 ± 2.77 | 293.68 ± 11.65* | 276.22 ± 21.65 | 64.52 ± 2.14 | 66.27 ± 1.93 | 245.97 ± 34.14 | 285.10 ± 44.78+ |
| Urea | mg/dL | 55.83 ± 4.01 | 74.37 ± 23.53 | 94.09 ± 23.63 | 46.51 ± 4.93 | 63.65 ± 12.42 | 49.21 ± 7.27 | 64.63 ± 9.84 | 50.51 ± 6.47 |
| Crea | mg/dL | 0.36 ± 0.05 | 0.30 ± 0.05 | 0.29 ± 0.02 | 0.26 ± 0.04 | 0.25 ± 0.02 | 0.33 ± 0.02 | 0.26 ± 0.04 | 0.33 ± 0.01 |
| Chol | mg/dL | 48.99 ± 4.82 | 46.76 ± 2.69 | 57.44 ± 9.16 | 57.22 ± 1.25 | 47.64 ± 4.16 | 53.73 ± 4.04 | 41.33 ± 3.01 | 54.70 ± 2.68 |
| HDL-Chol | mg/dL | 33.49 ± 1.35 | 40.88 ± 1.75+ | 42.30 ± 6.66 | 35.54 ± 1.85 | 21.02 ± 6.32 | 25.06 ± 6.32 | 29.57 ± 4.80 | 37.76 ± 2.56+ |
| LDL-Chol | mg/dL | 6.36 ± 1.24 | 13.45 ± 3.67 | 4.24 ± 1.64 | 7.33 ± 0.12 | 7.09 ± 2.18 | 9.84 ± 2.13 | 12.83 ± 3.29 | 8.16 ± 2.66 |
| TL | mg/dL | 151.18 ± 5.95 | 116.47 ± 4.02 | 175.01 ± 19.23 | 153.51 ± 24.13 | 135.65 ± 4.40 | 137.65 ± 5.51 | 143.92 ± 6.62 | 238.97 ± 1.79+ |
| TG | mg/dL | 63.53 ± 7.76 | 94.39 ± 17.30 | 118.76 ± 31.19 | 107.62 ± 34.88 | 81.89 ± 0.76 | 56.95 ± 8.09+ | 94.52 ± 5.80 | 226.43 ± 7.97+ |
| UA | mg/dL | 0.85 ± 0.02 | 0.83 ± 0.01 | 0.87 ± 0.03 | 0.84 ± 0.03 | 0.83 ± 0.01 | 0.80 ± 0.01 | 0.87 ± 0.02 | 0.98 ± 0.04+ |
| UA | g/dL | 7.47 ± 0.38 | 7.92 ± 0.99 | 8.80 ± 0.36 | 8.37 ± 0.75 | 8.16 ± 0.37 | 8.93 ± 0.37 | 7.64 ± 0.28 | 8.46 ± 0.15 |
Values were obtained at the end of the eight weeks of treatment from fresh blood serum isolated immediately after animal sacrifice; they represent the mean ± SEM of n = 4-6 rats. ANOVA with post-hoc Tukey-HSD test. Abbreviations: Gluc, Glucose; Crea, Creatinine; Chol, Cholesterol; HDL-Chol, High density lipoproteins; LDL-Chol, Low density lipoproteins; TL, Total lipids; TG, Triglycerides; UA = Uric acid; TP = Total protein.
Values are mean ± SE of at least n = 4-6. *p ≤ 0.05 versus DM + WEE or C + WEE groups. + p ≤ 0.05 versus DM or C groups.
Source: Author's own elaboration.
Table 3 shows the levels of hematocrit and hemoglobin in the different groups. The results showed that these parameters were not affected by treatment with either varieties of damiana; it also depicted CBC. The results showed that WBC was only lower in DM and DM + WEE groups (4938 Cel µL-1 ± 180 Cel µL-1 and 4312 Cel µL-1 ± 926 Cel µL-1, respectively) from damiana var. diffusa; the rest remained unchanged. In all cases, lymphocytes were lower than reference values. Some animals were found in the upper limit according to reference values in monocytes except in C group from damiana var. aphrodisiaca value (15.6%), which was very high. Eosinophyls were unchanged in all experimental groups. Basophils were very high in all groups. Neutrophyls on band and metamyelocytes were out of range according to the reference values.
Table 3 Effects of Turnera diffusa var. diffusa and Turnera diffusa var. aphrodisiaca on cell blood counts
| Concentration | T. difusa Var. diffusa | T. difusa Var. aphrodisiaca | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| References | C | C + WEE | DM | DM + WEE | C | C + WEE | DM | DM + WEE | ||
| Hct | % | 40-55 | 44.5 ± 3.5 | 41.7 ± 0.8 | 44.5 ± 3.6 | 47.2 ± 1.3 | 46.2 ± 5.5 | 42.4 ± 1.4 | 45.1 ± 4.0 | 48.5 ± 2.1 |
| Hb | g/dL | 10.22-23.52 | 14.2 ± 0.8 | 12.7 ± 0.2 | 12.6 ± 0.7 | 14.0 ± 0.3 | 16.0 ± 1.2 | 15.8 ± 0.4 | 12.3 ± 0.9 | 14.6 ± 0.6 |
| WBC | Cel µ-1 | 7300-15600 | 9218 ± 1632 | 6219 ± 1632 | 4938 ± 180* | 4312 ± 926* | 11775 ± 1987 | 9275 ± 602 | 7229 ± 1121 | 9062 ± 1028 |
| Nϕ | % | 1 - 29 | 25.8 ± 6.9 | 30.3 ± 2.4 | 28.8 ± 3.4 | 33.8 ± 5.3 | 27.8 ± 2.4 | 27.4 ± 3.0 | 26.2 ± 1.5 | 29.3 ± 5.1 |
| Ly | % | 64 - 99 | 42.0 ± 2.4* | 40.8 ± 6.8* | 54.8 ± 2.3* | 35.0 ± 4.8* | 31.8 ± 3.1* | 43.4 ± 3.0* | 44.7 ± 3.0* | 42.3 ± 4.1* |
| Mo | % | 0 - 7 | 8.3 ± 3.4 | 8.3 ± 1.8 | 5.0 ± 0.4 | 6.0 ± 2.0 | 15.6 ± 3.0 | 8.0 ± 0.9 | 7.3 ± 2.1 | 7.3 ± 1.5 |
| Eos | % | 0 - 7 | 1.5 ± 1.2 | 1.0 ± 1.0 | 3.0 ± 1.2 | 2.3 ± 1.3 | 2.4 ± 0.5 | 3.4 ± 0.9 | 1.7 ± 0.2 | 1.3 ± 0.6 |
| Bas | % | 0 | 19.5 ± 2.9* | 11.8 ± 2.8* | 6.5 ± 1.2* | 15.0 ± 4.4* | 19.6 ± 5.1* | 16.2 ± 3.6* | 17.0 ± 2.1* | 16.2 ± 1.6* |
| Nϕ band | % | 0 - 1 | 2.0 ± 0.7* | 2.5 ± 1.2* | 1.0 ± 0.7 | 1.0 ± 0.6 | 0.80 ± 0.01 | 0.8 ± 0.4 | 1.7 ± 0.4* | 2.7 ± 0.3* |
| Me | % | 0 | 1.3 ± 0.6* | 5.0 ± 4.0* | 1.0 ± 0.0* | 5.5 ± 3.3* | 0.8 ± 0.6* | 0.6 ± 0.4* | 1.3 ± 0.7* | 1.0 ± 0.5* |
Values were obtained at the end of eight weeks of treatment from fresh blood isolated immediately after animal sacrifice; they represent the mean ± SEM of n = 4-6 rats. ANOVA with post-hoc Tukey-HSD test for unbalance observations was performed. Abbreviations: Hct, Hematocrit; Hb, Hemoglobin; WBC, Leucocytes; Nϕ, Neutrophils; Ly, Lymphocytes; Mo, Monocytes; Eos, Eosinophils; Bas, Basophils; Nϕ band, Band neutrophils; Me, Metamyelocytes.
Source: Author's own elaboration.
Discussion
Previous studies were made with cultivated T. diffusa var. aphrodisiaca for the hypoglycemiant effect, but it did not have the capacity to diminish BG. Findings in this study confirm our previous results with respect to the hypoglycemiant effects using cultivated T. diffusa var. aphrodisiaca once again and wild T. diffusa var. diffusa; both varieties of T. diffusa did not show the property of decreasing BG (Figures 1a and 1b), which agrees with Alarcon-Aguilar, Roman-Ramos, Flores-Saenz & Aguirre-Garcia (2002) but not with Pérez, Ocegueda, Muñoz, Avila & Morrow (1984) (Alloxan DM rodent models) who argue that damiana has anti-diabetic effects because it reduces glucose tolerance. Both studies employed different DM-rodent models and perhaps another variety of T. diffusa. Nevertheless, several mechanisms have been investigated to explain the beneficial use or action of medicinal plants in DM, which include modulation of oxidative stress. In our previous research (Esquivel-Gutiérrez, Alcaraz-Meléndez, Salgado-Garciglia & Saavedra-Molina, 2017), damiana var. aphrodisiaca was tested for its antioxidant effects in the same STZ-DM rat model; the antioxidant effects were assessed by measuring MDA and NO• in kidney mitochondrion during three and five weeks, showing that damiana var. aphrodisiaca had a positive effect on oxidative stress generated in mitochondria by reducing the MDA and NO• levels. Other studies have shown that DM is associated with increased formation of free radicals and decreased antioxidant potential, leading to damage in lipids and proteins (Valko et al., 2007). For instance, this work assessed the antioxidant effects of WEE from wild damiana var. diffusa, and conversely to our previous results with cultivated damiana var. aphrodisiaca, damiana var. diffusa only achieved to significantly diminish lipid peroxidation levels as TBARS (Table 1) in the DM group, but no nitric oxide changes were observed. In control groups, the TBARS and NO• levels remained unchanged, which means damiana var. diffusa did not affect this parameter when it was consumed.
Hyperglycemia affects functionality in various organs, including kidneys. Although the pathophysiology of the DN is not well defined, it is clear that oxidative stress plays a key intermediate role in the development of DN (Zheng et al., 2016). Nonetheless, in our studies, no alterations in mitochondrial oxidative stress in the kidneys were observed when levels of TBARS and NO• were measured. In this sense, observing part of the antioxidant defense by measuring the SOD activity in kidney mitochondria was important. Statistically changes were not identified in enzyme activity of mitochondrial SOD although there was a tendency to increase when controls and induced DM rats were treated with WEE from damiana var. diffusa (data not shown). Additionally, it is important to consider that genetic and phenotypic variability modifies the content of total phenolic compounds reflecting the total antioxidant capacity in wild damiana. Presumably, domestication processes and cultivation conditions increased the antioxidant properties (Soriano-Melgar et al., 2012) effect reflected when cultivated damiana var. aphrodisiaca was provided. The yield percentage of extract residues in damiana var. diffusa and damiana var. aphrodisiaca was ~1.8 and ~1.6, respectively on dry weight basis, a similar level considering that the first one is a wild variety and the second one cultivated in a greenhouse, but it does not mean that the content in antioxidant compounds is greater in damiana var. diffusa than in damiana var. aphrodisiaca.
When DM groups were treated with WEE from either variety of damiana, BW remained unchanged. The results agreed with those observed in our previous study when WEE from damiana var. aphrodisiaca were provided. The treated C groups tended to lose BW over time when either variety of damiana was administered (Figures 2a and 2b), which also agrees with that result previously observed for damiana var. aphrodisiaca. This effect may be related to inhibition in the absorption of nutrients as lipids although not directly with glucose metabolism. Probably T. diffusa has compounds with these properties in its chemical composition. DM patients tend to lose weight and regain it after the treatment (Diabetes Prevention Program Research Group, 2009). Natural antioxidants from medicinal plants occur in all parts of the plants, it includes flavonoids, monoterpenes, phenyl propanoids, sesquiterpenes, among others; they are present in T. diffusa var. aphrodisiaca (Alcaraz-Meléndez et al., 2004), confirming that damiana has a positive effect in DM by reducing available intake calories because patients treated with a herbal preparation containing damiana lost BW (Andersen & Fogh, 2001). Diabetes causes DN (Zheng et al., 2016). Measurement of nitrogenous compounds in serum can be considered as a marker of renal functionality (Chirumbolo, 2016). Unchanged nitrogenous compound levels in damiana groups suggest that both varieties of damiana have a positive meaning in kidney function since high concentrations of urea, creatinine, and uric acid have been reported to have a negative effect in DM. Blood lipids are altered in DM patients, for example, elevation in total cholesterol and triglycerides are the most common factors associated with the risk of cardiovascular complication associated with DM (Noriega-Cisneros et al., 2011). The DM and C groups were treated with WEE in this study to evaluate lipid profiles trying to explain the beneficial use of this medicinal plant, whose results have shown that damiana var. diffusa but not var. aphrodisiaca helps to mitigate negative effects in diabetic people by lipid control (Table 2). Further studies need to be done to normalize blood lipids when WEE from damiana is administered.
Hyperglycemia affects cellular immunity by impairing activity in lymphocytes in STZ-DM models. Lymphocytes are diminished in circulation (Table 3), but histological studies are needed to ensure infiltration in tissues as reported before in pancreas by direct STZ beta cell toxicity (Rossini, Like, Chick, Appel & Cahill, 1977). DM can induce susceptibility to pathogens as intestinal parasites. Moreover, DM is characterized as an inflammatory disease involving basophiles and other WBC (Chirumbolo, 2016). Nonetheless, it was not unusual that basophiles were very high in all the groups tested because animals might present cross-infection between groups (DM and C) because they share fomites and other tools for maintenance. In this sense, intake of extracts from either variety of damiana did not show any benefits on inflammatory disorder given by the high number of basophils observed in WBC.











 text new page (beta)
text new page (beta)


