INTRODUCTION
In recent years, the world’s silver mine production has shown a slow but steady increase from 22 200 t in 2009 to 25 000 t in 2020. However, this supply does not correlate to a higher profit for industrial companies, since the silver price has dramatically dropped from USD 35.12 per ounce in 2011 to USD 16.21 per ounce in 2019, more than 50 % of value decrease in eight years (Garside 2020, 2021). As a result, it has become imperative to equilibrate the deficit of silver production by developing more efficient and profitable processes while following environmentally friendly practices, especially for economies that rely on this field of the mining industry for its financial support.
An area of opportunity is to recover silver from mining tailings. These byproducts of a primary extraction process still contain 100-140 g/ton of silver, which in some companies (particularly in developing countries) are currently not being properly exploited due to economical unfeasibility. One of the main setbacks is that certain elements are considered impurities, for example manganese (Mn), which can remain in tailings as part of minerals like pyrolusite due to its chemical affinity with silver. Unfortunately, a main characteristic of the mining industry is to act as a refractory agent, which affects the efficiency of the conventional cyanidation recovery process by damaging the internal walls of the furnaces (Jiang et al. 2004).
A possible alternative to optimize silver extraction could be to develop a bioleaching process for the removal of manganese (IV) as manganese (II) oxide, which is mostly soluble, or to fracture the shell matrix to increase silver extraction during a second cyanidation process. Bioleaching is a process that dissolves metals into a liquid phase through the metabolic routes of microorganisms in a highly oxidative environment. Also, it is a low-cost and environmentally friendly process if its byproducts (e.g., the acid bioleaching medium) are treated properly (Núñez-Ramírez et al. 2019, Zhang et al. 2019, Vargas-Rubio et al. 2021). Several authors indicate that chemical reactions occur on the mineral surface by an oxidation agent (Bosecker 1997, Diao et al. 2014, Ghosh et al. 2016). Microorganisms like Thiobacillus ferrooxidans, Acidithiobacillus thiooxidans, Acidithiobacillus ferrooxidans, and Leptospirillum ferrooxidans have oxidative characteristics in sulfide minerals from by-products and wastes of the mineral recovery industry that improve the solubility of manganese and other minerals (Rawlings et al. 1999, He et al. 2010, Gómez-Ramírez et al. 2021). This research aimed to evaluate manganese removal and silver extraction from mining tailings using native microorganisms as bioleaching agents and a flooded system for 18 days.
MATERIALS AND METHODS
Tailings obtained from La Encantada mine, located in Ocampo, Coahuila, Mexico (Fig. 1), were used for this research. These tailings are by-products obtained from a cyanidation process that is currently being performed to recover silver. The mining tailings consist of oxidized material which was chosen because there is approximately a volume of 12 000 00 t in the tailings dams without further treatment.
Elemental composition
Elemental quantification was realized for each processed sample and also for a sample without any biological treatment, as a control. Firstly, 0.25 g of mining waste previously dried (80 ºC for 3 h) and pulverized was mixed with 2 mL of HNO3 at room temperature (25 ºC). Then, it was heated to 70 ºC for 30 min. Afterwards, 6 mL of HCl were added to the sample and it was kept at 70 ºC for 2 h. After this time, the sample was cooled down. The volume was adjusted to 17 mL and the sample was centrifuged at 3000 rpm. Samples were analyzed by triplicate with a Perkin Elmer 5300DV inductively coupled plasma optical emission spectrometer (ICP-OES).
X-ray diffraction (XRD)
To determine the tailings’ components from their crystalline structure, X-ray diffraction was used. In a muffle, 100 g of tailings were dried at 50 ºC for 24 h. The samples were crushed later and the product was sieved (50 µm). The sample was analyzed by triplicate using a MiniFlex 600 X-ray Diffractometer Rigaku (0-90º in 2Ө, 0.02º pass, 0.7º/min, 40 kV, 15 mA).
Isolation of microorganisms
For the isolation of native microorganisms, a sample of tailings was taken from wet areas of the mine. Afterwards, 9K medium was added (Silverman and Lundgren, 1959) with the following composition: 3 g/L (NH4)SO4, 0.1 g/L KCl, 0.5 g/L K2HPO4, 0.5 g/L MgSO4·7H2O, 0.01 g/L Ca(NO3)2 and 44.22 g/L FeSO4·7H2O. The consortium was maintained by changing the culture medium every 600 mV of oxidation-reduction potential (ORP). The consortium was kept at 30 ºC with an aeration pressure of 1 kgf/cm2.
Molecular identification of the isolated consortium
DNA extraction
DNA extraction was carried out according to Zazueta-Álvarez et al. (2018); 1 mL of an overnight culture (1 × 108 cell/mL) was centrifuged at 13 000 rpm for 2 min. The cell pellet was resuspended in 300 µL of lysis buffer (EDTA, 0.05 M; NaCl, 0.1 M; pH 7.5) by gentle vortexing for 30 s. Afterwards, 100 µL of lysozyme solution (10 mg/mL) and 30 µL of SDS solution (20 % w/v) were added. The mix was incubated at 37 ºC for 5 min. DNA was purified using a mix of phenol-chloroform-isoamyl alcohol (25:24:1, v/v/v). The extraction was carried out by triplicate.
PCR
The extracted DNA sample was used as a template to amplify a conserved 16S ribosomal DNA (rDNA) region for total bacteria by PCR, using a primer pair forward 5′-CCGTCAATTCCTTTGAGTTT-3′ and reverse 5′-GTGCCAGCAGCCGCGGTAA-3′. Approximately 20 ng of purified DNA were used per reaction combined with 2.5 μL of 10X PCR buffer, 1 μL of MgCl2 (25 mmol), 1 μL of dNTP mixture (20 mmol), 1 μL for each primer (10 mmol), 0.25 μL (5 U/μL) of GoTaq DNA polymerase (Promega); all dissolved in 18.25 μL of sterile Milli-Q water. PCR was programmed as follows: initial DNA denaturation at 95 ºC for 5 min, followed by 10 cycles of nesting (denaturation 30 s at 94 ºC, annealing from 65-55 ºC for 30 s, lowering temperature by 0.5 ºC for each cycle, and a final extension of 30 s at 72 ºC). The PCR product was sequenced and compared using a basic local alignment search tool of the National Library of Medicine (NCBI 1988, Altschul et al. 1990). The 16S rRNA amplicons were sequenced. From the data obtained from the identified cultures of ribosomal genes, taxonomically related sequences of the determined genera were collected for each isolate in the NCBI Taxonomy browser database.
Bioinformatic analysis
With the previous sequences multiple alignments were performed with CLUSTAL W (Larkin et al. 2007) and T-coffee (Magis et al. 2014). The shared regions of the 16S rRNA genes were included in phylogenetic trees and analyzed using Jukes-Cantor nucleotide substitution models in the MEGA 6 program (Tamura et al. 2011). The evolutionary history was inferred using the Neighbor-Joining method (Saitou and Nei 1987), which is used to reconstruct phylogenetic trees. Phylogenetic trees were constructed using the unweighted pair group method with arithmetic mean method (UPGMA) with a bootstrap statistical analysis of 1000 replicates (Hillis and Bull 1993). Percentages of similarity were calculated with BioEdit 7.0.5.2 software. Sequences with identity scores greater than 97 % in BLAST and verified in EZBiocloud (< 3 % divergence) were resolved at the species level, while scores between 95 and 97 % at the genus level (Rosselló-Mora and Amann 2001). The evolutionary distances were computed using the Maximum Composite Likelihood method (Tamura et al. 2004) and were also in the same units of the number of base substitutions per site. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches (Felsenstein 1985). This analysis involved eight nucleotide sequences. All ambiguous positions were removed from each sequence pair (pairwise deletion option). There were a total of 1570 positions in the final dataset. Evolutionary analyses were conducted in MEGA X (Kumar et al. 2018).
Flooded system
The bioleaching process was performed in containers of 9.43 × 10-3 m3 to simulate the structural configuration of the terrain in a flooded system. A Box-Behnken experimental design was used with the following variables: ferrous ion concentration (2-14 g/L), pH (2-6), and agitation time at 100 rpm (2-6 min/day). Experimentation was performed for 18 days (Zazueta-Álvarez et al. 2018). Analyzed responses (in percentage) were manganese removal and silver extraction. All experiments were performed with a water-tailing ratio of 2:1 and subsequent inoculation of 10 % (v/v) of the isolated consortium.
Cyanidation process
To extract silver, the residue from the bioleaching process was washed and leached with sodium cyanide at a concentration of 1000 ppm for 72 h (Núñez-Ramírez et al. 2018).
RESULTS AND DISCUSSION
Elemental results and XRD
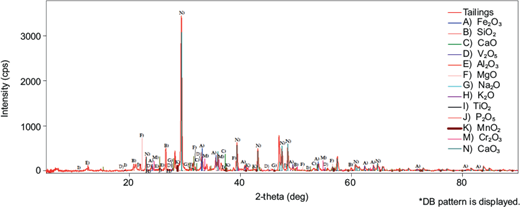
Figure 2 shows the diffractogram of the mining tailings indicating the presence of many mineral species and their crystalline characteristics. It can also be inferred that the surface of the mineral contains specific components for microorganisms to adhere in search of nutrients for their proliferation.
The XRD results in tailings are shown in Table I highlighting the presence of manganese in the form of pyrolusite (manganese oxide IV, MnO2). It has been reported that this material presents refractory properties causing processing problems (Jiang et al. 2004, Kong et al. 2015), sometimes appearing with iron species as ferromanganese compounds (Yuan et al. 2020). Mn also has an affinity for silver (Ramachandran 1996, Jiang et al. 2004), which affects conventional cyanidation by decreasing the solubility of silver and resulting in extractions ≤ 30 %, thus generating large quantities of tailings with considerable silver concentration.
TABLE I CHEMICAL COMPOSITION OF THE TAILINGS OBTAINED BY INDUCTIVELY COUPLED PLASMA OPTICAL EMISSION.
| Component | Content (%) |
| Silicon oxide (SiO2) | 9.67 |
| Aluminum oxide (Al2O3) | 2.23 |
| Ferric oxide (Fe2O3) | 18.6 |
| Magnesium oxide (MgO) | 0.49 |
| Calcium oxide (CaO) | 32.1 |
| Sodium oxide (Na2O) | 0.85 |
| Potassium oxide (K2O) | 0.04 |
| Titanium oxide (TiO2) | 0.18 |
| Phosphorus oxide (P2O5) | 0.1 |
| Manganese oxide (MnO2) | 3.78 |
| Chromium oxide (Cr2O3) | < 0.01 |
| Vanadium oxide (V2O5) | < 0.01 |
The results of elemental analysis obtained through ICP-OES are shown in Table II indicating that the presence of heavy metals (Pb, Zn, As) in reasonable concentrations generates an extreme environment for the proliferation of microorganisms (Ye et al. 2021) with a highly oxidative environment (Johnson et al. 2020, Amar et al. 2021). However, the stress generated by the characteristics of the mining tailings causes the microorganisms to present a state of adhesion to the surface of the mineral as protection.
Microorganisms isolation and identification
Figure 3 shows that the isolates encoded as ITDBC2, ITDBC5 and ITDBC6 were identified as Leptospirillum ferriphilum, with GenBank accession numbers MZ190834, MZ190833, and MZ190832, respectively. The activity performed by L. ferriphilum in reductive processes has been reported for Mn with the help of other biological species (Dan et al. 2016) as well as for iron oxidation (Ozkaya et al. 2007). The acquired knowledge from this research of L. ferriphilum capabilities under extreme conditions on mine sites may represent a factor to be considered in the scale-up design of a subsequent bioleaching process.
The evolutionary history was obtained using the Neighbor-Joining method (Saitou and Nei 1987), which is used to reconstruct phylogenetic trees. Figure 3 shows the optimal tree with the sum of branch length = 0.66187444. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree.
This stage of the research will allow the use of indigenous microorganisms for the in-situ treatment of mining tailings, thus avoiding the use of commercial strains or a combination of strains characterized by other types of materials.
Bioleaching process
Table III shows the removal of manganese, which reached up to 70 % under anaerobic/microaerophilic conditions, thus creating high stress for microorganisms that belong to the native consortium of the genus Leptospirillum and in a greater proportion of identity to L. ferriphilum (Fig. 3). This confirms the ability of the analyzed strains (ITDBC2, ITDBC5, ITDBC6) to present a system capable of reducing the oxidative stress generated by elements like arsenic or lead and, consequently by other potential toxic elements (PTE) contained in mining waste (Li et al. 2010). Also, this genus is capable of using elements found on the surface of the tailings as energy sources such as sulfur and iron during the biohydrometallurgical process (Pradhan et al. 2008, Vera et al. 2013). By generating a decrease in the Mn concentrations of the solid phase it becomes feasible to recover silver with a second cyanidation process. This is also shown in Table III, since the silver that could be obtained with the second cyanidation process was found as a residue.
TABLE III MANGANESE REMOVAL AND SILVER EXTRACTION AFTER THE BIOLEACHING PROCESS USING THE RESPONSE SURFACE BOX-BEHNKEN EXPERIMENTAL DESIGN.
| Run | Ferrous sulfate (g/L) | pH | Agitation (min/day) | Manganese removal (%) | Silver extraction (%) |
| 1 | 7 | 6 | 6 | 57.37 | 28.61 |
| 2 | 7 | 6 | 2 | 60.00 | 25.45 |
| 3 | 2 | 2 | 4 | 59.47 | 19.15 |
| 4 | 7 | 4 | 4 | 63.68 | 36.96 |
| 5 | 7 | 4 | 4 | 65.26 | 33.69 |
| 6 | 7 | 2 | 2 | 68.16 | 4.71 |
| 7 | 2 | 4 | 2 | 58.95 | 22.20 |
| 8 | 2 | 4 | 6 | 57.37 | 17.72 |
| 9 | 7 | 2 | 6 | 63.95 | 26.70 |
| 10 | 14 | 4 | 2 | 71.58 | 3.61 |
| 11 | 14 | 6 | 4 | 65.00 | 55.53 |
| 12 | 14 | 4 | 6 | 71.84 | 37.40 |
| 13 | 2 | 6 | 4 | 55.00 | 31.93 |
| 14 | 14 | 2 | 4 | 71.84 | 43.10 |
| 15 | 7 | 4 | 4 | 63.68 | 32.18 |
It has been reported that the use of reducing agents might change some mineral species, such as certain components with Mn, which could be removed by solubilizing them (Pakarinen and Paatero 2011). High-temperature processes (Shekhar et al. 2021) or the use of acid solutions (Ismail et al. 2004) require high energy consumption and the addition of substances that could generate an environmental impact.
In this research, the bioleaching performed by L. ferriphilum had an optimal acidic pH (2.0) environment for bacteria but due to the concentration of carbonates used in the cyanidation process (Table III), the removal of manganese reached 60 % even at pH 6, probably because the bacteria produce biogenic sulfuric acid allowing the solubilization of Mn. Also, due to their autotrophic metabolism, the bacteria produce organic acids and sugars that allow the oxidation of the Mn species present in tailings (Tian et al. 2010, Zhao et al. 2010).
Table IV shows the results of the analysis of variance (ANOVA) indicating that the concentration of ferrous sulfate and pH has a significant positive effect (p < 0.05) on the removal of manganese by generating an indirect oxidative mechanism (Acharya et al. 2003), while the stirring time does not influence the removal (p > 0.05). This could indicate that the process could take place under anaerobic or microaerophilic conditions. These low oxygen conditions demonstrate the adaptability of L. ferriphilum to use manganese (IV) as an electron acceptor in the respiratory chain by applying a direct mechanism on the mineral surface (Li et al. 2005).
TABLE IV REGRESSION COEFFICIENTS OF RESPONSES OF THE BIOLEACHING PROCESS (CODED FACTORS).
| Response | Intercept | Lineal | Quadratic | Interactions | R2 | ||||||
| X1 | X2 | X3 | X1 1 | X2 2 | X3 3 | X1X2 | X1X3 | X2X3 | |||
| Mn | 59.1 | 6.184* | -3.922* | -0.972 | -0.451 | -1.974* | 0.132 | -0.514 | 0.568 | 0.395 | 0.978 |
| Ag | -55.1 | 9.39* | 5.98* | 3.70 | 2.83 | -1.17 | -11.74* | -0.03 | 2.44 | -4.71 | 0.931 |
*Indicates significant differences (P < 0.05).
X1: ferrous sulfate (g/L), X2: initial pH, X3: agitation (min/day), R2: coefficient of determination, Mn: manganese removal (%), Ag: silver extraction (%).
This study demonstrated the adaptability of L. ferriphilum to proliferate in extreme environments in the presence of reactive oxygen substances (ROS) such as hydrogen peroxide (H2O2), superoxides and even highly reactive hydroxyl radicals (Cárdenas et al. 2012), which are produced by constant changes in the oxidation states of the metals present in mining tailings. This is possible due to the genes involved in the resistance to oxidative stress in these environments, for example Rubrerythin (Rbr), which is implicated in the expulsion of ROS (Khaleque et al. 2020). The thioredoxin system (TrxA/TrxB) for the repairment of proteins allows to maintain a reducing environment within the cell during the transport of electrons through the membrane (Lee et al. 2012). The periplasmic thiol oxidation system (DsbA/DsbB) is used in the proper folding of proteins by maintaining the formation of disulfide bonds (Norambuena et al. 2012). The formation of peroxiredoxin (Bcp) creates an antioxidant effect by controlling peroxide levels (Ferrer et al. 2016). The UvrABC complex catalyzes the recognition and processing of DNA lesions (Ullrich et al. 2016) and DNA glycosylases participate in this DNA repair activity (Wang et al. 2019). The heavy metal resistance systems includes genes TnLfArs and arsRBC, which encode the exit pumps for the arsenite-arsenate species (Tuffin et al. 2006, Christel et al. 2018).
On the other hand, it is important to mention the sessile mechanism of L. ferriphilum during the formation of biofilms generated by the production of extracellular polymeric substances (EPS) that act as a protection against the adverse environmental conditions where microbial growth takes place (Liu et al. 2017, Saavedra et al. 2021). All these survival characteristics of L. ferriphilum allow it not only to proliferate but to generate changes in the surface of the mining tailings to subsequently obtain the silver contained in this byproduct.
Other researchers have reported higher percentages of manganese removal using different operating conditions as well as other species of microorganisms. Lan et al. (2020) obtained the optimal conditions for a 98 % bioleaching of Mn highlighting a pH of 3.5, temperature of 45 ºC and pulp percentage (w/v) of 5 % using molasses as a carbon source. Keshavarz et al. (2021) obtained up to 80 % of Mn bioleaching under optimal conditions in 30 days; however, the pulp percentage ratio (w/v) was 0.1 %. Also, non-biological processes with optimal temperatures up to 700 ºC were evaluated by Eghbali et al. (2021). Our results show a higher Mn bioleaching in less time (18 days) compared to previous reports.
In addition to processing a higher pulp content (w/v) than the one evaluated at the laboratory level (50 %), this research also used low operating temperatures (37 ºC) without the addition of organic compounds due to the characteristics of the native microbial consortium, thus presenting a process with a high probability of success in the industrial scale-up.
After the bioleaching process, heavy metals present in the waste generated by the mining industry can transform and migrate to different areas than where they were deposited (Zazueta-Álvarez et al. 2018). Such risk could be avoided by using bioleaching, thus generating an eco-friendly process (Zheng et al. 2019, Zhang et al. 2022) that could prevent the migration of toxic species contained in the tailings towards the aquifers or water bodies that are used for human or animal consumption.
The acquired knowledge from this research of the capabilities of L. ferriphilum under extreme conditions, such as mining sites, may represent a factor to be considered in the scale-up design of future bioleaching processes.
Silver extraction in heap leaching
After the bioleaching process, each sample of the experimental design was placed in cyanidation for 72 h. Table IV shows that ferrous sulfate concentration (g/L), initial pH (both in their linear terms), and agitation (min/day) (in its quadratic term) had a significant effect (P < 0.05) on silver recovery, thus indicating that the removal of manganese carried out by the biotechnological process allowed the subsequent recovery of silver.
The interaction between the energy source of the microorganisms (concentration of ferrous sulfate), favorable growth conditions for the microorganisms (initial pH), and homogenization of the multiphase system (agitation time) can be observed in Table IV, with the following highlights:
The growth of L. ferriphilum promoted the recovery of silver from mining tailings. A highly oxidizing environment was generated with the presence of ferrous sulfate, however, these conditions allow L. ferriphilum to shelter on the surface of the mineral, degrading it during its growth.
To recover silver by cyanidation, pH values below 6.0 are unnecessary, indicating that microorganisms can adapt to pH values above the optimum for their growth. However, it is important to maintain adequate proliferation conditions, such as the presence of a direct energy source for its adaptation to the system (ferrous sulfate) and an aerobic environment.
An increase in the agitation times per day favored the extraction of silver; however, manganese removal is preferred under microaerophilic conditions.
Table V shows the maximization generated by the software Minitab (v. 17) for silver extraction. It is shown that the removal of manganese from the solid phase is unnecessary since the fracture of the crystalline matrix would be sufficient for the cyanidation process to improve the recovery of silver.
TABLE V SILVER EXTRACTION OPTIMIZATION.
| Setting value | D value | Ferrous sulfate (g/L) | Initial pH | Agitation (min/day) |
| Optimal | 0.9464 | 14.0 | 6.0 | 4.1414 |
Microaerophilic conditions are important; however, daily agitation allows the homogenization of the system and possibly avoids the excessive formation of biofilm that could later be undesirable.
The interaction of L. ferriphilum with the mineral surface is necessary. L ferriphilum can survive oxidative stress not only due to the number of protons present in acid media but also through the production of enzymes capable of regulating the oxidative stress generated during adhesion to the mineral surface. In this way, various mechanisms occur in the bioleaching process which allow the survival of L. ferriphilum, thus the recovery of silver contained in an industrial waste can be carried out.
The novelty and contribution of this research relies on the improvement of a biohydrometallurgical process using native microorganisms that generate bioaugmentation with the isolated and identified strain and use microorganisms found in the soil where the tailings are located, which has the advantage that the use of commercial strains is not necessary.
On the other hand, it has already been reported that the removal of manganese and the subsequent extraction of silver are also carried out in a stirred tank. However, using a flooded system under stressful conditions for the microorganisms generates a higher removal of the refractory material (Mn). This flooded system may allow an improvement in the energy consumption of the process. Also, it should be noted that the bioleaching process can be used on both mine tailings and concentrates.
CONCLUSIONS
The isolates ITDBC2, ITDBC5, and ITDBC6 were identified as Leptospirillum ferriphilum from a mining tailing. These bacteria acted as bioleaching agents under oxidative stress conditions in an extreme environment, showing high adaptability and generating a combined action of mechanisms on the pyrolusite matrix by allowing a greater action of the cyanidation process for the recovery of silver from mining waste. Bioleaching through a flooded system was able to remove 70 % of the manganese and 55.53 % of the extracted silver. The obtained results could be used to initiate larger-scale processing based on the foreseeable economic benefits that result from the optimized extraction of silver from mining waste with the help of native microorganisms, thus generating a process that does not require high energy consumption while being environmentally friendly.











 text new page (beta)
text new page (beta)