INTRODUCTION
In Mexico, about 1200 hectares of fig fruit (Ficus carica L.) are cultivated, with an estimated production rate of 5380 tons per year (SIAP 2007). The state of Morelos is the leading fig producer in the republic, with 50% of the total production. Today, there are commercial agreements for the export of this product to Canada, the United States of America and Japan (CESVMOR 2020). Currently, there are no records anywhere in the world on the presence of pesticide residues in fig crops. This partly because in almost all the countries where this product is grown, pest control is carried out through biological control (Wohlfarter 2011, NIPHM 2015, Moniruzzaman 2017, Nilda 2019). However, in Mexico various types of pesticides are used to control pests on this crop. Some of them are classified as highly dangerous, according to the list prepared by the Pesticide Action Network International (PAN 2016), which implies severe ecological effects and negative impacts on the health of the local population and consumers (Palacios-Nava 1999).
Despite knowing these products’ adverse effects, they continue to be used indiscriminately. Farmers generally apply pesticides without using good safety practices, i.e., mainly using excessive amounts of pesticides several times throughout the production process, potentially contaminating the crop before sending their produce to market (SENASICA 2017). In order to comply with the requirements of different markets around the world regarding the residual levels of toxic substances, the State Committee for Plant Health of Morelos (CESVMOR, for its acronym in Spanish) implemented a training program in the production units for the application of Good Use and Management of Agrochemicals (CESVMOR 2020). However, a wide range of banned substances are still detected in various fruits and vegetables (Aldana-Madrid 2008, Pérez 2013, Angeles-Núñez 2014).
Some of those substances are highly persistent. They can remain in the environment for long periods before breaking down. These substances can be highly mobile and can bioaccumulate (UNEP 2006). Its persistence in the environment depends on some environmental factors such as pH, temperature, humidity, and solar radiation, as well as the action of some microorganisms (Belfroid 1998). These factors promote the excitation, rupture, or rearrangement of chemical bonds, generating partial or total transformations of the original molecules. When the degradation process is 100 percent, mainly CO2 and H2O are produced, reducing pesticides’ toxic effects (Raymond 2001). Therefore, this work aims to assess the occurrence of pesticide residues in figs from 15 different parcels located in Morelos in order to determine the degree of dissipation and health risk.
Chemicals and materials
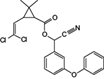
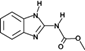
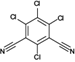
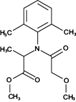
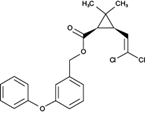
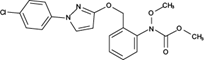
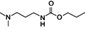
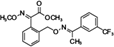
The analyzed pesticides were cypermethrin 99.2%, carbendazim 99.2%, chlorothalonil 99.1%, metalaxyl-M 99.1%, permethrin 97.8%, pyraclostrobin 97.1%, propamacarb 99.2%, trifloxystrobin 99.1%, thiophanate-methyl 99.1%, and atrazine as an internal standard (Table I) (AccuStandar Inc., New Haven, CT, USA). All solvents (toluene, acetonitrile, methanol, acetic acid, formic acid, and water, pesticide grade) were obtained from TEDIA High Purity Solvents (Carson City, CA, USA). The QuEChERS Dispersive Solid Phase Extraction kit was purchased from Waters Corporation, Mexico.
TABLE I TARGET ANALYTES IN THIS STUDY.
CAS: Chemical Abstracts Service.
Field experiments
Field experiments were conducted on 15 parcels for pesticide monitoring (Table II). Plants were randomly selected from each crop, and approximately 20 pieces (2 kg) were taken. They were then placed inside sterile polyethylene bags, refrigerated, and transported to the laboratory for analysis. Once in the laboratory, samples were processed per the guiding document on validation procedures and quality control of analytical methods for determining pesticide residues in food and feed (EURL 2019).To evaluate the dissipation behavior of some of the pesticides, the cultivate “Arias 2” was selected, located in the municipality of Axochiapan, Morelos (México) (Latitude: 18.5002, Longitude: -98.7497 18 º30 ′ 1 ″ North, 98 º44 ′ 59 ″ West). The fig crop was raised in 3.75 × 18.00 m plots, with spacing 3.75 × 2.00 m (columns x rows). Four treatments, each replicated twice, were planned with a randomized block design. Each block had eight plants; therefore, 32 plants were sampled for all sampled blocks. Five pesticides were selected to assess dissipation (cypermethrin, pyraclostrobin, thiophanate-methyl, chlorothalonil, and carbendazim). These pesticides were selected because they were detected more frequently in the fig fruit. A mixture of the pesticides was sprayed with a pump at the fruit formation stage at a rate of 6.66 L per block. The spray doses ranged from 2.564 g/block to 3.223 g/block. For residue analysis, fig fruit samples from each replicate were collected on days 0 (2 h after spray), 5, 10, 15 and 20 after the pesticide application. These were placed inside polyethylene bags, refrigerated, and transported to the laboratory to be analyzed. The samples were processed in the same way as when the pesticides were monitored.
TABLE II GEOGRAPHICAL LOCATION OF THE PLOTS.
| Number | Municipality | Parcel name | Location | Coordinates |
| 1 | Ayala | Las Rayas | Tenextepango | 18º42′ 06″ North, 98º59′ 24″ West |
| 2 | Ayala | Los Limones | Campo Texcalamate | 18º42′ 37″ North, 98º59′ 24″ West |
| 3 | Ayala | Los limones | Campo Texcalamate | 18 45 49 North, 98 52 46 West |
| 4 | Ayala | Tierra 2 | Campo Casa Blanca Moyotepec | 18º42′ 37″ North, 98º59′ 24″ West |
| 5 | Ayala | Eufrosina | Campo Casa Blanca Moyotepec | 18º42′ 37″ North, 98º59′ 24″ West |
| 7 | Ayala | El Jaguey | Ejido Jalostoc Ayala | 18º47′ 31″ North, 98º57′ 04″ West |
| 8 | Ayala | El Jaguey | Ejido Jalostoc Ayala | 18º47′ 31″ North, 98º57′ 04″ West |
| 9 | Ayala | Premier 1 | Ejido Jalostoc Ayala | 18º43′ 09″ North, 98º53′ 09″ West |
| 10 | Ayala | El Amate Amarillo | Ejido Jalostoc Ayala | 18º43′ 9″ North, 98º54′ 04″ West |
| 12 | Ayala | El Jaguey | Ejido Jalostoc Ayala | 18º47′ 31″ North, 98º57′ 04″ West |
| 13 | Ayala | Huerto Huizachera | Ejido Jalostoc Ayala | 18º43′ 9″ North, 98º54′ 04″ West |
| 14 | Tepalcingo | Los Higueros | Ixtlilco | 18º31′ 30″ North, 98º49′ 57″ West |
| 15 | Tepalcingo | El Guamúchil | Ixtlilco el Grande | 18º31′ 38″ North, 98º49′ 38″ West |
Sample preparation and clean up
The extraction and clean-up procedure used was based on quick, easy, cheap, effective, rugged and safe (QuEChERS) sample preparation method for pesticides (Waters Co., Mexico). The fig fruit was first prepared by homogenizing 2.0 kg in a blender. Then 10 g of homogenized sample was placed in a 50 mL polytetrafluoroethylene (PTFE) centrifuge tube with 10 mL of acetonitrile containing atrazine (internal standard 0.000133 mg/kg), 1 g of sodium citrate (Na3C6H5O7), 1 g of sodium chloride (NaCl) and 4 g of magnesium sulfate (MgSO4). Each tube was stirred for 2 min, and later taken to a bath of ultrasound for 5 min. Then the samples were centrifuged at 3500 rpm for 3 min. From this solution, an aliquot of 5 mL was taken in a plastic tube which contained 900 mg of magnesium sulfate (MgSO4), 150 mg of PSA (primary and secondary amine), 150 mg of resin C18 and 80 g of activated carbon. It was then stirred in a vortex and centrifuged at 3500 rpm for 3 min. The supernatant was filtered through a nylon membrane (0.2 µm). The filtrate was divided into two equal aliquots; one was used for analysis by gas chromatography and one for liquid chromatography.
Analytical method performance
Starting with individual stock solutions of pesticides (1 mg/mL) a series of dilutions were made until a stock solution of 20 ng/mL was obtained, using acetonitrile as a solvent. The calibration curves were prepared by appropriate dilution of the stock solution in a blank sample to correct the matrix effect. The concentrations of the calibration curves ranged between 0.00011, 0.00032, 0.00107, 0.00218, 0.00641, and 0.01202 mg/kg. The ratios of the concentration of each compound divided by the concentration of the internal standard were plotted versus the relationships of the areas of each standard over the area of the internal standard, and fit by simple linear regression to obtain the equation for the standard graphs for the tested pesticides.
A mean recovery test was performed using spiked blank fig samples at three different concentration levels of selected pesticides (0.005, 0.2, and 1.0 mg/kg). The spiked samples were allowed to settle for 2 h at room temperature before the extraction step; this procedure was performed to distribute the pesticide evenly and ensure complete interaction with the sample matrix. The spiked samples were processed as explained above.
Gas chromatography-tandem mass spectrometry (GC-MS/MS) analysis
Cypermethrin, chlorothalonil, metalaxyl-m and permethrin were analyzed by gas chromatography triple quadrupole mass spectrometer system (GC/QqQ, Agilent, Santa Clara, CA, USA), with electron ionization (EI). The column was a column HP 5, 15 m, 250 mm ID, 0.25 µm film thickness (Agilent, Santa Clara, CA, USA). The oven temperature was set at 90 ºC and held for 2 min, then ramped at 15 ºC/min to 180 ºC (2 min), then ramped 5 ºC/min to 330 ºC (5 min). Nitrogen was used as the QqQ collision gas (99.9999%, Infra, Mexico City, Mexico) at 1.5 mL/min and the carrier gas was Helium (99.9999%, INFRA, Mexico City, Mexico) at 2.25 mL/min. EI energy was 70 eV, and quadrupole temperatures were set at 150 ºC. Product ion and collision energy experiments were performed to determine the optimum two product ions, collision energies, and ratios between quantifier and qualifier ions. The mass selective detector (MSD) transfer line was at 250 ºC and ion source was set at 320 ºC. Table III summarizes the multiple reaction monitoring (MRM) transitions and collision energy for each transition.
TABLE III SUMMARY OF THE OPTIMIZED PARAMETERS FOR EACH PESTICIDE BY GC-MS/MS AND UPLC-MS/MS.
| Analyte | Analysis mode | First transition (m/z) | Collision energy (V) | Second transition (m/z) | Collision energy (V) |
| Cypermethrin | GC-MS/MS | 181→152 | 20 | 163 →127 | 5 |
| Chlorothalonil | GC-MS/MS | 265 → 133 | 40 | 263 → 168 | 25 |
| Metalaxyl-M | GC-MS/MS | 234→174 | 10 | 220 → 192 | 10 |
| Permethrin | GC-MS/MS | 183→153 | 15 | 183 → 77 | 35 |
| Carbendazim | UPLC-MS/MS | 192→160 | 10 | 192 → 132 | 27 |
| Pyraclostrobin | UPLC-MS/MS | 388 → 194 | 17 | 388 → 163 | 33 |
| Propamacarb | UPLC-MS/MS | 189→ 102 | 23 | 189 → 144 | 17 |
| Trifloxystrobin | UPLC-MS/MS | 409 → 186 | 25 | 409 → 145 | 60 |
| Thiophanate-methyl | UPLC-MS/MS | 343→151 | 25 | 343 → 311 | 11 |
GC-MS/MS: Gas chromatography-tandem mass spectrometry.
UPLC-MS/MS: Ultra-performance liquid chromatography-tandem mass spectrometry.
Ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analysis
Carbendazim, pyraclostrobin, propamacarb, trifloxystrobin and thiophanate-methyl analysis was done in a Waters UPLC-MS/MS (XEVO TQ-MS Mass Spectrometer, Waters, Milford, MA, USA) in electrospray ionization in positive mode (ESI). For desolvation, nitrogen gas was used at a flow of 100 L/h (500 ºC) and argon as collision gas at a flow of 0.15 mL/min (Column C18, Acquity, UPLC BEH, 1.7 µm, 2.1 x 100 mm, Waters, Milford, MA, USA), at a temperature of 60 ºC. The injection volume was 10 µL. The mobile phases A and B were acetonitrile and 0.1% formic acid (70:30 (v/v)), at a constant flow rate of 0.30 mL/min. Collision cell energy and fragmentation voltage were optimized in the dynamic multiple reaction monitoring mode (MRM) (Table III).
Weather conditions
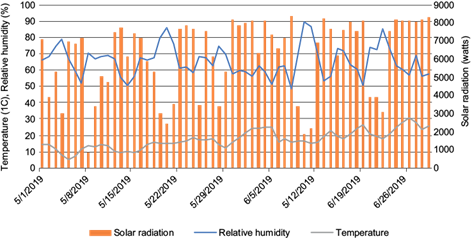
During the development of the study (May-June 2019), the weather conditions presented the following average values: temperature 17.4 ºC (max: 31.0, min: 5.0 ºC); relative humidity RH 65.0% (max: 89.0, min: 48.5); solar radiation 6421.0 Watts (max: 8377.6, min: 1869.9); and sunshine duration 564.7 minutes (max: 960.6, min: 0; Fig. 1).
Risk health index analysis
The potential risk to human health was assessed based on the concentration of pesticide residues in fig fruit, according to the methodology established by the European Food Safety Authority (EFSA 2020). Estimated daily intake (EDI, equation 1) was calculated by multiplying the residual pesticide concentration (mg/kg) by the food consumption rate (kg/day) and dividing it by a body weight of 60 kg for the adult population. The average daily fig fruit intake for adults (LP) considered was 0.0235 kg/person/day, according to the Agrifood Atlas for Mexico (SENASICA 2017).
LP (kg/day): is the highest large portion reported (97.5 th percentile of eaters)
HR (mg/kg): is the highest residue in composite sample of edible portion found in the supervised trials used for estimating the maximum residue level.
bw (kg): is the body weight
The risk health index (RHI) of the residues was computed using the results and other statistics followed by equation (2), modified after EFSA (EFSA 2020).
EDI is estimated daily intake. ADI is acceptable daily intake. If RHI value > 1, this is considered as not safe for human health (WHO 2008).
Dissipation
The results of the residues of the selected pesticides were subjected to the first-order kinetics model.
Where C r is the concentration after a time t, C 0 is the initial concentration and k the dissipation constant. Additionally, the half-life time (t 1/2 ) was calculated from the regression equation, using the following formula:
RESULTS AND DISCUSSION
Analytical method performance
Good linearity and reproducibility of calibration curves were achieved (r > 0.95). The recovery of all the pesticides at three concentration levels ranged from 84.0 to 90.6%. The reproducibility of recovery results, as indicated by relative standard deviations (RSD) < 20%, confirmed that the method is sufficiently reliable for pesticide analysis (SANTE 2019).
The limits of detection (LOD) and quantification (LOQ) were calculated using signal-to-noise criteria (S/N); LOD= 3 (S/N) and LOQ = 10 (S/N) (Miller and Miller 2004). The LOD were between 3.3E-06 and 6.3E-05 mg/kg for permethrin and cypermethrine, respectively (Table IV).
TABLE IV SUMMARY OF THE QUALITY PARAMETERS OF THE OPTIMIZED METHOD FOR THE ANALYSIS OF PESTICIDES.
| Analyte | LOD | LOQ | Recovery percentage | ||
| mg/kg | mg/kg | 0.005 (mg/kg) | 0.2 (mg/kg) | 1 (mg/kg) | |
| (n=3) | (n=3) | (n=3) | |||
| Cypermethrin | 6.30E-05 | 2.10E-04 | 84 ± 9 | 86 ± 7 | 89 ± 8 |
| Carbendazim | 6.00E-06 | 2.00E-05 | 84.4 ± 7 | 88.1 ± 10 | 88.8 ± 10 |
| Chlorothalonil | 5.00E-06 | 1.67E-05 | 88.1 ± 6 | 89.2 ± 8 | 89 ± 11 |
| Metalaxyl-M | 5.00E-06 | 1.67E-05 | 88.3 ± 7 | 90.1 ± 7 | 90.6 ± 10 |
| Permethrin | 3.30E-06 | 1.10E-05 | 85.8 ± 9 | 87.2 ± 11 | 88.1 ± 8 |
| Pyraclostrobin | 1.50E-05 | 5.00E-05 | 85.4 ± 6 | 86.5 ± 8 | 87.2 ± 8 |
| Propamacarb | 1.60E-05 | 5.33E-05 | 85.6 ± 8 | 87.5 ± 7 | 87.2 ± 10 |
| Trifloxystrobin | 1.70E-05 | 5.66E-05 | 84.7 ± 8 | 86.4 ± 6 | 88.2 ± 9 |
| Thiophanate-methyl | 1.80E-05 | 5.99E-05 | 86.8 ± 6 | 87.1 ± 9 | 89.2 ± 9 |
LOD: Limit of Detection; LOQ: Limit of Quantification.
Occurrence of pesticides in fig crops
Of the 15 parcels sampled, each had at least one pesticide residue. The pesticides with the highest concentrations were thiophanate-methyl (0.733 mg/kg), chlorothalonil (0.445 mg/kg), propamocarb (0.395 mg/kg) and carbendazim (0.313 mg/kg). Almost an order of magnitude below were metalaxyl-M (0.047 mg/kg), cypermethrin (0.038 mg/kg), trifloxystrobin (0.036 mg/kg), permethrin (0.034 mg/kg) and pyraclostrobin (0.02 mg/kg).
The pesticides most frequently detected were chlorotalonil, pyraclostrobin and cypermetrhrin (four times), followed by metalaxyl-M, permethrin, carbendazim and thiophanate-methyl (three times), trifloxystrobin (twice) and propamocarb (once; Table V). Although some pesticides have been banned in other countries, in México they are still used. Such is the case with paraquat, dimethoate, lindane, parathion, malathion and endosulfan, cypermethrine, chlorothalonil and permethrin, among others (Arellano and Rendón 2016).
TABLE V RESIDUAL CONCENTRATIONS AND MAXIMUM RESIDUAL LIMITS OF THE PESTICIDES DETECTED IN FIG CROPS IN MORELOS (mg/kg).
| Common name | No. of positive samples | Range (mg/kg) | No. of samples exceed MRLs | MRL EURL a | MRL Japan b | MRL Codex c |
| Chlorotalonil | 4 | 0.04-0.445 | 3 | 0.01 | 2.0 | 2.0 |
| Metalaxyl-M | 3 | 0.014-0.047 | 0 | 0.05 | 0.7 | 0.2 |
| Permethrin | 3 | 0.024-0.034 | 0 | 0.05 | 0.5 | 0.1 |
| Pyraclostrobin | 4 | 0.014-0.020 | 0 | 0.02 | 0.05 | 0.02 |
| Cypermethrin | 4 | 0.022-0.038 | 0 | 0.05 | 0.8 | 0.07 |
| Trifloxystrobin | 2 | 0.032-0.036 | 2 | 0.01 | 0.3 | 0.05 |
| Carbendazim | 3 | 0.014-0.313 | 3 | 0.1 | 2.0 | 0.5 |
| Propamocarb | 1 | 0.395 | 1 | 0.01 | 0.5 | 0.5 |
| Thiophanate-methyl | 3 | 0.018-0.733 | 3 | 0.1 | 2.0 | 0.1 |
a Values taken from the database from the European Commission (EURL 2019).
b Values taken from the database from the Japan Food Chemical Research Foundation FCRF 2019).
c Values taken from the database from the CODEX Alimentarius (CODEX 2019).
MRL : Maximum Residue Limits; EURL : EU Reference Laboratories.
In Mexico there are no values of maximum permissible values (MRL) for pesticide residues in fig cultivation. For this reason, in this study we take as reference the values established by the European Commission (EURL 2019), the CODEX Alimentarius (CODEX 2019) and the Japan Food Chemical Research Foundation for similar fruits (FCFR 2019). When comparing the results obtained with the MRL reported by these three agencies, it is observed, in general terms, they are below these MRL.
In Mexico, there are no official data on the number of pesticides, the amounts used and the number of times that they are applied per year, which makes it difficult to accurately monitor compounds of interest. For example, in a report made by the CESVMOR in 2015, they mention that for the control of pests in the cultivation of figs, only the following pesticides are used: carbendazim, permethrin, chlorpyrifos and dimethomorph (CESVMOR 2015). However, the results obtained in this study reveal the presence of other additional compounds, indicating that there is still no control over the type of pesticides used by farmers, which in part can be explained by the clandestine sale of unauthorized products, given that in some local markets they are usually cheaper than when purchased from an authorized distributor.
One of the causes of the high levels of pesticide residues is the resistance that pathogens acquire to some of these compounds, which has caused an excessive use of such compounds. For example, the most used fungicides for the control of black Sigatoka in banana cultivation are mancozeb, chlorothalonil, benzimidazole, imazalil, carbendazim, trifloxystrobin, pyraclostrobin, and pyrimethanil. There are records that 30 to 35 applications of fungicides such as mancozeb and chlorothalonil are carried out in the Pacific Center (Aguilar-Barragán 2014).On the other hand, using commercial mixtures indiscriminately can generate problems of physical or chemical incompatibility; this can lead to inactivity of the active principle, that is to say, a decrease in the effectiveness of pest control.
Dietary risk index assessment
The impact of pesticide on human health is not an easy and precise process to determine due to differences in the periods and the levels of exposure, the type of pesticide or mixtures used in the field, the geographic and meteorological characteristics of the agricultural areas where pesticides are applied, and others variables.
Table VI shows the EDI, ADI, and RHI. All RHI values are observed to be less than 1, which does not represent a risk to human health. However, it is noteworthy that dietary pesticide intake estimated in this study considered only exposures from fig fruit and did not include other fruits, vegetables, grains, dairy, fish, and meat, among others, nor did it consider, for example, drinking water, residential or occupational exposures. The above suggests that this calculation is underestimated and that in order to get closer to reality, aspects such as the processing of the fruits, once harvested, must be evaluated; that is, if they are washed, peeled, or cooked, as well as the groups of people who consume them (adults, children, pregnant women).
TABLE VI HEALTH RISK ASSESSMENT BASED ON ACCEPTABLE DAILY INTAKE (ADI) OF PESTICIDE RESIDUES IN FIG FRUIT.
| EDI mg/kg | ADI d mg/kg | RHI | |
| Chlorothalonil | 1.85E-04 | 0.02 | 9.27E-03 |
| Metalaxyl-M | 1.96E-05 | 0.08 | 2.45E-04 |
| Permethrin | 1.42E-05 | 0.05 | 2.83E-04 |
| Pyraclostrobin | 8.33E-06 | 0.03 | 2.78E-04 |
| Cypermethrin | 1.58E-05 | 0.02 | 7.92E-04 |
| Trifloxystrobin | 1.50E-05 | 0.04 | 3.75E-04 |
| Carbendazim | 1.30E-04 | 0.03 | 4.35E-03 |
| Promacarb | 1.65E-04 | 0.04 | 4.11E-03 |
| Thiophanate-methyl | 3.05E-04 | 0.08 | 3.82E-03 |
dWorld Health Organization (WHO 2008); EDI: Estimated Daily Intake;
RHI: Risk Health Index.
Environmental fate of pesticides
Most of the pesticides found in this study have slightly to high environmental persistence (15-120 days). The exceptions are carbendazim and propamacarb, which persist for more than 120 days and increase the risk of human exposure (Footprint 2006). Likewise, it is essential to mention that systemic pesticides such as carbendazim, metalaxyl, methylthiophanate, and propamocarb tend to be more persistent in plants, which increases the risk of bioaccumulation. Because some pests become resistant to certain active substances, farmers have to make various applications during the year (Mena-Espino and Couoh-Uicab 2015). One way to express the magnitude of bioconcentration or bioaccumulation of pesticides is the bioconcentration factor (BCF), which depends on the hydrophobic characteristic interpreted by the octanol-water partition coefficient (Kow) of the pesticide and the lipid content of the organism. Increased hydrophobicity (lipophilicity) leads to increased bioaccumulation (USEPA 1996, USGS 2007). In this sense, the BCF reported in various studies indicate that carbendazim, chlorothalonil, metalaxyl-M, propamacarb and thiophanate-methyl have a light value (BCF < 100). The rest (cypermethrin, permethrin, pyraclostrobin, trifloxystrobin) present BCF values between 100-5000 (moderate to high), suggesting that these have a high capacity to accumulate in fatty tissue (ODA 2014). This situation is worrying if we consider that some of the pesticides detected have chronic toxic effects on the health of the exposed population (Table VII).
TABLE VII TOXICOLOGICAL CHARACTERISTICS OF THE PESTICIDES DETECTED IN THIS STUDY.
| Compound | USEPA acute toxicity (oral, rats, mg/kg) | USEPA acute toxicity (dermal, rats, mg/kg) | USEPA acute toxicity (inhalation, rats, mg/L) | Classification | Topical toxicity | Chronic toxicity | ||||||
| Carbendazim | practically non-toxic | moderately toxic | practically non-toxic | U (WHO) | III (USEPA) | Ocular (positive) | Dermal (positive) | Allergenic capacity (negative) | Neurotoxicity (requires further study) | Mutagenicity (requires further study) | Carcinogenicity (nd, IARC) | Endocrine disruption (category 1) |
| Chlorothalonil | slightly toxic | nd | moderately toxic | U (WHO) | I (USEPA) | Ocular (positive) | Dermal (positive) | Allergenic capacity (positive) | Neurotoxicity (nd) | Mutagenicity (negative) | Carcinogenicity (2B, IARC) | Endocrine disruption (nd) |
| Cypermethrin | moderately toxic | moderately toxic | practically non-toxic | II (WHO) | nd (USEPA | Ocular (positive) | Dermal (positive) | Allergenic capacity (negative) | Neurotoxicity (level 4) | Mutagenicity (negative) | Carcinogenicity (nd, IARC) | Endocrine disruption (category 2) |
| Metalaxyl-M | moderately toxic | moderately toxic | practically non-toxic | nd (WHO) | I (USEPA) | Ocular (positive) | Dermal (positive) | Allergenic capacity (negative) | Neurotoxicity (nd) | Mutagenicity (negative) | Carcinogenicity (nd, IARC) | Endocrine disruption (nd) |
| Permethrin | slightly toxic | slightly toxic | slightly toxic | II (WHO) | III (USEPA) | Ocular (positive) | Dermal (positive) | Allergenic capacity (positive) | Neurotoxicity (level 4) | Mutagenicity (negative) | Carcinogenicity (3, IARC) | Endocrine disruption (category 2) |
| Propamacarb | slightly toxic | slightly toxic | practically non-toxic | U (WHO) | nd (USEPA | Ocular (positive) | Dermal (positive) | Allergenic capacity (nd) | Neurotoxicity (level 2) | Mutagenicity (negative) | Carcinogenicity (nd, IARC) | Endocrine disruption (nd) |
| Pyraclostrobin | slightly toxic | moderately toxic | slightly toxic | nd (WHO) | nd (USEPA) | Ocular (negative) | Dermal (positive) | Allergenic capacity (negative) | Neurotoxicity (nd) | Mutagenicity (negative) | Carcinogenicity (nd, IARC) | Endocrine disruption (nd) |
| Thiophanate-methyl | practically non-toxic | practically non-toxic | practically non-toxic | U (WHO) | III (USEPA) | Ocular (negative) | Dermal (negative) | Allergenic capacity (nd) | Neurotoxicity (nd) | Mutagenicity (positive) | Carcinogenicity (nd, IARC) | Endocrine disruption (nd) |
| Trifloxystrobin | slightly toxic | moderately toxic | practically non-toxic | nd (WHO) | III (USEPA | Ocular (positive) | Dermal (positive) | Allergenic capacity (positive) | Neurotoxicity (nd) | Mutagenicity (negative) | Carcinogenicity (nd, IARC) | Endocrine disruption (nd) |
WHO (2020): World Health Organization; USEPA: U.S. Environmental Protection Agency; level 2: Neurotoxic agents causing measurable biochemical alterations; level 4: Neurotoxic agents that cause morphological alterations in the cells of the Central Nervous System; IARC: International Agency for Research on Cancer: nd: there is no information; U: no danger of acute effects; I: highly toxic; II: moderately toxic; III: slightly toxic; 2B: possible human carcinogen; category 1: sufficient evidence of endocrine disruption; category 2: suspected endocrine disruption
Human health toxicity
The acute toxicity of a chemical substance refers to the adverse effects that manifest after the oral or cutaneous administration of a single dose of that substance or multiple doses administered over 24 hours or as a consequence of exposure by inhalation for 4 hours.
In this sense, the acute toxicity of the pesticides detected is varied, from non-toxic to moderately toxic. Regarding topical toxicity, some of these compounds (chlorothalonil, permethrin and trifloxystrobin) can cause local effects such as eye irritation, skin irritation and allergies, while propamocarb, thiophanate methyl, permethrin, cypermethrin affect the nervous, cardiovascular and musculoskeletal systems. Effects of greatest concern are those resulting from chronic exposure, such as hepatotoxicity (trifloxystrobin) and carcinogenicity (chlorothalonil and permethrin). It has also been reported that the intake of some of these substances can induce endocrine disruption (carbendazim, cypermethrin and permethrin) (IARC 2008, USEPA 2008) (Table VII).
Dissipation behavior
For the compounds with the highest frequency, an evaluation of their dissipation in the fruit was carried out. The residual concentrations of cypermethrin, pyraclostrobin, thiophanate-methyl, carbendazim and chlorothalonil were monitored from time zero (t = 0) to day 20 (t = 20). The pesticide dissipation behaviors are shown in table VIII.
TABLE VIII KINETIC PARAMETERS OBTAINED FOR EACH OF THE PESTICIDES STUDIED.
| Compound | Regression equation | Half-life (days) | Determination coefficient R2 |
| Thiophanate-methyl | Cr = 0.6295exp(-0.135t) | 9.07 | 0.8184 |
| Chlorothalonil | Cr = 0.4598exp(-0.056t) | 5.02 | 0.9328 |
| Carbendazim | Cr = 1.2905exp(-0.120t) | 5.79 | 0.8589 |
| Cypermethrin | Cr = 0.48exp(-0.168t) | 6.93 | 0.9845 |
| Pyraclostrobin | Cr = 0.38exp(-0.131t) | 5.29 | 0.976 |
The initial concentration of cypermethrin residues on day zero was 0.494 mg/kg. It decreased by 58.4% in the first five days; on day 10, it had decreased by 84.0%; and on day 20, the concentration had dissipated by 99.0%. These results were higher than that Suman-Gupta et al. (2011) observed in the dissipation of cypermethrin in tomato cultivation, which was 15 days for the dissipation of 95.0% of the amount initially added (0.098 mg/kg). The half-life in the present study was 6.41 days. This value was higher than that reported by Suman-Gupta et al. (2011), 3.6 days in tomato cultivation. It is essential to mention that the initial concentration they applied was almost an order of magnitude less than the amount applied in the fig crop (0.494 mg/kg). For its part, on day zero the determined residual concentration for pyraclostrobin was 0.395 mg /kg. Five days after pesticide application, the concentration was reduced by 56.0%. On day 20 the dissipation was 99.0%. These results were similar to what was found by Wu et al. (2018), who reported a total dissipation time for pyraclostrobin of 21 days in pepper crops. Meanwhile, the half-life calculated in fig fruit was 5.93 days. This value is lower than those observed in other studies where the half-lives of pyraclostrobin were 10.3-11.2 days in peanut plants (Zhang et al. 2012), 16.7-17.2 days in bananas (Zhao et al. 2015) and 15.4-16.5 days in apples (Shi et al. 2015). In the case of thiophanate-methyl, 60% of the initial concentration (0.733 mg/kg) was dissipated during the first five days, and on day 15 practically 99% was dissipated. Similar results were reported by Malhat et al. (2020) in strawberry crops in Egypt (14 days). The half-life calculated in fig fruit was 9.07 days. This result was lower than that reported by Bassam and Sumir (2018), 12-14 days in cucumber, but higher than the value reported by Dalia et al. (2017) in apples 5.23-6.03 days.
Meanwhile, chlorothalonil (0.445 mg/kg) dissipated 90% in the first five days, and on day 15 the concentration was practically zero. This behavior was similar to that observed by Fan Hou et al. (2016) in cabbage (14 days). The half-life calculated was 5.02 days. This value was similar to that reported by Jankowska et al. (2016) in tomatoes (5.32 days).
Carbendazim (0.313 mg/kg) dissipated 99% of the initial concentration in 15 days. This was similar to what was reported by Bhattacherjee et al. (2009) in mango (15 days). The calculated half-life was 5.79 days, a value lower than that reported by Bhattacherjee et al. (2009), 7 days.
The differences observed with other studies, in part is due to the applied doses, the different types of formulations, the prevailing climatic conditions in each of the regions and the morphological characteristics of the cultivated products. In this sense, several authors have reported that the dissipation of pesticides occurs faster in tropical and subtropical climates than in temperate conditions (Savage and Jordan 1980, Barua et al. 1990). It is worth mentioning that the season in which this study was carried out the temperate climate predominated, with significant increases in temperature in late May, as well as solar radiation and relative humidity presenting high levels, which probably allowed for periods of relatively short dissipation for some of the pesticides.
CONCLUSIONS
The present is the first study to be carried out in Mexico on the level of pesticide residues in fig crops. The most disturbing findings was that residue of some pesticides, which are prohibited and not approved for use on particular fruits and vegetables, such as chlorothalonil, permethrin, and cypermethrin, were found in some samples. The rate of dissipation of the pesticides in the fig crops can be represented by first-order kinetics. The presence of pesticide residues, which are prohibited, represents a latent risk to the health of consumers since there is no control over the quantities used or the frequency with which they are applied. These findings suggest the need for constant monitoring of pesticide residues for long periods, not only in fig crops but also in other vegetables grown in Morelos, as well as the search for biological alternatives for controlling pests in agricultural products.











 text new page (beta)
text new page (beta)