Introduction
In previous demonstrations published predominantly under this general topic (Monković, Petruševski, Bukleski, 2006; Petruševski, Bukleski, Stojanovska, 2007; Petruševski and Bukleski, 2008; Bukleski and Petruševski, 2009, Baloska and Petruševski, 2010) various phenomena were addressed. First, the equilibrium between NO2 and its dimer, N2O4, was demonstrated (Monković et al., 2006). Phenomena of continuous (Petruševski et al., 2007) and discontinuous thermochromism (Petruševski and Bukleski, 2008) were discussed, followed by a synthesis of another so-to-say ‘bithermochromic’ compound, allegedly [(CH3)2NH2]2NiCl4 (Bukleski and Petruševski, 2009). Finally, a novel compound, Ni[HgI4], was synthesized (Baloska and Petruševski, 2010) that also proved to be thermochromic. In all of the above demonstrations, an approach was employed that allows the setup (a glass ampoule) to be prepared once (putting the substance in question in a glass tube that was subsequently sealed) and then performing the experiments unlimited number of times. Finally, an identical approach was used in an experiment to demonstrate melting of iodine (Stojanovska, Petruševski, Šoptrajanov, 2012), where crystalline iodine was put and sealed in a test-tube, to be used ‘ad infinitum’.
The phase transition phenomena are well known and they are part of the physical chemistry courses in the undergraduate studies. The phase diagrams of solid-solid, solid-liquid, liquid-gas and liquid-liquid transitions are studied and designed by students (Atkins and de Paula, 2006). There are also procedures for making diagrams that show the dependence of the critical solution temperature of a binary system on its composition (Senland and Pettitt, 1995). A demonstration of a liquid-liquid phase transition can be made much easier by a method described in this paper.
In binary systems, two critical temperatures can occur. The systems can have upper or lower critical solution temperature of the phase transition, but some of them can have both upper and lower critical solution temperatures [Moore, 1975; Glasstone 1975]. The lower critical solution temperature (tlower) - LCST is the lowest temperature at an exactly specified composition when the binary system exists as two phases. Below this temperature, the two components forming the binary system are fully miscible. The upper critical solution temperature - UCST (tupper) is the highest temperature at which the binary system is not miscible for certain composition of the binary mixture. Above this temperature, the two substances are miscible. An example of a system with LCST is triethylamine and water or isobutyl alcohol and water (Atkins and de Paula, 2006). Systems with UCST are isobutiric acid and water (Senland, 1995; Johnson, 2006) or hexane and nitrobenzene (Atkins and de Paula, 2006). As mentioned previously, there are mixtures that have both upper and lower critical temperatures. The most characteristic example for such mixture is nicotine and water (Glasstone, 1975; Atkins, 2006).
The reason for the existence of UCST is due to the high thermal motion of the particles that form the system. This thermal motion overcomes the potential energy that molecules of the same type have. The thermodynamic interpretation of this phenomenon suggests that the miscibility is correlated with the Gibbs energy and it depends on the temperature of the system. According to the equation:
the miscibility would be possible if the Gibbs energy of mixing has negative value. Intuitively we can conclude that the entropy of the two liquids that mix would have positive value so the product T·ΔS is positive. For the process of mixing to be spontaneous, the value ΔH, should be low enough (lower than the product T·ΔS) so the difference between ΔH and T·ΔS is negative. If the enthalpy of mixing has value that is larger than the product T·ΔS, then the process of mixing would not be spontaneous. In this case, by increasing the temperature T, we contribute the product T·ΔS to become higher than the ΔH value. So the value of ΔG would be negative again and the two liquid phases would be miscible again.
This thermodynamic interpretation can be used only for describing those systems that have UCST. The systems with LCST are different and they show negative deviation from the Raoult’s law. In this case the two liquid components form a weak complex (Atkins and de Paula, 2006) stable at low temperatures, but unstable at temperatures higher than the LCST. When this kind of system is heated, the complex breaks and the components are less miscible.
The behavior of systems formed by two liquids that have both upper and lower critical temperature can be explained using both explanations about the systems with upper and systems with lower critical temperature. After the weak complex is formed, the increase in temperature results in complex disruption leading to partial miscibility. Further temperature increase leads to homogenization as a result of thermal motion so the two liquids become miscible again.
Ideal solutions are solutions that obey Raoult’s law and they tend to mix in all proportions. For these systems the entropy change can be written as (Logan, 1998):
where R is the universal gas constant, and x is the mole fraction. In this case, the enthalpy change is zero, so
transforms to:
The mole fraction is number between 0 and 1, so the natural logarithm from x always has negative value. This means that the Gibbs energy change has negative value and the ideal liquids are fully miscible.
For real systems the enthalpy change is non-zero; therefore the Gibbs energy can have either negative or positive values. For real systems of two components, the change in Gibbs energy can be calculated according to the equation (Logan, 1998):
where A and B are coefficients of proportionality dependent on the nature of the substances.
When mixing two different liquids, the Gibbs free energy will also be dependent on the degree of thermal expansions of the liquids. That is because the two liquids generally have different free volumes (Patterson and Robard, 1978; Patterson, 1972). In the case where liquid polymer is formed when mixing the two liquids, the free volume is small due to the chain connectivity. On the other hand, the pure liquids have greater free volume so the difference is quite large. So two liquids that have different free volumes will undergo mixing contraction and it will result in negative contribution to ΔH and ΔS (Patterson, 1982).
Experimental
Preparation of the ampoules
The first step of this experimental procedure is the preparation of the ampoules. The ampoules are prepared from standard test tubes. For this purpose, the test tube is heated 3 to 4 cm below its opening. During the heating, the test tube is rotated and after the glass becomes soft enough the test tube is being pulled on both sides (Fig. 1a-c). The formed ampoule is left to cool to room temperature. The previously measured volumes of the corresponding two liquids are inserted into the ampoule using a funnel with long and thin neck or by syringe and needle. Afterwards, the ampoule is heated again at its narrow point and then sealed. One should be aware when sealing flammable liquids. To prevent flammable vapors from catching fire during the sealing, the ampoule is first filled with the two liquids and then CO2 is added (e.g. when sealing n-hexane and nitrobenzene). Please see the “Hazards and safety” section below!
Conducing the experiment
The sealed ampoules can be used many times for performing the experiment of liquid-liquid phase transition. This experiment can be set up by placing the ampoule in beakers that are filled with ice-water and boiling water, respectively. Before use, the ampoules are shaken vigorously and are put in the beaker with ice-water for less than a minute.
Results and discussion
Depending on the chosen system, the liquids can either become “homogeneous” or a homogeneous liquid can be separated into two liquids by increasing the temperature. The systems with UCST that were studied are shown in Table 1. Besides the value for the UCST, composition at which this temperature occurs is also given.
Table 1 Systems with UCST. The mole fraction refers to the first component as it is written.
| System | tupper/ °C | x | Reference |
|---|---|---|---|
| cyclohexane – methanol | 49.1 | 0.71 | (Glasstone, 1975) |
| 2-methylbutane – phenol | 51.0 | 0.51 | (Campbell, 1958) |
| carbon disulfide – methanol | 35.0 | 0.11 | (McKelvy and Simpson, 1922) |
| water – phenol | 65.9 | 0.66 | 1922)(Glasstone, 1975) |
| hexane – nitrobenzene | 21.0 | 0.62 | (Atkins, 2006) |
| water – aniline | 167.0 | 0.15 | (Glasstone, 1975) |
| aniline – hexane | 59.6 | 0.45 | (Glasstone, 1975) |
| isobutyric acid – water | 26.7 | 0.11 | (Johnson, 2006) |
All systems with their compositions given in Table 1 are slightly miscible at room temperature, except the system hexane-nitrobenzene which can also be homogeneous depending on the exact value of the room temperature. When the prepared ampoules are well shaken, emulsion forms. After approximately two minutes, two layers can be seen with a distinct interface. Because of the slight solubility of each phase within the other, they are not yet in thermodynamic equilibrium. When placing the ampoules in beaker with ice water, the systems are still heterogeneous, but when placed in hot water they become homogeneous.
The opposite happens in systems with LCST given in Table 2. When the prepared ampoules with this kind of systems are placed in ice water, the existence of only one phase can be noticed, but when placed in a beaker with hot water, the two phases are separated and two layers are formed. Many more examples with LCST for different glycol ethers - water systems are given and investigated by Christensen, Donate, Frank, LaTulip, Wilson, 2005.
Table 2 Systems with LCST. The mole fraction refers to the first component as it is written.
| System | tlower/°C | x | Reference |
|---|---|---|---|
| diethylamine – water | 1.4 | 0.17 | * |
| triethylamine – water | 18.5 | 0.41 | (Campbell, 1969) |
|
Diethylene glycol n-penthyl ether – water |
40.0 | 0.03 | (Christensen et al., 2005) |
*experimentally determined by the authors
In Table 3, systems with both upper and lower critical temperatures are mentioned.
Table 3 Systems with both upper and lower critical temperature. The mole fraction refers to the first component as it is written.
| System | tupper/°C | tlower/°C | x | Reference |
|---|---|---|---|---|
| nicotine – water | 208.0 | 60.8 | 0.57 | (Atkins, 2006) |
| methyl-ethyl ketone – | 133.0 | –6.0 | 0.50 | (Campbell, 1958) |
| water1-methylpiperidine –water | >250.0 | 48.0 | 0.27 | (Glesstone, 1975) |
| 2-methylpiperidine –water | 227.0 | 79.3 | 0.24 | (Flaschner, 1908) |
| 4-methylpiperidine –water | 189.0 | 85.0 | 0.30 | (Flaschner, 1909) |
| 2,4,6-trimethylpyridine–water | 225.0 | 6.0 | 0.33 | (Flaschner, 1909) |
| 3-methylpyridine – water | 152.5 | 49.4 | 0.26 | (Flaschner, 1909) |
| glycerol –m-toluidine | 120.0 | 6.7 | 0.49 | (Dickman, 2002) |
| ethylene glycolen-butyl ether – water | 129.0 | 47.3 | 0.10 | (Christensen et al.,2005) |
Systems with UCST that have been heated in order to become homogeneous, once subjected to cooling, will respond with a phase separation of the two liquids that form the system. The moment when the homogeneous system reaches the critical temperature, the system becomes opalescent (Fig. 2). Soon after the meniscus appears, the two layers are still cloudy. After few minutes, the layers become clear and they both contain dissolved liquid from the other layer. The miscibility of the two liquids can be decreased by cooling the system and opalescence reappears in both layers.
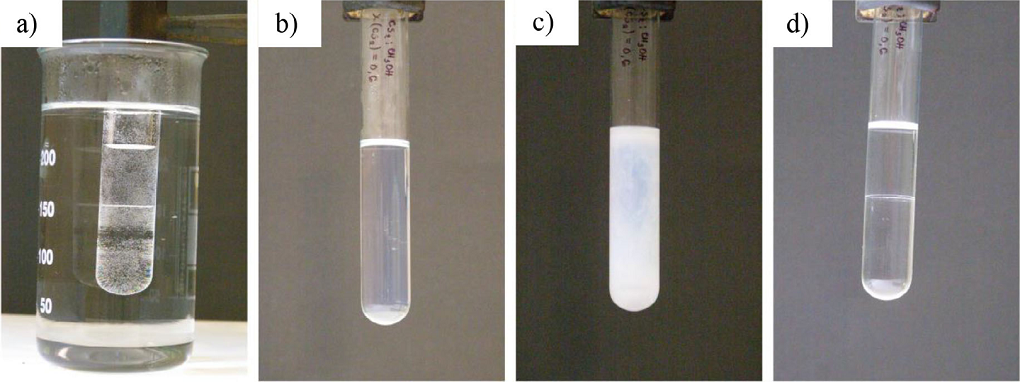
After being heated, (a) the system becomes homogeneous (b) and when it reaches the critical temperature, it becomes opalescent (c). After few minutes, the system separates in two layers (d).
Figure 2 System with UCST, binary mixture of: CS2-CH3OH.
The same thing happens with the systems of LCST. The system is being cooled to form homogeneous layer and after that, it is left at room temperature. As the temperature of the system reaches the critical temperature, opalescence occurs. After a while when two distinct layers are formed, the system is in equilibrium. This equilibrium can be distorted if the system is heated (Fig. 3). Then the miscibility of the liquids decreases and opalescence occurs again.
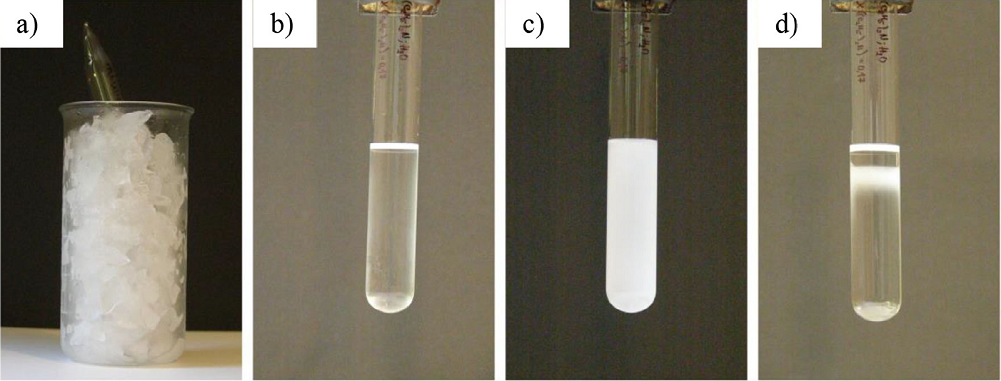
After being cooled, (a) the system becomes homogeneous (b) and when it reaches the critical temperature, it becomes opalescent (c). After few minutes, the system separates in two phases (d).
Figure 3 System with LCST, binary mixture of: (C2H5)3N-H2O.
The opalescence is a result of the light dispersion from the micro droplets formed in the system when the one phase system separates into a two-phase system. The droplets from the two liquids move in opposite direction according to their density. The transition from one phase to a two-phase system is spontaneous.
According to the values of the critical temperatures given in Tables 1-3, it is impossible to reach some of the required temperatures without decomposition of the substances. For that reason, according to the appropriate phase diagrams, we recommend determination of the optimal temperature that leads to the phase transition. For example, when preparing the system carbon disulfide-methanol it is best to take 1.0 mL carbon disulfide and 7.7 mL methanol, that is when x(CS2) = 0.08, and the temperature of the phase transition is at 36.1 °C. According to this, one can conclude that critical solution temperature is depended on the composition of the mixture (Atkins and de Paula, 2006; Christensen et al., 2005). By varying the composition, the critical solution temperature can be lowered or raised, as shown on Figure 4 where the plot representing the dependence of the critical solution temperature on the composition of the mixture for systems having only UCST or LCST or both, are shown.
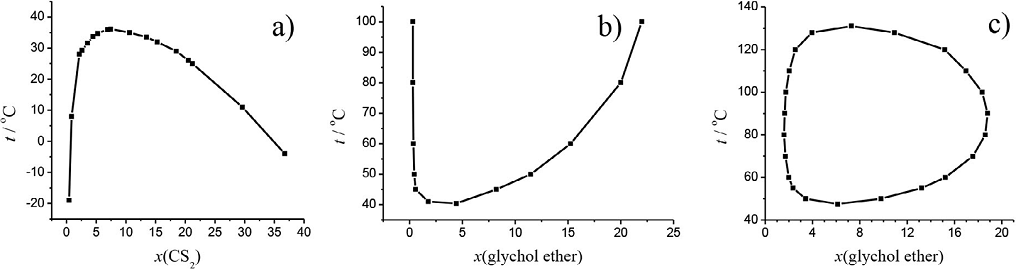
Figure 4 Diagrams representing systems with a) UCST, CS2-CH3OH (McKelvy and Simpson, 1922), b) LCST, diethylene glycol n-penthyl ether-water, c) both upper and lower critical solution temperature, ethylene glycole n-butyl ether-water (Christensen et al., 2005).
It is important to notice that the above-mentioned temperatures (in Tables 1-3) are for normal pressures. In the ampoules, the pressure will be higher so the temperatures may differ slightly from those given in the tables.
Performing the experiments and sealing the liquids for the combinations given in Table 1 (for UCST) is relatively safe and the demonstrations can be conducted only by using hot and cold water. The same applies to the system (C2H5)3N-H2O with LCST (Table 2). In order to demonstrate the phase transitions in the systems with both upper and lower critical temperature, it is necessary to heat the ampoules above 120 °C. To reach temperatures above 100 °C one should use oil bath. The authors do not recommend heating the content of the sealed ampoule above 120 °C since the pressure build-up inside of the ampoule can lead to explosion. However, if the content of the ampoule is heated to about 120 °C prior to sealing, the ampoules can be heated up to 190 °C without any danger of explosion. However, this is only recommended for experienced and good experimenters. The positive side of having the content of the systems with high value of the UCST sealed in an ampule is that by increasing the pressure, the UCST of the system drops (Glasstone, 1975). This means that the phase transition could be achieved at lower temperatures than those given in Tables 1-3.
Taking into consideration the pedagogical aspect of performing these demonstrations, it would be much better if one could find a way (substance) to color only one of the two layers in the combination of two colorless liquids (Fig. 5). This way the visibility would be better and the separation of the layers could be more visible especially when the audience is greater. Finding the adequate substance (colorant) in this case is not an easy job.
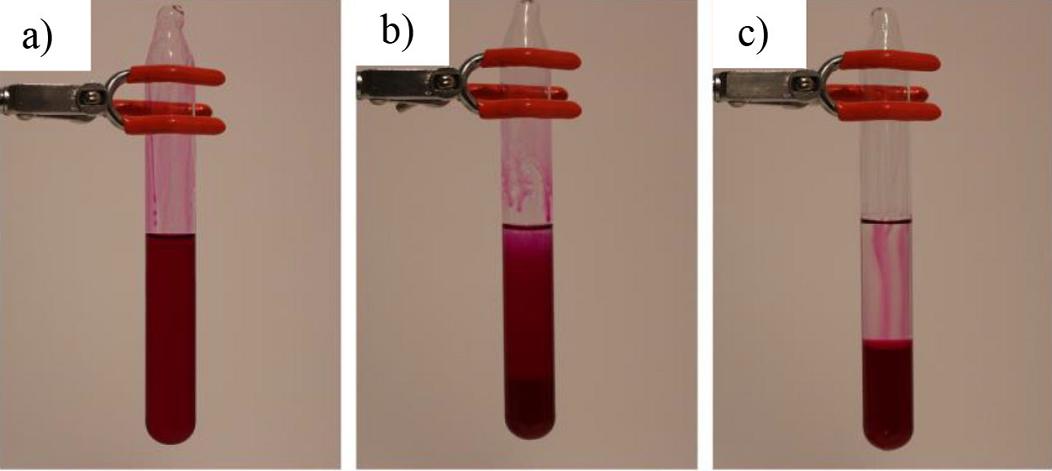
a) after the system is cooled at 4.3 °C it becomes homogeneous; b) when left at room temperature two layers start to form; c) after few minutes the separation is (more or less) complete.
Figure 5 It was determined that phenolphthalein is an excellent colorant for the system (C2H5)3N-H2O since it colors only the water layer while the organic layer stays colorless after the system reaches the critical temperature:
Phenolphthalein is the substance used for coloration of the system triethylamine - water. The solubility of the indicator in water at 25 °C is 400 mg/L (Yalkowsky, He, Jain, 2010), the solubility of triethylamine in water at 25 °C is 68.6 g/L (Yalkowsky et. al., 2010), and phenolphthalein is seemingly unsoluble in triethylamine at room temperature (no data available). Based on the given solubility it is expected that triethylamine will not be colored even though it is basic. The water itself will not be colored based on both the solubility and the pH value. However, when triethylamine dissolves in water, the pH of the water increases and the phenolphthalein transits to its anionic form that is water-soluble and turns the water layer pink. This is the reason why phenolphthalein can be used for coloration of this system.
Conclusion
This paper shows a way of conducing demonstration experiments for the phenomenon of liquid-liquid phase transitions and it makes the experiment simple as well as economic. The ampoules with the liquids are prepared once and they can be used unlimited number of times. Since the phenomenon of liquid-liquid phase transition is part of the undergraduate curriculum, the described way of performing the experiment can be used as a lecture demonstration. The setup enables the lecturer to demonstrate the phenomenon quite easy, fast and with no additional preparation. Also, there is no waste after the performed demonstration. Besides the economic aspect, there is also the positive environmental impact. Since many of the chemicals used to demonstrate the phase transitions are hazardous to not only human but also to the environment (CS-2, ketones, pyridines, piperidines, phenol, nicotine…), the described way of demonstration is very convenient to protect the environment and the experimenter as well. The ampoules are sealed so the hazardous vapors do not endanger the experimenter. In addition, after the demonstration, the ampoules are kept and stored for another use and no hazardous chemical waste is produced by the experiment itself.
This paper not only does describe safe and economic way of performing liquid-liquid phase transition experiment, but it also gives more systematic overview of dozen combination of different substances that show transitions at different temperatures from simple substances to more specific ones. It also gives a simple explanation of the observations behind this phenomenon.
The demonstration of the liquid-liquid phase transitions for the systems with higher UCST is the only drawback of the proposed method of performing this kind of economic demonstrations.
Hazards and safety
Sealing the ampoules can be dangerous since some of the chemicals used are highly flammable. For that reason, protective glasses must be worn and the sealing must be done in a hood with the ventilation system on. It is highly recommended that the narrow part of the ampoule is dry prior to sealing. The best way to avoid wetting the narrow part is by placing the liquids in the ampoules by syringe and needle. Great concern must be taken when sealing the ampoules with nitrobenzene and hexane. When sealing the ampoule with this system and systems that contain derivates of piperidine, the test tube is filled with carbon dioxide generated from Kipp apparatus for obtaining inert atmosphere. Some of the chemicals used are also toxic. Nicotine is very toxic and dangerous for the environment. 2,4,6-trimethylpiridine is flammable and harmful upon inhalation, in contact with skin and if swallowed, it is irritating to the eyes, the respiratory system and the skin. All derivates of piperidine are highly flammable and irritant to the skin and eyes.
The best way (as explained above) to prepare the ampoules that need to be heated over 60 °C is by heating the system to the required temperature and then sealing it. When the ampoule is prepared in this way, high pressure does not build up inside. It is not recommended to heat the glass ampoules above 120 °C.











 text new page (beta)
text new page (beta)



