INTRODUCTION
Differences in growth potential between species are essential in selecting tree species for commercial plantations and ecological restoration; however, in order to select the appropriate species for a geographically and ecologically complex and highly biodiverse country like Mexico, it is very important to know why a tree species has more growth potential and capabilities to adapt to predicted climate scenarios that other species in a given environment. This requires a more detailed approach in the study of growth dynamics, e.g. growth rate, shoot elongation rates and timing and duration of the growing period, as well as a possible association to climatic variables (Rehfeldt, 1988).
Climate change represents a challenge to match genotypes to new environments (Ledig and Kitzmiller, 1992; Sáenz-Romero et al., 2016), since the appropriate climatic habitat will become a “moving target” (Harris et al., 2006). Mexico will have an increase in the mean annual temperature of 2.3 °C and a decrease in precipitation of 9 % by the year 2060 compared to the average of 1961-1990 (Sáenz-Romero et al., 2010). This will cause mismatches between the populations of forest species and the climate to which they are adapted, inducing a gradual decay in populations, particularly in the lower limits of their natural altitudinal distribution (Allen et al., 2010; Breshears et al., 2005; Jump et al., 2006; Peñuelas et al., 2007; Rehfeldt et al., 2009). This phenomenon will cause a decline in forest cover and a decrease in productivity in Mexico (López-Toledo et al., 2017; Sáenz-Romero et al., 2020).
The forest of the Nuevo San Juan Parangaricutiro (NSJP) indigenous community, state of Michoacan, Mexico is dominated by P. pseudostrobus Lindl., P. leiophylla Schiede ex Schltdl. & Cham. and P. devoniana Lindl. (Medina et al., 2000) distributed in a sequential and overlapping altitudinal range, from the high altitude populations of P. pseudostrobus (upper limit at 2900 masl) to the lower altitude populations of P. devoniana at 1900 masl inside the NSJP forest (Castellanos-Acuña et al., 2015), but the species can be found as low as 1650 m of altitude outside the community (Sáenz-Romero et al., 2012a). These three species are of great importance in the region due to their wide distribution, high quality of wood and resin production; however, P. pseudostrobus is by far the most abundant in the NSJP region, and the most valuable economically for its faster growth rate, straight stem form and wood quality (López-Upton, 2002).
Estimations of the impacts of climatic change on the NSJP forest indicate that suitable climatic habitat of P. pseudostrobus will move to higher altitude due to climate change, leaving low-lying populations in the rear exposed to an unsuitable warmer and drier climate (Sáenz-Romero et al., 2012b), at what is called the “xeric limit” (Mátyás, 2010). Forest declination (widespread tree defoliation followed by pest attacks leading to sudden tree death) has recently been observed in populations of P. pseudostrobus in their lower altitudinal limit of the NSJP forest, specifically on sites with shallow soils, which induces more drought stress during the dry season (López-Toledo et al., 2017; Sáenz, 2015). Thus, such low-altitude populations might need to be replaced, with human assistance, by P. devoniana, the next species distributed at lower altitudes, occupying drier sites than P. pseudostrobus (Castellanos-Acuña et al., 2015; Gómez-Pineda et al., 2020).
Pinus devoniana is distributed in drier and warmer sites than P. pseudostrobus (Gómez-Pineda et al., 2020), so it is likely that the first specie has more drought resistance. It seems that P. devoniana is an alternative to replace P. pseudostrobus at the xeric limit. We asked the question: What characteristics could make P. devoniana more drought resistant than P. pseudostrobus? Therefore, the aim of the present study was to inspect the growth dynamics of Pinus pseudostrobus, P. devoniana and P. leiophylla shoots, and relate it to contemporary and future climate projections of the sites where they distribute, in order to understand why those species could occupy overlapping but differentiated altitudinal range distributions along the mountains of the Mexican Neovolcanic Belt, and then use that knowledge to design management plans that reduce the risk of forest decline under climate change scenarios.
MATERIALS AND METHODS
Seed collection
Cones were collected from P. devoniana (two populations), P. leiophylla (three populations) and P. pseudostrobus (two populations) along an altitudinal transect, from 2110 (mean natural distribution of P. devoniana) to 2520 masl (middle part of the altitudinal distribution of P. pseudostrobus and close to the upper limit of P. leiophylla in NSJP) (Table 1). All sites had the same exposure (Southeast). At each site, five cones with open-pollinated seed were obtained from each of the 11 trees of each species. Trees were randomly selected from those with mature cones spaced 50 m apart to reduce relatedness. Hereafter, population is a group of trees represented by the samples, and the location of each population is the provenance.
Table 1 Collection sites of cones from three species of pines in the region of Nuevo San Juan Parangaricutiro, State of Michoacan, Mexico.
| Site | Latitude N | Longitude W | Altitude (masl) | Species (Pinus) |
| 1 | 19°27’34.4” | 102°11’43.6” | 2520 | P. pseudostrobus |
| 3 | 19°26’24.6” | 102°10’29.6” | 2310 | P. pseudostrobus, P. leiophylla, P. devoniana |
| 4 | 19°26’05.1” | 102°10’08.3” | 2217 | P. leiophylla |
| 5 | 19°25’42.1” | 102°09’34.6” | 2110 | P. leiophylla, P. devoniana |
Experimental design
Seeds were removed from the cone and germinated in a growth chamber (Lumina ICP-18, Mexico) at a constant temperature (25 °C), alternating light and darkness in periods of 12 h. The germinated seeds were transplanted into 20-cm long rigid plastic containers of 350 cm3 (Beaver Plastics®, Mexico), containing a mixture of peat moss, perlite and vermiculite (2:1:1) and a slow release fertilizer (Osmocote, Scotts, USA), 1 L m-3 of mixed substrate.
At eight months of age, the plants were established in a raised rectangular wooden bed filled with a 40 cm layer of a 4:1 mixture of Andosol forest soil and Creciroot® commercial substrate. The mixture was placed on a 20 cm layer of small extrusive volcanic stones to improve drainage. The raised nursery bed was built inside a shade house (35 % shade) in the facilities of the Instituto de Investigaciones sobre los Recursos Naturales, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Michoacan, Mexico (19° 41’ 23.02’’ LN, 101° 15’ 0.87’’ LW; altitude 1900 masl).
A randomized complete blocks experimental design with four replications was used. In each block three species were placed in large plots and within them the provenances in small plots of five seedlings in a row. Seedlings were spaced at 17 × 17 cm. The first and the last plots were flanked by a protective row of randomly chosen seedlings. Seedlings were watered as needed to measure the full expression of the seedlings growth under favorable environmental conditions, but little irrigation was required during the rainy season from June to October 2012.
Data recording and statistical analyses
Starting in January 2012 (12 months of age) and until the end of the year (23 months of age), plant height measurements were made every 15 days. Height measurements were made in great detail, to the millimeter, using an aluminum bar as a support for the rule in order to avoid variation in the substrate surface. A modified logistic growth function was fitted for each individual seedling height using PROC NLIN of SAS Ver. 9.4 (SAS Institute, 2014):
where Yi is the observation on the i th seedling (total height); β0, β1 and β2 are regression parameters and X is the measurement date (Julian day).
Regression parameters (β0, β1 and β2) were used to estimate a growth curve of predicted values for each individual seedling, using the following model:
where Pi is the predicted growth (total height) for the i th seedling; β0, β1 and β2 are regression parameters, X is the measurement date (Julian day) and Z is the total elongation (mm).
From these regression models, it was possible to obtain several growth variables for use in analyses of genetic variation, providing the ability to compare the timing of the shoot elongation between species, regardless of the amount of total shoot elongation during the growing season, which was expected to be very different between species. The growth variables were: total elongation, the difference between the final and the initial seedling height (TElongation); day during the growing season on which 2 mm of height growth had occurred for each seedling, i.e., the start of growth period in Julian days (Start); day on which all the growth has occurred, except the last 2mm, i.e., the end or cessation of growing period in Julian days (End); the elongation rate between 20 and 80 % of the total elongation, i.e., the maximum growth rate (Rate); and the number of days between the start and the end of growth, the duration of growth (Duration).
At 30 months of age (July 2013), the plants were harvested for assessment of aerial biomass. This consisted in the separation of stem, branches and needles and oven drying (Model FE-290, Felisa Technologies, Wyckoff, New Jersey, USA) for 72 hours at a temperature of 75 °C. Later, they were weighed on an analytical balance (Scout, Model RS232, Parsippany, New Jersey, USA), and the proportions of each part in relation to the total biomass were calculated.
All growth and biomass variables were subjected to analysis of variance to test for differences between species and between populations within species using PROC GLM of SAS (SAS Institute, 2014). Ratio of variance component to total variance was estimated by using PROC VARCOMP METHOD = REML (SAS Institute, 2014). These analyses used the following statistical model:
where Y ijkl is the observation on the l th seedling of the k th population of the j th species in the i th block, µ is the overall mean, B i is the effect of the i th block, S j is the effect of the j th species, P k (S j ) is the effect of the k th population nested into the j th species; B i × S j is the interaction of blocks by species, B i × P k (S j ) is the interaction of blocks by populations nested into species, and ε ijkl is the error term; i = 1, ... b, j = 1, ... s, and k = 1, ... t and l = 1, … n, where b = 4, s = 3, t = 2 for P. devoniana and P. pseudostrobus and t = 3 for P. leiophylla; n = 5, which are the number of species, blocks, populations, and seedlings-per-plot, respectively.
Estimation of climatic variables
Mean monthly temperature and precipitation, monthly degree days (> 5 °C), a monthly aridity index (ratio of monthly degree days to monthly precipitation), and length of the frost-free period were estimated for all provenances for the contemporary climate (period 1961-1990). Climate estimations were obtained from spline climate surfaces fitted to monthly average temperatures (mean, maximum and minimum) and monthly precipitation from numerous meteorological stations (Sáenz-Romero et al., 2010); point estimations are available at http://charcoal.cnre.vt.edu/climate/.
In order to visualize how climate change will affect the growth dynamics of these species, climate projections were made for the year 2060 using an ensemble of General Circulation Models (GCMs) for the rcp60 scenario, which projects greenhouse-effect gas concentrations on medium to high pathways (van Vuuren et al., 2011). Using only one GCM implies some uncertainty in the projections for the future, since not all GCMs use the same weight for climate variables; however, this can be corrected by using an ensemble of these, which in this case calculates the mean of 17 GCMs, and has been shown to work better for modeling with plants (Fordham et al., 2012).
RESULTS
Differences between species
Statistically significant differences were found between species for total elongation, end, duration, rate and plant height (Table 2). Pinus pseudostrobus and P. leiophylla showed greater elongation, longer period of elongation, and later growth cessation, compared to P. devoniana (Figure 1). The growth dynamics as absolute values of growth, and also as a proportion of the total growth is shown in Figures 2 and 3, respectively. It is shown that at the second year of age, P. pseudostrobus grew about 25 cm, P. leiophylla 23 cm and P. devoniana only 8 cm (Figure 2).
Table 2 P-values of the analysis of variance for growth traits related to shoot elongation from a species/provenance common nursery tests of Pinus pseudostrobus, P. leiophylla and P. devoniana.
| Source of Variation | Degrees of freedom | Total elongation | Start | End | Duration | Rate | Height |
| Species | 2 | 0.0062 | 0.1148 | 0.0361 | 0.0547 | 0.0444 | 0.0003 |
| Block | 3 | 0.1701 | 0.6017 | 0.0727 | 0.0995 | 0.4583 | 0.2549 |
| Population/Species | 4 | 0.1811 | 0.8959 | 0.4053 | 0.6023 | 0.3653 | 0.0138 |
| Species × Block | 6 | 0.0095 | 0.0689 | 0.4285 | 0.3591 | 0.2087 | 0.0054 |
| Pop/Species × Block | 12 | 0.4323 | 0.4456 | 0.8443 | 0.7641 | 0.1121 | 0.7999 |
| Error | 110 |
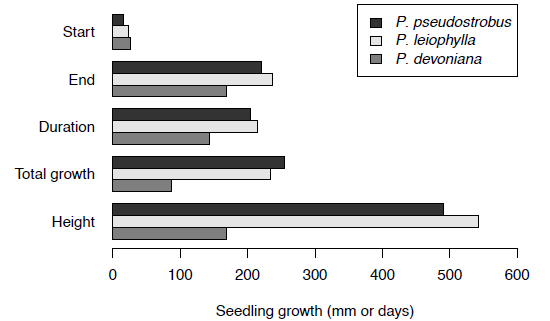
Figure 1 Mean by species of date of growth start (“Start”, Julian day), date of growth end (“End”, Julian day), duration of shoot growth (“Duration”, days), total elongation (“T. Elongation”, mm) and final height (“Height”, mm) of Pinus pseudostrobus, P. leiophylla and P. devoniana.

Figure 2 Logistic growth model expressed as absolute growth of means per species (second year of growth) in P. pseudostrobus, P. leiophylla and P. devoniana.
For biomass distribution, significant differences (P ≤ 0.05) were found between species for branch dry weight and also for biomass allocation to branches, needles and stem as a percentage of total biomass. Pinus leiophylla was the species that allocated the most biomass to branches (16 %), while P. devoniana allocated very little (2.8 %). In contrast, P. devoniana was the species that allocated the most biomass to stem (42.3 %) and needles (54.8 %), while P. leiophylla (stem 38 %, needles 46 %) and P. pseudostrobus (stem 36.1 %, needles 49.9 %) had similar percentages of allocation in these traits.
Differences between populations within species
When performing the ANOVA by species, significant differences were found between populations only in P. devoniana for needles biomass (P = 0.0306) and total biomass (P = 0.0341), and nearly significant for stem biomass (P = 0.0569) and plant height (P = 0.0527). The population originated at an altitude of 2310 masl, showed a larger growth and biomass; however, no differences were found in biomass allocation percentages between this population and the one from 2110 m of altitude, which shows that the architecture of these populations is similar.
Relationship with climatic variables and potential impacts of climatic change
The onset of growth, recognized as shoot elongation on the second year after germination for P. devoniana occurs mainly after the dry season ends, in late May (Julian day 150; Figure 3), as the dry season in the area occurs mainly from November to May. The onset of shoot elongation for P. pseudostrobus and P. leiophylla occurs in early March and April, as soon as the frost season ends (45-50 Julian days; left arrows in Figure 3). Considering the climate of 1961-1990, P. pseudostrobus and P. leiophylla are exposed to a less arid climate during the dry season (the value of the monthly aridity index reaches a maximum value of 1.60 and 1.8, respectively) than P. devoniana with a monthly aridity index with a peak reaching 1.86 (larger values of the aridity index mean a warmer and drier climate), because the latter species is distributed at lower altitudes.
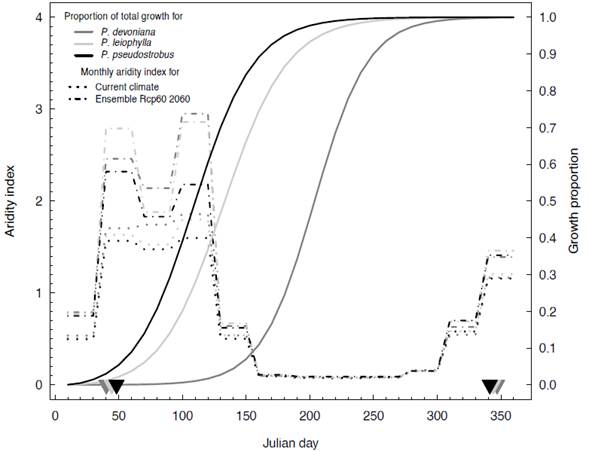
Figure 3 Logistic growth model (solid lines), expressed as a proportion of total growth (averaged per species; second year of growth) and monthly aridity index (broken lines; color corresponds to their respective species) of contemporary (average of period 1961-1990; dotted lines: . . . .) and future climate (decade center in year 2060; dotted and dashed broken lines: . - . - . -), for Pinus devoniana (dark gray), P. leiophylla (light gray) and P. pseudostrobus (solid black). Arrows (bottom triangles) mark the Julian day when the frost season under contemporary climate ends (February; notice on a day earlier for P. devoniana, late for P. pseudostrobus, intermediate for P. leiophylla) and starts (December; notice the opposite sequence: on a day late for P. devoniana, earlier for P. pseudostrobus, intermediate for P. leiophylla). Larger values of monthly aridity index mean warmer and drier climate than lower values. Climate values were averaged per species based on their altitudinal distribution in the Nuevo San Juan Parangaricutiro forest, Michoacan, Mexico.
Projected climate estimates centered on the year 2060 indicate a substantial increase in aridity during the dry season for all three species (Figure 3). The increase in aridity is notable when P. pseudostrobus and P. leiophylla would be starting the elongation of their shoots at this sensitive stage (Figure 3).
DISCUSSION
Pinus pseudostrobus and P. leiophylla exhibited during the second year of growth approximately three times more growth in plant height (Total elongation) than P. devoniana. This is an indicator that P. devoniana exhibits a moderate grass stage during the second year of age. It is referred to as “moderate” because 8.0 cm is too much for a full grass stage (for example, P. devoniana has been reported to elongate shoots by 2.5-2.6 cm in 11-month-old plants, for the best provenance (Musálem and Sánchez-Cruz, 2003), but too few for a normal growth pattern. The higer growth potential of P. pseudostrobus and P. leiophylla was also shown on the previous nursery stage (Castellanos-Acuña et al., 2013).
The very small biomass allocation to branches and larger allocation to stem and foliage in P. devoniana, compared to other two species, is another characteristic that confirms its grass-stage type seedling architecture.
The growth patterns of these three species seem to be genetically related to the timing of the dry season and the frost-free period, but under different adaptive strategies. Pinus devoniana seems to delay its growth start until after the dry season ends, while P. pseudostrobus and P. leiophylla start their growth just after the end of the frost period, which occurs much earlier in the year than the end of the dry season. This does not necessarily mean that P. pseudostrobus or P. leiophylla are more resistant to drought stress than P. devoniana, because the first two species are found at higher altitudes (cooler and moister sites) than P. devoniana (Gómez-Pineda et al., 2020; Perry, 1993; Sáenz-Romero et al., 2012a; 2015;), as confirmed by the values of the annual aridity index; thus, the difference in shoot elongation time suggests that, at least for the second year of growth, it is an adaptation to avoid drought stress in the case of P. devoniana, and probably an adaptation to tolerate frost damage in the case of P. pseudostrobus and P. leiophylla because of the differential altitudinal distribution of the species. This also supports the idea that the grass stage of P. devoniana is an adaptative strategy against harsh conditions (Keeley and Zedler, 1998; Koskela et al., 1995; 1999), likely conferring an adaptive advantage during the critical stage of seedling establishment.
The delayed start of the growth strategy of P. devoniana might imply an adaptive advantage in projected future climates, because it likely confers the ability to avoid dry season drought stress to a greater extent during the young seedlings stage, a critical period for natural regeneration seedling recruitment (Guzmán-Aguilar et al., 2020). In contrast, P. pseudostrobus and P. leiophylla, which appear to be more adapted to resist frost damage rather than drought damage, could experience a more stressful condition during shoot elongation in future climates. It has been showed that by changing P. pseudostrobus provenances to altitudes higher than their origin, seedlings showed no signs of frost damage (Gómez-Pineda et al., 2021).
Assisted migration is needed to sites that will become drier, transferring species more tolerant to drought stress. In this case, P. leiophylla and P. pseudostrobus would need to migrate to sites at higher altitud, which will have aridity index values that match those that currently occur at the contemporary natural distribution (Sáenz-Romero et al., 2015; Gómez-Pineda et al., 2020); then, the sites left empty at lower elevations would be suitable for P. devoniana.
Such a proposed change in management, that assumes that P. devoniana is more resistant to drought compared to P. leiophylla and P. pseudostrobus, should ideally be preceded by experiments comparing the growth ability of these three species under formal drought stress treatments, preferably in the field, or at least in growth chambers. Reciprocal altitudinal common nursery trials have been conducted in the field (Castellanos-Acuña et al., 2015), but emphazing the shift of species and populations to higher altitudes to test the feasibility of assisted migration to higher altitudes as an option for adaptation to climate change; in addition, more experimentation is also needed with an emphasis on the shift of population and species to lower altitudes, as a way to visualize the effect of higher temperatures and lower precipitation, as an indicator of projected future climates, as was done recently for Abies religiosa (Cruzado-Vargas et al., 2021). Furthermore, a broader representation of the natural distribution of each species will be highly desirable by including more provenances in the field experiments, especially including those from the extremes of the natural distribution.
CONCLUSIONS
Differences between species were found for total elongation, end of growth, duration of growth, rate of growth and plant height. Pinus pseudostrobus and P. leiophylla showed greater elongation, longer period of elongation, later cessation of growth and greater plant height than P. devoniana. The growth patterns of these species were significantly related to climatic variables, mainly the monthly aridity index and the duration of the frost season. For P. devoniana, it appears that while in a grass stage, the start of growth occurs only after the dry season ends (late May). Pinus pseudostrobus and P. leiophylla begin their growth as soon as the frost season ends (end of February), in the middle of the dry season. Pinus pseudostrobus and P. leiophylla would be under drought stress during shoot elongation if climate changed to a warmer and drier season from March to May. Pinus devoniana may be planted instead those two pines at their lower altitudinal limits.











 text new page (beta)
text new page (beta)


