Introduction
Pseudo-cereal quinoa (Chenopodium quinoa Willd.) is an ancient indigenous plant native to the Andean region, it is considered as one of the oldest crops from the Americas (Fischer et al., 2013). This annual herbaceous plant is reported as resistant to different abiotic stresses such as cold temperatures, drought and salinity (Fischer et al., 2017). Worldwide, there are approximately 250 species of Chenopodium (Sezgin and Sanlier, 2019). Its large genetic variability contributes to wide adaptation and growth under adverse environmental conditions such as drought, hail, frost and high altitude (Abderrahim et al., 2015). At present, quinoa is widely cultivated in Peru, Bolivia, Chile, Ecuador, Colombia and Argentina; furthermore, its high adaptability allows the small-scale cultivation, which is also found in Europe (Bhargava et al., 2006).
Quinoa seeds are the main edible part of the plant, varying in color from white to black, although they are commonly light yellow (Sezgin and Sanlier, 2019); they constitute an excellent raw material for healthy and tasty foods, being an excellent example of ‘functional food’. The content of fiber, essential amino acids, fatty acids, minerals, vitamins, antioxidants, and especially phytochemicals content in quinoa, provide this grain a great advantage over other crops in terms of health care (Nowak et al., 2016).
Among its phytochemicals, phenolics stand out for being mainly responsible for antioxidant and anti-inflammatory properties of quinoa (Abderrahim et al., 2015). Another feature is the presence of saponins, which are glycoside compounds with pharmacological properties (Nickel et al., 2016). Although saponins impart a bitter taste to the grain, they have a variety of biological effects including antifungal, antiviral, anticancer, hypocholesterolemic, hypoglycemic, antithrombotic, diuretic, and anti-inflammatory activities (Vega-Galvez et al., 2010).
The nutritional composition of quinoa seeds and their bioactive compounds may differ between varieties (Abderrahim et al., 2015), in this sense, despite the fact that quinoa is an ancient crop, its available technical information is limited in terms of its functional and chemical composition, which is also influenced by the different environments.
Currently, in Mexico, interest in quinoa cultivation is increasing, and it is gradually being cultivated and commercialized in different regions of the country; however, there is little information on the basic nutritional content and antioxidant properties of quinoa grown in Mexico. For this reason, the present study aims to characterize nutritional value and antioxidant properties of seeds of different commercial varieties of quinoa from Peru: Yellow, Black, Red and an organic Yellow quinoa, to compare them with the Yellow quinoa produced in the state of Aguascalientes, México.
Materials and methods
Raw material
For this study, the traditional Chilean Biobio Yellow Quinoa (AYQ) variety was grown in a 6600 m2 plot located at the municipality of Asientos, state of Aguascalientes, Mexico (22° 06’ 50.94 “N - 102° 05’ 56.31” W, at an altitude of 2,024 masl). Soil texture is sandy loam, pH 8, with low organic matter content and high salinity. The crop was drip-irrigated once a week. In addition, four commercial Peruvian quinoa samples: Red quinoa (RQ), Yellow quinoa (YQ), Black quinoa (BQ), and an organic Yellow quinoa (OYQ) were purchased in a local supermarket in Zacatecas, Mexico.
Sample preparation
Seeds were ground in a blender (Blenda BL-200, China) for 30 seconds and stored at room temperature in hermetic glass jars until analysis.
Proximal characterization
Proximal analyses (water content, protein, ash, fiber and fat) were performed on samples through the methods proposed by the Association of Official Analytical Chemists (AOAC, 1990). A factor of 6.25 was used to estimate protein content from nitrogen content. Total carbohydrate content was estimated by difference. All results were expressed in g 100 g-1 on a dry basis (db).
Saponin detection
A frothing test was used for the qualitative evaluation of saponins following the procedure described by Tandon et al. (2011), in which water extracts (1 g of quinoa/20 mL of H2O) were obtained by boiling for 15 min in a water bath (Lab Companion, Billerica, Massachusetts, USA). The extract was cooled and filtered through Whatman 1 cellulose filter; then, 2 mL of extract were transferred into a test tube and shaken vigorously by hand, after which it was left to stand for 10 min and the result was noted. A thick persistent froth indicated the presence of saponins.
Phenolics extraction
Phenolic compounds were extracted (Tomás-Barberán et al., 2001) by homogenizing 5 g of quinoa sample for 10 min with 20 mL of methanol; 5 mL of HCl 6 N and 2 mg of NaF were added to inactivate polyphenol oxidases and prevent phenolic degradation. After extraction, the mixture was centrifuged (3095 × g; 4 °C) for 10 min (Sigma 3-16KL, Germany). The supernatant was stored for 24 hours in opaque vials at 4 °C until analyzed. Extractions were replicated thrice.
Determination of total phenolic content
Total phenolic content (TPC) was quantified using the Folin-Ciocalteu test (Li et al., 2006); briefly, 250 μL of extract were mixed with 15 mL deionized water and 1.25 mL of Folin-Ciocalteu phenol reagent (Sigma-Aldrich, St. Louis, Missouri, USA). After 5 min at room temperature, 3.75 mL of Na2CO3 7.5 % were added and leveled to 25 mL with deionized water. Sample absorbance was measured at 765 nm in a spectrophotometer UV-Vis Thermo Scientifc 10S (Thermo Fisher Scientifc Inc, Waltham, Massachusetts, USA). Results were reported as mg of gallic acid equivalents (GAE)/100 g of db.
Antioxidant capacity
The same extract obtained for TPC quantification was used to evaluate the antioxidant capacity (AC). Total antioxidant potentials are best determined by a combination of methods, since individually or collectively, phytochemicals contribute to the overall antioxidant capacity in different ways (Fisher et al., 2013). In this study, the AC was determined by the ABTS•+, DPPH and FRAP methodologies as follows.
ABTS•+ radical scavenging ability
The AC was determined through a modification of the spectrophotometric technique (Re et al., 1999), using the ABTS•+ radical (Sigma-Aldrich, St. Louis, Missouri, USA) generated by 2.45 mM K2S2O8. The mixture was kept in the dark at room temperature (~20 °C) for 16 h before use, and then the ABTS•+ solution was diluted to give an absorbance of ca. 0.7 ± 0.01 at 734 nm. Afterwards, 100 μL of extract were mixed with 900 μL of the ABTS•+ diluted solution and, after a 2.5 min reaction at 20 °C, the absorbance was measured at 734 nm. The results were expressed as μmol equivalents of Trolox (TEAC)/100 g of db.
DPPH radical scavenging activity
A sligh modification to the method described by Brand-Williams et al. (1995) was used to analyze the AC of samples: 100 μL of quinoa extract were added to 1 mL of 0.0076 mM 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Sigma-Aldrich, St. Louis, Missouri, USA). Free radicals scavenging activity using the free radical reaction DPPH was evaluated by measuring the absorbance at 515 nm, after a 2.5 min reaction at 20 °C, in a spectrophotometer UV-Vis Thermo Scientifc 10S (Thermo Fisher Scientifc Inc, Waltham, Massachusetts, USA). Results were expressed as TEAC/100g of db.
FRAP (ferric reducing antioxidant power)
The FRAP assay followed a procedure previously described (Benzie and Strain, 1996). Briefly, the FRAP reagent was prepared fresh on a daily basis with 2.5 mL of sodium acetate buffer (300 mmol L-1, pH 3.6), 2.5 mL of 10 mmol L-1 TPTZ [2,4,6-Tris(2-pyridyl)-s-triazine] (Sigma-Aldrich, St. Louis, Missouri, USA) solution (40 mmol L-1 HCl as solvent) and 25 mL of 20 mmol L-1 Fe (III) and warmed to 37 °C in a water bath prior to use; 100 μL of the sample was added to 1 mL of the FRAP reagent. Mixture absorbance was measured at 593 nm after 30 min. Results were expressed as TEAC/100g db.
Statistical analysis
All the analyses were carried out by triplicate and results were expressed as mean ± standard deviation. A one-way ANOVA was carried out to determine statistical significances and, if significant, a Tukey test was applied (P ≤ 0.05). Furthermore, a regression analysis between the antioxidant activity and total phenol content was carried out. All the statistical analyses were performed using Statsgraphics® Centurion XV (Statpoint Technologies Inc., Warrenton, Virginia, USA).
Results and discussion
Proximal composition
The nutritional characterization of the quinoa samples is shown in Table 1. Considering the obtained values, quinoa samples were characterized by high carbohydrate contents, followed by proteins, lipids and fibers. In general, statistically significant differences were found (P ≤ 0.05) in the proximal analysis. In detail, it was observed that water content ranged from 10.5 g/100 g db in BQ to 8.7 g/100 g db in the Peruvian Yellow organic sample. On the other hand, lipid content varied between 5.2 and 9.6 g/100 g db, RQ showing the highest (P ≤ 0.05) fat concentration. Protein content was found to be between 13.4 and 16.4 g/100 g db. In regard to the yellow varieties, quinoa grown in Mexico (AYQ) stood out for its greatest protein content compared to the other yellow varieties. On top of its high protein content, quinoa proteins are considered as high quality due to its balanced pattern of essential amino acids (Vilcacundo and Hernández-Ledesma, 2017). As far as fiber content, significant differences were found (P ≤ 0.05). The highest values were found in BQ and YQ (8.8 and 8.5 g/100 g db, respectively). Regarding ashes, values ranged from 2.18 to 2.5 g/100 g db, although no significant differences (P > 0.05) were found among varieties. Carbohydrate content fluctuated between 58.8 and 65.22 g/100 g db, being the Peruvian Yellow organic sample the one presenting the highest (P ≤ 0.05) concentration.
Table 1 Mean values and standard deviations in the different nutritional variables analyzed according to the variety, expressed as g/100 g dry sample (n = 3).
| Component | OYQ | RQ | BQ | YQ | AYQ |
| Water content | 8.7 (0.01) a | 10.1 (0.01) c | 10.5 (0.1) e | 9.3 (0.1) b | 10.3 (0.1) d |
| Lipids | 5.9 (0.6) a | 9.6 (1.3) b | 5.2 (0.3) a | 6.0 (0.19) a | 5.7 (1.18) a |
| Protein | 14.0 (1.2) ab | 15.5 (0.1) bc | 14.2 (0.4) ab | 13.4 (0.1) a | 16.4 (0.5) c |
| Fiber | 3.1 (0.07) a | 3.3 (0.6) a | 8.8 (0.1) b | 8.5 (0.3) b | 3.3 (0.5) a |
| Ash | 2.2 (0.05) a | 2.3 (0.01) a | 2.4 (0.01) a | 2.3 (0.04) a | 2.5 (0.28) a |
| Carbohydrate | 65.22 (1.01) c | 59.2 (0.8) a | 58.8 (0.7) a | 60.4 (0.2) ab | 61.7 (0.4) b |
Means followed by the same letter in the same row are not statistically different (Tukey, P ≤ 0.05). OYQ: Organic yellow Quinoa, RQ: Red Quinoa, BQ: Black Quinoa, YQ: Yellow Quinoa, AYQ: Chilean Biobio Yellow Quinoa grown in Mexico.
In general, the chemical values determined in this study differed as a function of the genotype and growing conditions of quinoa seeds. According to Nowak et al. (2016), fluctuations or differences in nutritional value can be explained not only by different quinoa varieties, or different geographical conditions, but also by different agronomic factors, such as mineral concentration in the growing soil, fertilizer application and other environmental conditions. Previous reports showed that protein and carbohydrate contents were significantly different between different colored quinoa varieties (Red, White and Black) (Pereira et al., 2019); however, some of the commercial samples could probably have been washed out, causing the outer endosperm to be removed or damaged, losing some proteins to water (Fisher et al., 2017). Regarding the fiber content, in addition to the genetics of quinoa, there are different causes that could affect its composition, such as growing locations (Miranda et al., 2011) or processing (Repo-Carrasco-Valencia and Serna, 2011).
There is a very small difference in water content between our data as compared to other studies published elsewhere. Chirinos et al. (2013) reported a 6.2 % water content in Peruvian Quinoa, Kowalski et al. (2016) 8.39 % in quinoa var. Cherry Vanilla (produced in Albion Washington, USA), Agza et al. (2018) 9.5 % in Quinoa cv. Titicaca, Miranda et al. (2011) reported a range of 7.74 to 15.18 % in six ecotypes of Chilean Quinoa (Ancovinto, Cancosa, Cahuil, Faro, Regalona and Villarica), while Pellegrini et al. (2018) reporteded a range of 5.27 to 8.24 %(White Spanish quinoa, White Peruivian quinoa, Red and Black Bolivian Real quinoa, and two different brands of white Bolivian Real quinoa and two different brands of white Bolivian Real quinoa).
It is known that quinoa is rich in macronutrients such as proteins, carbohydrates and good-quality lipids. Lipid content coincided with those reported by Kowalski et al. (2016) of 6.96 %, while Agza et al. (2018) reported 6.3 %, Navruz-Varli and Sanlier (2016) 6.07 % and Li and Zhu (2017) a range of 3.2 to 6.93 % in seven types of commercial quinoa seeds collected from Peru, Bolivia and China; Miranda et al. (2011) reported 5.88 to 7.15 % and Pellegrini et al. (2018) from 4.87 to 6.48 % lipids. In regard to fat composition, other studies highlight the lipid profile of quinoa, indicating that monounsaturated fatty acids represent the highest concentration (~ 40 %), followed by polyunsaturated fatty acids (30 %), and saturated fatty acids (27-29 %) (Pereira et al., 2019). In this sense, different epidemiological studies support that consumption of foods rich in polyunsaturated fatty acids such as quinoa, results in the prevention of many diseases, including cardiovascular disease, cancer, and autoimmune diseases (Zhang et al., 2016). Moreover, quinoa has higher lipid yield compared to cereal grains, including wheat (2.0 %), rice (1.9 %), millet (2.9 %), maize (3.9 %) and sorghum (3.3 %) (Charalampopoulos et al., 2002).
As for protein, data were also very similar to 12.39 %, reported by Kowalski et al. (2016), while Agza et al. (2018) reported 13.57 %, Navruz-Varli and Sanlier (2016) 14 %, Li and Zhu (2017) 11.7 to 13.7 %, Miranda et al. (2011) 11.32 to 16.1%, while Pellegrini et al. (2018) found a range of 11.62 to 13.66 % protein. The nutritional value of quinoa, its protein and, hence, amino acid richness is probably its main attraction for consumers. The total protein content of quinoa is higher than that of rice, barley, maize, rye and sorghum, and it is close to that of wheat (Navruz-Varli and Sanlier, 2016). The proteins in quinoa are mainly composed of albumins (35 %) and globulins (37 %), they also contain low concentrations of prolamins, with likely variations of these percentages between species (Abugoch, 2009). Quinoa provides a protein value similar to casein in milk (Vega-Gálvez et al., 2010) and higher content than that of cereals such as wheat, rice and maize (Tang et al., 2015). It is particularly rich in histidine and lysine, which are deficient in most cereals and it is also gluten-free, thus offering a variety of nutritious and suitable food products for consumers with food allergies such as celiac disease (Tang and Tsao, 2017). All the aforementioned gives quinoa the reputation of one of the best sources of plant protein.
In regard to fiber content, some differences were found when comparing the quinoa fiber content of this study with the composition reported by other authors for the same pseudo-cereal. Results were lower than the 18.98 % reported by Kowalski et al. (2016). They coincided, however, with Agza et al. (2018) who reported 3.00 %, Li and Zhu (2017) 7.7 to 15 %, and Miranda et al. (2011) 1.33 to 2.81 %. It is worth mentioning that in the present study, BLQ showed the highest fiber content (11.3 %). The total fiber in quinoa is about 10 %, which is higher than that of other grains and lower than legumes. This might be especially relevant for subjects with celiac disease because of their risk of suffering from fiber deficiency in their diet compared to people on a normal diet (Noratto et al., 2019).
For the percentage of ashes, the results coincide with those reported by Navruz-Varli and Sanlier (2016) of 2.7 %, Kowalski et al. (2016) 1.88 %, Agza et al. (2018) 2.43 %, Li and Zhu (2017) 1.77 % to 2.75 %, Miranda et al. (2011) 3.15 to 3.65 % and Pellegrini et al. (2018) 1.74 to 2.63 %. Ashes are related to mineral content, quinoa stands out for its potassium content; it also stands out for its higher content of calcium, magnesium, iron, copper, zinc than other cereals (Repo-Carrasco et al., 2003).
Regarding the carbohydrate content, values were comparable to those obtained by Miranda et al. (2011) with mean values of 56.7 to 68.3 %, Navruz-Varli and Sanlier (2016) with 64.1 %, and Agza et al. (2018) with 65.17 %, but lowest than those reported by Pereira et al. (2019) of 75.3 to 77 %. The main carbohydrate component of quinoa is starch (52-69 %), it also contains sugars (3 %), mostly maltose, D-galactose, D-ribose and low levels of fructose and glucose (Abugoch, 2009).
Saponins
The external layers of quinoa seeds also contain a class of compounds known as saponins, which have an intensely bitter flavor not desirable by consumers. Among the samples analyzed, only AYQ tested positive in the qualitative analysis of this component. Saponins are triterpenoid glucoside compounds present in many plant genera; most saponins have an intensely bitter flavour and all are potentially toxic if ingested in large quantities. The amount of saponins is highly variable between different quinoa varieties and, in accordance with the saponin concentration, quinoa varieties are distinguished in: “sweet quinoa” containing < 0.11 % of saponins and “bitter quinoa” containing > 0.11 % of saponins (Gómez-Caravaca et al., 2014). Saponins are traditionally removed by washing grains in running water or alkaline water, although other processing methods have been devised (Kowalski et al., 2016). Since saponins negatively affect the taste and digestibility of quinoa seeds, they should be removed before consumption. Regardless of their unpleasant taste, saponins have a variety of biological effects including antifungal, antiviral, anticancer, hypocholesterolemic, hypoglycemic, antithrombotic, diuretic and anti-inflammatory activities (Vega-Galvez et al., 2010). Yao et al. (2014) mentioned that saponin-rich quinoa seed extracts reduced inflammation by mediating the production of nitric oxide and inhibiting the oscillation of TNF-a and IL-6 inflammatory cytokines (Navruz-Varli and Sanlier, 2016).
Total phenolic content
In addition to macro and micronutrients, quinoa seeds provide a balanced combination of hydrophilic (e.g. phenolics, betacyanins) and lipophilic (phytosterols, carotenoids) compounds (Noratto et al., 2019), which may aid to reduce the risk of suffering from chronic diseases related to obesity. Among them, phenolic compounds are the main group of bioactive phytochemicals investigated in quinoa (Tang et al., 2015). As Figure 1 shows, the AYQ sample stood out for its TPC (242.9 mg of GAE/100g db), while the Yellow Peruvian samples OYQ and YQ, showed the lowest TPC (around 52 mg of GAE/100g db). Phenolics are compounds of a hydrophilic nature, located mainly in the seed coat, functioning as a chemical defense against insects and microorganisms (Multari et al. 2018). Phenolic compounds are mostly beneficial to health because of their antioxidant activity. As such, they are capable of scavenging free radicals, chelating metal catalysts, activating antioxidant enzymes and inhibiting oxidases (Heim et al., 2002). The quantity and quality of polyphenols in fruits and vegetables can also vary significantly according to different intrinsic and extrinsic factors, such as soil composition, growing conditions, stage of maturity and postharvest conditions, among others (Jeffery et al., 2003).
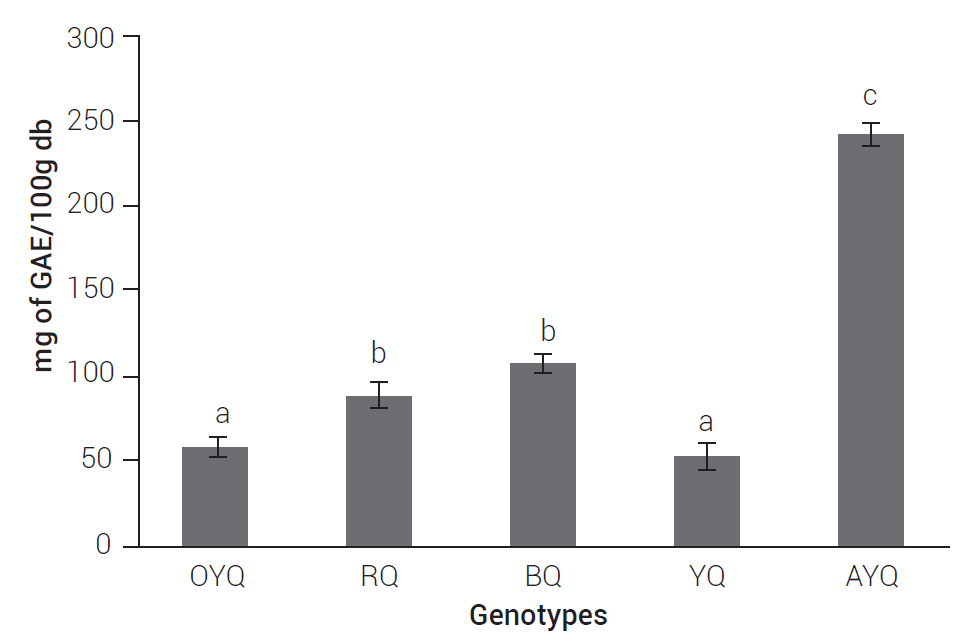
Figure 1 Mean values of total phenolic content expressed as mg of gallic acid equivalents (GAE)/ 100 g dry sample (db). Bars showing the same letters are not significantly different (Tukey, P ≤ 0.05). OYQ: Organic Yellow Quinoa, RQ: Red Quinoa, BQ: Black Quinoa, YQ: Yellow Quinoa, AYQ: Chilean Biobío Yellow Quinoa grown in Mexico.
Recent studies on quinoa seeds led to the identification of 23 phenolic compounds in free or conjugated forms, most of which were phenolic acids consisting of vanillic acid, ferulic acid and their derivatives, along with flavonoids quercetin, kaempferol and their glycosides (Tang and Tsao, 2017).
Chirinos et al. (2013) reported similar total phenolic values (103 mg of GAE/100g of db); however, much lower than those obtained by Kowalski et al. (2016) who found 20.6 mg/100g in quinoa flour. Dini et al. (2010) indicated that pre-cooked bitter quinoa seeds contain a larger amount of phenolics (864 mg of GAE/100g) than sweet quinoas (772 mg of GAE/100g), values that are much higher than those obtained in the present study. Miranda et al. (2011) reported 14.22 to 65.53 mg of GAE/100 g, and Pasko et al. (2009) reported values of 375 mg of GAE/100g in Bolivian quinoa seeds. The results obtained in BQ are similar than those obtained by Chirinos et al. (2013), who reported 130 mg of GAE/100g. Our TPC results are similar than those of common cereals, such as wheat (56 mg of GAE/100g), barley (88 mg of GAE/100g), millet (139 mg of GAE/100g) and rye (103 mg of GAE/100g), but lower than sorghum (413 mg of GAE/100g) (Ragaee et al., 2006).
Antioxidant capacity
The AC results were dependent on the method used, as Table 2 shows. Values between 41.8 and 109.2 μmol of TEAC/100g were obtained by ABTS·+ analysis, and these are lower than those obtained through the DPPH and FRAP methods. When comparing within the same method, the statistical analysis showed significant differences between varieties. The highest AC values were obtained in the AYQ samples when evaluated by the FRAP method (916.3 μmol of TEAC/100g).
Table 2 Antioxidant capacities of five quinoa genotypes as determined by three analytical methods, expressed as μmol equivalents of Trolox (TEAC)/100 g dry sample (n=3).
| Genotypes | ABTS·+ | DPPH | FRAP |
| OYQ | 61.4 (5.8) b | 149.6 (5.6) b | 320.1 (16.4) a |
| RQ | 109.2 (5.5) c | 147.7 (4.3) b | 549.8 (12.2) c |
| BQ | 95.7 (1.3) c | 148.6 (5.1) b | 580.1 (8.6) bc |
| YQ | 46.6 (7.8) ab | 155.3 (1.9) b | 395.2 (28.01) ab |
| AYQ | 41.8 (5.2) a | 121.6 (0.6) a | 916.3 (57.6) d |
Means followed by the same letter in the columns are not statistically different (Tukey; P ≤ 0.05). OYQ: Organic Yellow Quinoa, RQ: Red Quinoa, BQ: Black Quinoa, YQ: Yellow Quinoa, AYQ: Chilean Biobio Yellow Quinoa grown in Mexico.
Furthermore, some saponins may also exhibit antioxidant activity, capturing free radicals from many aqueous and hydrophobic phases, or indirectly stimulating antioxidant enzymatic systems and inhibiting the formation of complexes between free radicals and metal ions (Ribeiro et al., 2013). In this sense, saponins detected in AYQ could be responsible for the difference found in the antioxidant activity of this sample compared to the others, specifically when determined by the FRAP method. Saponins have been widely used as natural products for clinical drug development due to their various pharmacological properties, such as immunomodulatory, anti-oxidative, antiapoptotic, anti-diabetic, neuroprotective, and anti-cancer activity (Dong et al., 2019). Milling process is currently used to remove the saponin-inflicted bitter taste, thus, improving quinoa sensory attributes; however, causing a concomitant reduction in phenolics and their antioxidant activity (Han et al., 2019). Other studies also showed that bitter quinoa seeds had antioxidant capacity higher than that of quinoa seeds without saponins (Dini et al., 2010). Interestingly, the same authors found consistently higher FRAP values of bitter quinoa seeds than DPPH values. The probable reason for the lower DPPH values of bitter quinoa seeds could be due to the presence of compounds not reactive towards the DPPH free radical.
The antioxidant capacity of plant samples may be influenced by many factors, such as extraction solvent and test system, and cannot be fully described by one single method (Deng et al., 2012). The TEAC assay is based on the ability of the antioxidant to scavenge ABTS•+ and can measure antioxidant capacities of hydrophilic and lipophilic compounds in the same sample (Re et al., 1999). The FRAP assay is based on the antioxidant capacity to reduce ferric (III) to ferrous (II) ions (Benzie and Strain, 1996); it is a simple, inexpensive and reproducible method for the evaluation of antioxidant capacity (Li et al., 2008). The AC values found in this study were lower than those reported by Chirinos et al. (2013) who obtained 830 μmol of TEAC/100g with the ABTS•+ method and 530 μmol of TEAC/100g with the DPPH technique, while Pasko et al. (2009) reported ABTS•+ 2719 μmol/100g, DPPH 3884 μmol/100g. On the other hand, the AC quantification using the FRAP method showed the highest values, coinciding with that described by Dini et al. (2010), who found 830 μmol of TEAC/100g by the FRAP method. Antioxidant compounds, such as polyphenols, may be more efficient reducing agents for ferric iron, but some may not scavenge DPPH free radical so efficiently, due to steric hindrance. Genotype plays an important role in the antioxidant content in quinoa seeds, being the primary factor contributing to variation in antioxidant capacity of fruits and vegetables, as previously demonstrated (Fisher et al., 2013). Also, when comparing the antioxidant activity reported by other authors it is important to consider that samples must be analyzed under similar conditions (type of solvent, time of reaction, and forms of expressing the values), because such conditions affect valid comparison with values reported elsewhere.
Relationship between bioactive compounds and the antioxidant activity of quinoa samples
There is some controversy about the influence of the bioactive compounds present in fruits and vegetables on their antioxidant capacity. Chemical interactions that affect free radical scavenging properties between phytochemicals have not been extensively reported in vegetables, but both synergistic and antagonistic interactions can influence antioxidant capacity (Guo et al. 2003). The main classes of bioactive compounds found in quinoa seeds are carotenoids, vitamin C, saponins and polyphenolic compounds (phenolic acids, flavonoids, lignans, stilbenes, tannins) (Fischer et al., 2017). Regarding phenolic compounds, many authors have attributed their antioxidant properties to their relative abundance in plant tissues (Fares et al., 2010). In order to relate the bioactive compounds analyzed in this study and the antioxidant activity of quinoa samples, a regression analysis was performed. There were high determination coefficients observed between TPC and the AC determined by DPPH (R2 = 0.8932) and FRAP (R2 = 0.8698) essays; on the contrary, a very low determination coefficient was found (R2 = 0.0928) between TPC and the ABTS method. A high determination coefficient between antioxidant capacity and total phenolic content indicated that phenolic compounds could be the main contributor to the antioxidant capacities of quinoa.
On the other hand, there are several plausible explanations for the ambiguous relationship between the antioxidant activity and total phenolics: 1) the presence of other substances that act as antioxidants, such as ascorbic acid and carotenoids, 2) the synergism between antioxidants in the food matrix made antioxidant activity not only dependent on the antioxidant concentration, but also on the structure and interactions among antioxidants, that is why samples with similar concentrations of total phenolics may vary markedly in their antioxidant activity, 3) different methods used for measuring antioxidant activity based on different mechanisms may lead to different observations (Sun and Ho, 2005). Other studies also found higher determination coefficients between the total antioxidant activity of quinoa seeds and their total phenolics (R2 = 0.98), than that of carotenoid (R2 = 0.84) or ascorbate content (R2 = 0.55), indicating that the phenolic compounds are the major responsible for quinoa antioxidant properties (Dini et al., 2010).
Conclusion
The present study showed that the chemical composition of quinoa seeds differs according to genotype. Regarding the proximal composition; in general, samples presented high carbohydrate content, followed by that of proteins, lipids and fibers. Quinoa grown in Mexico stood out for its high total phenolic content, as well as for its antioxidant activity, although only the one quantified by the FRAP method, probably influenced by saponins. Overall, the quinoa samples demonstrated significant potential as a source of nutritional and functional components, as well as a potential ingredient to formulate nutritious and healthy foods.











 text new page (beta)
text new page (beta)


