Introduction
The potato/tomato psyllid (Bactericera cockerelli) is a pest of great economic importance of solanaceous crops worldwide and causes severe damage to these crops in Mexico, USA, Belize, Honduras and New Zealand (Page-Weir et al., 2011). This species attacks 20 botanical families (Liefting et al., 2009) and can complete its biological cycle on 40 plant species (Butler and Trumble, 2012). It constitutes a severe limitation to the production of pepper (Capsicum annuum L.), potato (Solanum tuberosum L.), tomato (Solanum lycopersicum L.) and husk tomato (Physalis philadelphica Lam.) (Garzón-Tiznado et al., 2009). Nymphs and adults feed on the buds producing toxemia. Symptoms include leaves yellowing, stunted plant growth and reduced size of fruit or tubers (Gharalari et al., 2009; Sengoda et al., 2010). B. cockerelli also transmits the bacteria Candidatus Liberibacter solanacearum, causing the zebra chip symptom (ZC) (Nachappa et al., 2011).
This disease was firstly detected in 1994 on potato crops from Saltillo, Coahuila, Mexico (Munyaneza et al., 2008), where yellowing foliage and necrosis of the tubers were observed (Muñoz et al., 2016). Also, potato tubers from infected plants develop a pattern of necrotic streaks after being fried (Gharalari et al., 2009; Munyaneza et al., 2008), thus, causing problems for the industry. In tomato, husk tomato and chili pepper it causes leaf curl, reduces growth rate and fruit weight loss (Brown et al., 2010; Page-Weir et al., 2011). If the attack is severe, the affected plant dies (Sengoda et al., 2010). The eggplant (Solanum melongena L.) can be a secondary B. cockerelli host, since it is used for feeding (Yang and Liu, 2009).
Habanero pepper is a vegetable of economic importance, demanded by the international and national market not only as food but also as a source of natural dyes and phitochemical compounds such as capsaicinoids (Chan et al., 2011). The market of this compound is increasing since capsaicin is widely used in medicine, cosmetics, paints and as tear gas (López-Gómez et al., 2017). The main pest of the habanero pepper is B. cockerelli (Sulc). This insect pest may cause 100 % loss of yield (Melgoza et al., 2018).
The use of tolerant crops and biological control against this insect pest is of limited efficacy (Butler and Trumble, 2012). Rojas et al. (2015) documented the existence in Mexico of the Tamarixia triozae parasitoid (Burks) (Hymenoptera: Eulophidae), affecting up to 85 % nymphs; however, Luna-Cruz et al. (2011) indicated that this parasitoid is highly susceptible to commonly used insecticides such as imidacloprid, abamectin, and spinosad, thus preventing their integrated use.
In 2005 and 2008, Bayer CropScience introduced the insecticides spiromesifen and spirotetramat, both tetronic acids, a group that act by inhibiting the synthesis of acetyl-CoA carboxylase, thus affecting the biosynthesis of lipids (Bielza et al., 2019; Nauen and Konanz, 2005). Spiromesifen is classified as a contact insecticide (Brück et al., 2009), although Kontsedalov et al. (2009) found some translaminar and limited systemic action when applied to the soil or the stem. Spirotetramat has bidirectional systemic action (Nauen et al., 2008), it translocates through both the xylem and phloem (Brück et al., 2009), controlling pests that feed on treated and untreated areas of the plant (Elizondo and Murguido, 2010; Tucuch-Haas et al., 2010). Both compounds exhibit toxicity against eggs and nymphs of the susceptible pests (Elizondo and Murguido, 2010). Additionally, treated females significantly decrease their fecundity and fertility (Nauen et al., 2008; Tucuch-Haas et al., 2010) and do not harm T. triozae (Liu et al., 2012). These insecticides were introduced into Mexican agriculture in 2005 as an alternative for controlling B. cockerelli; although they are effective against this pest, their impact on oviposition behavior is not well understood; thus, this research aimed to determine the effect of localized treatments of spirotetramat and spiromesifen on the behavior of B. cockerelli to lay eggs on treated and untreated foliage of habanero chili pepper.
Materials and methods
Insects and host
A population of B. cockerelli susceptible to insecticides, previously reproduced for about 103 generations (28 ± 3 °C , 50 ± 4% relative humidity, 10:14 h light:darkness), free of insecticide exposure, was used. The use of insecticide-susceptible populations avoids any behavioral interference due to a developed capacity to live and reproduce on environments contaminated by toxic compounds. Plants of habanero chili pepper were used as a host of this pest.
Insecticides
Commercial formulations were used. Oberon® 240 SC (spiromesifen, emulsifiable concentrate, 240 g ai L-1) and Movento® OD (spirotetramat, dispersible oil, 150 g ai L-1). For each insecticide, the dose of 1 mL of commercial product L-1 was used with distilled water as a diluent.
Plants
Habanero chili pepper plants cv. Criollo Naranja, 30 ± 3 cm in height, were used. This plant condition was reached at about 50 to 55 d after germination.
Application of insecticides
Before application, the foliage of the plant was divided into three strata: upper (21 to 30 cm distal), middle (11 to 20 cm below the upper stratum) and lower (1 to 10 cm proximal). A waxed thread delimited each stratum. The untreated strata were covered using a plastic bag (30 × 20 cm) secured with a rubber band. After that, the plant was placed in an acrylic container to be treated using a Potter Tower calibrated to apply 2 mg cm-2, as suggested by Hassan (1985). Thirty mL of the corresponding insecticide was used and applied at a pressure of 0.703 kg cm-2. One hour after the application, the protective plastic bags of untreated strata were removed, and the plants were introduced into entomological cages (70 × 70 × 100 cm); then, infested with 20 pairs of 20 to 24 h-old adults and allow them to lay eggs for 7 d, as suggested by Vega-Gutiérrez et al. (2008). The control was handled in the same way, except that it was treated with 30 mL of distilled water. After 7 days, the adults were removed from the plants and the number of eggs per leave on each of the three strata were counted. With this information, the percentage of eggs was calculated.
Experimental design and unit
The experimental design was a completely randomized blocks with seven treatments, including an untreated control. The experimental unit was a single plant and each treatment had 100 replications (100 plants), carried out on consecutive days.
Statistical analysis
Analysis of variance was performed using the SAS procedure (SAS Institute, 2012) and means were compared with Tukey test (P ≤ 0.05). To achieve homoscedasticity and normality, the response variable was transformed to log10 (x+1). The statistical significance of the studied factors was estimated using the F test (P ≤ 0.05) (Gomez and Gomez, 1984).
Results and discussion
Control
Eggs of B. cockerelli were observed in all strata of the plant (Figure 1). The percentage of eggs on the upper, medium and lower strata was 42, 32 and 16 %, respectively, with significant differences among them (P ≤ 0.05). Liu (2004) and Yang and Liu (2009) indicated that this species prefers to lay eggs on the top part or new growths of the plant. Abdullah (2008) and Garzón-Tiznado et al. (2009) argued that females of B. cockerelli colonize the apical parts of the plant because it provides nutritional advantages to offspring. Liu and Trumble (2006) indicated that females prefer to lay eggs between the first and fourth apical leaves of the chili pepper plant. The fourth and fifth instar nymphs are found at the bottom of the plant or on the undersides of the leaves, which is a severe constraint in the use of insecticides to control this pest (Gaskin et al., 2010; Liu, 2004).

Figure 1 Average number of B. cockerelli eggs per stratum of habanero pepper Capsicum chinense (Jacq) plant. The value in parenthesis represents the respective percentage, and the vertical lines indicate the standard error of the respective average. Lowercase letters indicate statistical significance (Tukey, P ≤ 0.05).
Knowledge about the mobility of insecticides within the habanero pepper provides an important basic awareness to consider about their rational use in field conditions. A systemic insecticide (which moves inside the plant) is useful to combat pests in crops whose plants are growing, because new shoots that were not contacted by the application are protected from the attack by the target insect pest; otherwise, insecticides should be used when the plant has completed its growth.
Spiromesifen
In all cases, the stratum with the lower number of eggs was the treated one (P ≤ 0.05) (Figure 2). The average number of eggs was significantly lower in the treated stratum in comparison to the untreated control (Figure 3). Gharalari et al. (2009) observed that when treated the upper part of potato (Solanum tuberosum L.) leaf with spiromesifen, the individuals of B. cockerelli living underside were not affected, indicating that this insecticide has contact activity. Also, Chauhan et al. (2018) and Luna-Cruz et al. (2015) argued that spiromesifen has contact activity, has no movement in the plant and is of low persistence; however, spiromesifen has proven effective in reducing Raoiella indica Hirst populations by more than 95 % at 7 d after application (Correa-Méndez et al., 2018).

Figure 2 Average number of B. cockerelli eggs per stratum of Capsicum chinense (Jacq) plant after 7 d of treating the respective stratum. Lower case letters indicate statistical significance (Tukey, P ≤ 0.05).
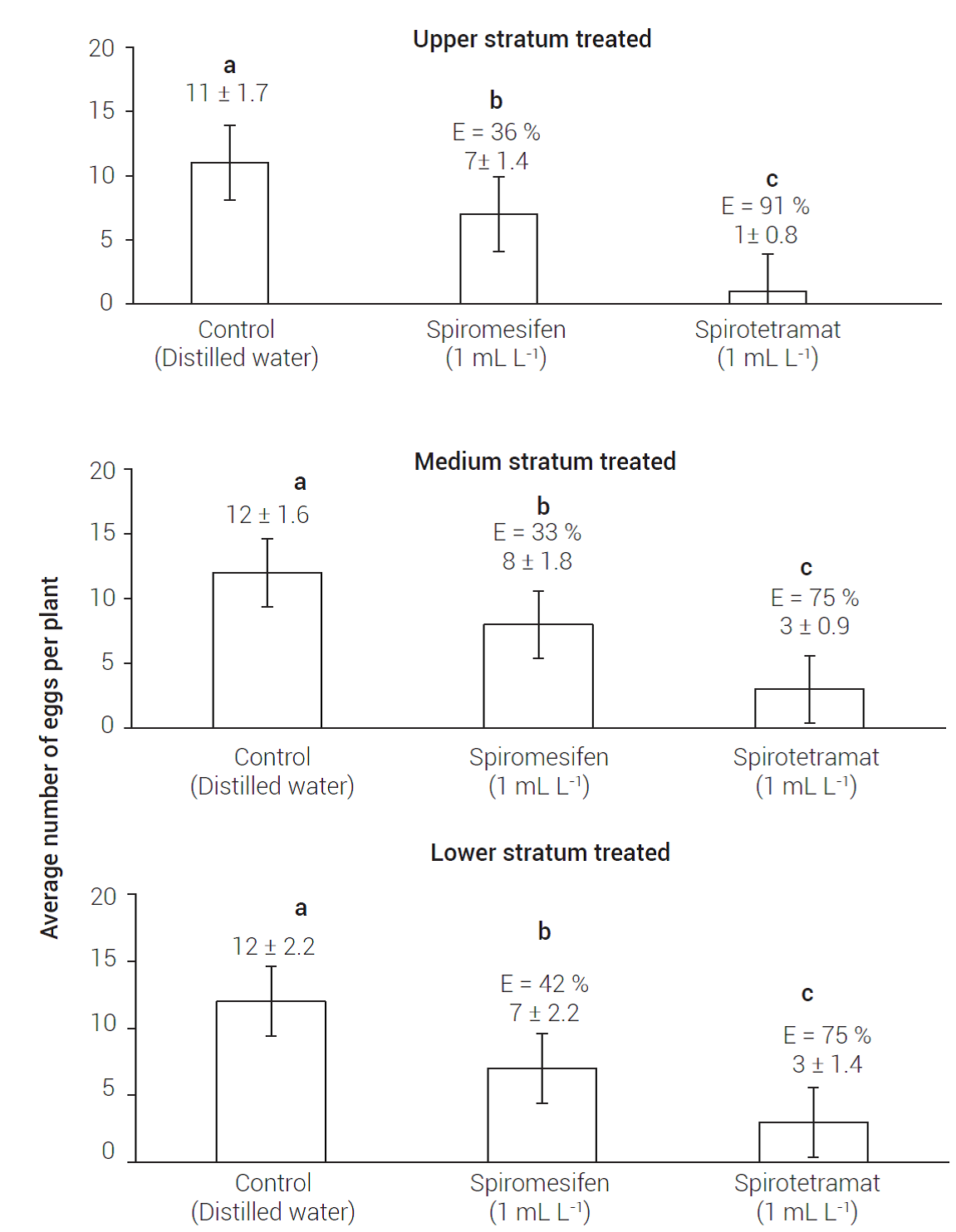
Figure 3 Average number of B. cockerelli eggs laid per plant of chili pepper [Capsicum chinense (Jacq)] and the respective treated stratum. The vertical lines indicate the standard error of the respective average. E: percentage by which control exceeds treatment. Lowercase letters indicate statistical significance (Tukey, P ≤ 0.05).
Liu (2004) documented that spiromesifen is more toxic to adults than to eggs in whitefly feeding on melon (Cucumis melo L.) and cabbage (Brassica oleracea L.) plants; moreover, the nymphs that emerged from eggs treated with spiromesifen were unable to molt successfully. Tucuch-Haas et al. (2010) found similar results when treated B. cockerelli with spiromesifen. The decrease in oviposition rate caused by spiromesifen has been, then, commonly found. The length of the bioactivity varies based on the dose used, but the effectiveness of the decrease of oviposition rate has been recorded for 15 days after treatment (Jamieson et al., 2010).
Spirotetramat
Regardless of the treated stratum, the average number of eggs decreased in all strata. No significant difference was observed in the number of eggs among strata (P > 0.05). Inhibition of oviposition was observed in both treated and in untreated strata, regardless of whether the untreated area was above or below the treated one (Figure 3). Once the eggs were laid, the level of mortality was less than 3 % on treated as well as on untreated parts of the plants. In consequence, the reduced number of eggs on all strata was not attributed to egg mortality caused by spirotretramat, but to an inhibitory activity on the oviposition of B. cockerelli. These findings (Figures 2 and 3) are in agreement with those of Page-Weir et al. (2011), who observed that spirotetramat was highly effective in reducing egg rate (P ≤ 0.05).
There is no scientific information about the effect of spiromesifen and spirotetramat on the oviposition of B. cockerelli in habanero pepper; however, the rate of 0.3 and 0.5 L ha-1 of commercial formulation of spirotetramat can reduce the oviposition rate of various species of plant pests, such as Bemisia tabaci (Gennadius), Thrips palmi (Karny), Tetranychus urticae (Koch) and Myzus persicae (Sulzer) (Elizondo and Murguido, 2010; Marcic et al., 2012). Spirotetramat has been used successfully to reduce the fecundity of B. tabaci in horticultural crops (Nauen et al., 2008) as well as that of Orchamoplatus citri (Jamieson et al., 2010). Also, Brück et al. (2009) found that aphids reduced their fertility when plants were treated with spirotetramat. Similarly, Marcic et al. (2012) documented that T. urticae females treated with 200 mg L-1 of spirotetramat reduced their gross fertility (43.7-93.3 %) and their net fertility (73.8-98.5 %) with respect to the control (distilled water).
The eggs laid by females of B. cockerelli, M. persicae, B. tabaci, and T. palmi treated with spirotetramat were unable to complete their biological cycle (Brück et al., 2009; Elizondo and Murguido, 2010; Fiaz et al., 2018; Liu, 2004; Page-Weir et al., 2011; Tucuch-Haas et al., 2010). Taking together, results of this study suggest that field applications of spirotetramat would be feasible to protect against susceptible pests when the plant is growing, and the application of spiromesifen when the plant has completed its growth.
Conclusions
The rate of inhibition of oviposition by B. cockerelli was higher in habanero pepper plants treated with spirotetramat compared to those treated with spiromesifen. The spirotetramat significantly reduced the oviposition rate of B. cockerelli in the treated and untreated strata of the habanero pepper, regardless of wheter the untreated area is above or below the treated one.











 text new page (beta)
text new page (beta)


