Introduction
Many plant species naturally produce compounds that exert therapeutic action for humans, so they have been called “medicinal plants” since antiquity. It is estimated that around 35,000 species that are used for medicinal purposes have been classified worldwide (Ghulam et al., 2017), and the World Health Organization (WHO) calculates that approximately 80% of the population around the world uses medicinal plants for the treatment of diseases (Rivera-Mondragón et al., 2017).
The total floristic wealth of Mexico is constituted of 23,314 species, placing it in the fourth place in the world (Villaseñor, 2016). Of this total, it has been reported that around 3000 species of angiosperm plants (approximately 15% of the total flora) have medicinal properties, i.e., one in seven species possesses some curative features (Ocegueda et al., 2005; Villaseñor, 2016). For these reasons, scientific attention has now been diverted to ethnomedicine, since in the last years an increase in demand for natural products from medicinal herbs products has developed, since the modern pharmacopoeia includes about 25% of the medicines that have been derived from this type of plants (Ghulam et al., 2017). For example, in Germany, there are two commercial phytopharmaceuticals used for the treatment of diabetes from endemic plants of the American continent, which are widely used in traditional Mexican medicine. The first one is Hando, which is based on nopal (Opuntia streptacantha Lem.) and is manufactured by the company Hando Austria. The second phytomedicine is Sucontral, which is produced from the medicinal plant Copalchi (Hintonia latiflora (DC) Bullock), and is manufactured by the company Harras Pharma, Munich. (Andrade-Cetto and Heinrich, 2005).
Therefore, it is urgent and necessary to conduct studies of medicinal plants for the identification and characterization of the active compounds of complex mixtures that allow different analytical and biomedical tests to indicate their biological activity (treatment of degenerative diseases such as diabetes among others), efficacy, and toxicity to prevent side effects. Diverse natural products based on medicinal plants (herbal medicines) exist on the market, with very little information about their chemical composition and pharmacological effects that can have adverse effects, different from those expected (positive effects on health).
Therefore, this work contains a compilation of different studies carried out in Cecropia obtusifolia Bertol., which is widely used for the treatment of diabetes mellitus type 2 (DM-2). This disease is characterized by the lack of sensitivity to insulin (resistance to insulin) and insufficient pancreatic secretion and unability to compensate for these alterations (dysfunction of β cells), resulting in hyperinsulinemia, hyperlipidemia, hypercholesterolemia, glucose intolerance and hypertension (Ross et al., 2004). Therefore, this plant has been widely used in traditional medicine in Mexico due to its hypoglycemic and hypolipidemic effects, which has led the scientific community to focus efforts to elucidate the modes of action of this species in DM-2.
Materials and Methods
A traditional manual search approach was used in which, by using keywords, reliable, accurate and up-to-date material related to phytochemical, pharmacological, toxicological and clinical studies of the medicinal plants, C. obtusifolia, were identified. Searches of scientific reports, books, as well as postgraduate theses were done mainly using three bibliographic databases (PubMed, Scopus, Web of Science) and one full-text database (Google Scholar). Literature was categorized, read and analyzed to summarize and synthesize some key points, which are discussed to highlight their importance and based on this previous knowledge identify/suggest areas of opportunity for future research.
Results
Importance of medicinal plants in the ethnopharmacology
Natural products research is commonly related to the discovery and development of drugs based on ethnobotanical information, where the plants have formed the base of sophisticated systems of traditional medicine, based on empirical findings, and the knowledge associated to their uses has been transmitted for hundreds and thousands of years (Buenz et al., 2017) (Fig. 1).
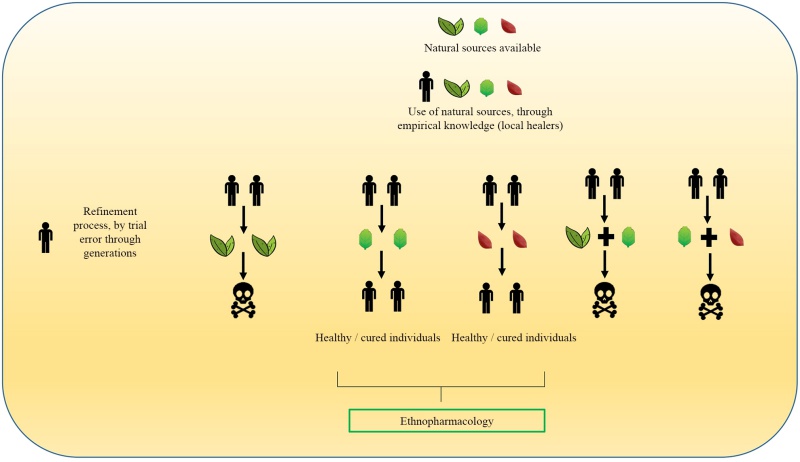
Figure 1: Methodology traditionally used for the identification of medicinal plants through life experiences between generations. Design by J. D. Cadena-Zamudio. Based on the adaptation of Buenz et al., 2017.
Natural products contain bioactive phytochemicals that are becoming increasingly important in foods such as Nutraceuticals (NC) (Foster et al., 2005). Therefore, ethnopharmacological research has focused on obtaining natural products and generating libraries with them, since they have shown a higher success rate compared to the libraries obtained by combinatorial chemistry. Each combinatorial approach has its own exclusive high-performance coding and selection strategy, which leads to different success and performance rates between each method (Sukuru et al., 2009; Liu et al., 2017). Since the primary and secondary metabolites examined and obtained from plants have already presumed different biological functions, and although these libraries have fewer compounds than those obtained by combinatorial chemistry, the percentage of products with biological activity is higher in natural products, which has been corroborated thanks to the use of techniques such as high performance like high-throughput screening (HTS), where the timely detection of natural products has reported higher rates (Sukuru et al., 2009; Harvey et al., 2015; Buenz et al., 2017). This suggests that the historical ethnopharmacological uses of plant-based drugs can be used as a preliminary screening criterion for the identification of bioactive compounds, since natural products and their derivatives represent around 50% of the total number of drugs in clinical use in the world, being the angiosperms 25% of the total; causing a quarter of all medical prescriptions to be formulations based on substances derived from plants or synthetic analogues derived from natural compounds (Castillo-España et al., 2009).
Unfortunately, the continuous loss of knowledge in traditional medicine and biodiversity, as a consequence of human population growth, has resulted in the loss of original resources (natural sources), which has motivated the scientific community to increase efforts to restore and document the ethnopharmacological use of natural products in the original communities. These tools are of great utility for future discovery of new biologically active compounds (Martínez-Francés et al., 2015; Buenz et al., 2017).
Species used for the treatment of DM-2
Mexico is considered one of the countries with the greatest biodiversity in the world; it is estimated that there are 23,400 vascular plants in this country, of which around 3000 have medicinal effects (Villaseñor, 2016). Of these plant species, 1200 have hypoglycemic activity and are used in traditional medicine for treatment with DM-2 (Andrade-Cetto et al., 2010). The reports suggest that the hypoglycemic activity is conferred by some kind of secondary metabolite that is biosynthesized by these plants (Hernández-Galicia et al., 2007; Romero-Cerecero et al., 2009; Andrade-Cetto et al., 2010; Alonso-Castro et al., 2016). The chemical and pharmacological evaluations to confirm or discard the antidiabetic properties (and/or hypoglycemic effect) have been carried out in 5% of these reported species only, and the pharmacological mechanism of action has been studied in very few of them (Alarcón-Aguilar et al., 2002; Ocegueda et al., 2005).
In Mexico, seven species stand out for being widely used in traditional medicine to treat DM-2; phytochemical and pharmacological studies exist for all of them, proving their activity, both in vivo or in vitro (Pérez et al., 1984; Román-Ramos et al., 1991; Alarcón-Aguilar et al., 2002; Andrade-Cetto and Heinrich, 2005). Within this select group of plants, most of them are now included in the Herbal Pharmacopoeia of the United Mexican States (FEUM), such as the “nopal” (Opuntia streptacantha) and “tronadora” (Tecoma stans (L.) Juss. ex Kunth). Hypoglycemic activity of O. streptacantha was demonstrated in healthy rabbits with temporal hyperglycemia and in rabbits with moderate diabetes (Andrade-Cetto and Heinrich, 2005). On the other hand, Mellado and Lozoya (1985) showed the hypoglycemic effect of T. stans infusion through intravenous administration in dogs, and likewise, Aguilar-Santamaría et al. (2009) identified antidiabetic activities in streptozotocin (STZ) rats, including the inhibition of intestinal glucosidase and postprandial antihyperglycemic effects, as well as hypocholesterolemic and hypotriglyceridemic effects.
Other plants studied are commonly known in Mexico as “trompetilla” (Bouvardia ternifolia (Cav.) Schltdl.), “hierba dorada” (Brickellia veronicifolia (Kunth) A. Gray) and “cuajilote” (Parmentiera aculeata (Kunth) Seem.), their aqueous extracts showing hypoglycemic activity administered to normoglycemic and induced diabetic mice (Peréz-Gutiérrez et al., 1998). Similar studies have been conducted in Ibervillea sonorae (S. Watson) Greene and Parkinsonia aculeata L., species commonly known as “wereke” and “retama”, respectively (Alarcón-Aguilar et al., 2002, 2005; Hernández-Galicia et al., 2007; Leite et al., 2007). Finally, the Urticaceae family has considered its wide use of traditional medicine, of which the empirical treatment of DM-2 stands out, where the most used for this condition are Cecropia peltata L. and Cecropia obtusifolia (Giovannini et al., 2016).
Cecropia obtusifolia, geographical distribution and traditional use
Cecropia obtusifolia (Fig. 2), a plant of the family Urticaceae, popularly known as “guarumbo”, “ghancarro”, “hormiguillo”, “chiflon” and “koochlé”, is used in Mexico by the traditional healers for DM-2 treatment (Martínez-Toledo et al., 2008). This species is originally from Central America and it is distributed from Mexico to northern South America; it grows in tropical, warm, semi-warm and temperate climates, from regions bordering the coasts and up to 1500 m above sea level. Cecropia obtusifolia grows in tropical forests and in secondary vegetation of deciduous, subperennial or evergreen forests that have been disturbed; sometimes it is associated with xerophilous scrub of cacti or cedar, in pasture and in mixed oak-pine forest (Argueta et al., 1994; Aguilar et al., 1998; Berg et al., 2005). In Mexico, it is distributed on the Mexican Gulf slope, from the states of Tamaulipas to Quintana Roo, and in the Pacific, from Sinaloa to Chiapas, Puebla, Hidalgo and Guanajuato (Argueta et al., 1994; Andrade-Cetto and Heinrich, 2005).

Figure 2: Guarumbo (Cecropia obtusifolia Bertol). Photo by J. D. Cadena-Zamudio, from a plant growing in the “Los Tuxtlas” Tropical Biology Station-UNAM, San Andrés Tuxtla, Veracruz, Mexico.
The use of C. obtusifolia to treat diabetes was reported in the states of Hidalgo, Guerrero, Veracruz, Yucatán, Campeche, Tabasco, México, Oaxaca and Chiapas (Andrade-Cetto and Heinrich, 2005). The dried leaves (~15 g) are boiled in water (~500 ml), and the resulting infusion should be cooled and then filtered, to drink it throughout the day as “agua de uso” (Andrade-Cetto and Heinrich, 2005). The inhabitants of the community of Tlanchinol in the state of Hidalgo also use the tree bark for the treatment of the same disease (Andrade-Cetto, 1999). Furthermore, infusion of C. obtusifolia is used for the treatment of nerve conditions, as fever reducer (antipyretic), cardiac conditions, liver and pulmonary diseases, asthma, flu, wounds, scorpion and ant bites, bone fractures, kidneys, rheumatoid arthritis and wart removal, among many others (CONABIO, 2017).
Chemical compounds reported in Cecropia obtusifolia
Due to the properties mentioned above, some studies have been carried out to ascertain its chemical composition, the presence of sterols, tannins and monosaccharides, e.g., L-rhamnose, D-glucose and D-xylose was demonstrated; the presence of saponins and phenolics has also been reported, as well as the absence of alkaloids, flavonols and cardiotonic glycosides (Trejo, 1983). Recent studies reported the presence of 18 new compounds identified from C. obtusifolia leaves (Table 1), which have been shown to have both antidiabetic and anti-inflammatory activities.
Table 1: Compounds identified in Cecropia obtusifolia Bertol. and its potential biological activities. DCME: dichloromethane extract; NR: not reported or very low concentrations. Modified from Rivera-Mondragón et al. (2017).
| Compound | Part of the plant | Concentration | Biological activity | References |
| 4,22-Cholestadien-3-one | leaves | DCME | NR | Guerrero et al. (2010) |
| 4-Cholestene-3,24-dione | leaves | DCME | NR | Guerrero et al. (2010) |
| β-Sitosterol | leaves | NR | Anti-inflammatory | Andrade-Cetto and Heinrich (2005); Loizou et al. (2010) |
| Stigmast-4-en-3-one | leaves | DCME | Anti-inflammatory, Anti-diabetic | Jamaluddin et al. (1995); Alexander-Lindo et al. (2004); Guerrero et al. (2010); Tewtrakul et al. (2010) |
| Stigmasterol | leaves | NR | Anti-inflammatory | Andrade-Cetto and Heinrich (2005); Gabay et al. (2010) |
| Aloe-emodin | leaves | NR | Anti-inflammatory | Choi et al. (2013); Yan et al. (2013); Kshirsagar et al. (2014); Park et al. (2016) |
| Chrysophanol | leaves | NR | Anti-inflammatory | Choi et al. (2013); Yan et al. (2013); Kshirsagar et al. (2014); Park et al. (2016) |
| Emodin | leaves | NR | Anti-inflammatory | Choi et al. (2013); Yan et al. (2013); Kshirsagar et al. (2014); Park et al. (2016) |
| Physcion | leaves | NR | Anti-inflammatory | Choi et al. (2013); Yan et al. (2013); Kshirsagar et al. (2014); Park et al. (2016) |
| Rehin | leaves | NR | Anti-inflammatory | Choi et al. (2013); Yan et al. (2013); Kshirsagar et al. (2014); Park et al. (2016) |
| 1-(2-Methyl-1-nonen-8-il)- aziridine | leaves | NR | NR | Andrade-Cetto and Heinrich (2005) |
| 2-Methylbenzaldehyde | leaves | DCME | NR | Guerrero et al. (2010) |
| 2,3-Dihydrobenzofuran | leaves | DCME | NR | Guerrero et al. (2010) |
| 3´-Methoxyacetophenone | leaves | DCME | NR | Guerrero et al. (2010) |
| 4-Ethyl-5-(n-3valeroil)-6-hexahydrocoumarin | leaves | NR | NR | Andrade-Cetto and Heinrich (2005) |
| 4-Vinyl-2-methoxy-phenol | leaves | DCME | NR | Guerrero et al. (2010) |
| Palmitic acid | leaves | DCME | Anti-inflammatory | Guerrero et al. (2010)) |
| Stearic acid | leaves | DCME | Anti-inflammatory | Guerrero et al. (2010); Pan et al. (2010) |
Biological activity of the main phytochemicals identified in Cecropia obtusifolia
As mentioned above, different parts like leaves and tree bark of C. obtusifolia are used to treat various illnesses, mainly DM-2; the hypoglycemic effect was attributed to two secondary metabolites in particular, chlorogenic acid (CGA) and isoorientin (ISO) (Andrade-Cetto and Wiedenfeld, 2001), due to the stimulation in the glucose uptake 2-NBD and the inhibition of glucose-6-phosphatase, resulting in an inhibition of the production of hepatic glucose and increase in the tolerance to glucose (Hemmerle et al., 1997; Andrade-Cetto and Wiedenfeld, 2001).
Pharmacological studies
Pérez et al. (1984) tested the hypoglycemic effect of C. obtusifolia in mice with induced diabetic with Alloxan (toxic compound that acts on the islets of Langerhans, used in experiments to induce diabetes mellitus in animal models). The same hypoglycemic effect was subsequently observed in hyperglycemic rabbits (Román-Ramos et al., 1991). In 1997, Hemmerle et al. tested the effect of CGA and its derivatives in the microsomal activity of glucose 6-phosphatase, demonstrating inhibition of glycogenolysis, gluconeogenesis, and reduction of liver glucose production. Andrade-Cetto and Wiedenfeld (2001) carried out studies on diabetic rats induced with STZ and treated with C. obtusifolia aqueous and butanolic extracts at doses of 90 mg/kg and 150 mg/kg for the first one, and 9 mg/kg and 15 mg/kg for the second one, finding a significant decrease in plasma glucose levels three hours after treatment. Sezik et al. (2005) showed that ISO, administered by oral route for two weeks in diabetic rats induced with STZ, decreased their glucose, cholesterol and triglycerides levels from the fifth day of treatment, keeping it up to 12 days after the suspension of the administration of the compound. Nicasio-Torres et al. (2005) reported concentrations of 19.84±1.64 mg of CGA/g in the dry leaves of C. peltata and C. obtusifolia; the extract of the last one showed a decrease in plasma glucose levels (45.6%) between two and four hours after the oral administration. This proved that the hypoglycemic effect is correlated with the CGA concentrations in plant leaves. Andrade-Cetto et al. (2008) showed that the butanolic extract of C. obtusifolia leaves decreases the postprandial hyperglycemia in rats pre-treated with STZ; this effect was associated with the intestinal α-glucosidase enzyme inhibition, thus preventing the splitting of complex carbohydrates and delaying the absorption of glucose. Similarly, Alonso-Castro et al. (2008), reported that the CGA is able to stimulate the production of glucose in sensitive and insulin resistant adipocytes. In 2010, Andrade-Cetto and Cárdenas Vázquez found that the mechanism of the hypoglycemia effect of C. obtusifolia is due to the inhibition of the enzyme glucose 6-phosphatase in the gluconeogenesis, which results in a reduction in the production of hepatic glucose. Sezik et al., in 2005, reported the hypoglycemia and hypolipidemic effects of ISO in STZ-induced diabetic rats, dosing orally for two weeks, demonstrating that ISO can reduce the levels of glucose, cholesterol, and triglycerides from the fifth day of treatment and hold it up to ten days after the suspension of the treatment.
Toxicological studies
In the toxicological studies, Pérez-Guerrero et al. (2001) quantified the lethal dose (LD50) in rats, using the aqueous extract of C. obtusifolia leaves and continued to administer intraperitoneally, obtaining a dose of 1.45±0.07 g/kg (equivalent to 11.21±0.52 g/kg of dry leaf per body weight). Another study of this type, but with a genotoxic approach, was carried out by Martínez-Toledo et al. (2008), evaluating the possible effects of the aqueous extract of C. obtusifolia in somatic mutation tests in Drosophila melanogaster Meigen wings and a micronucleus assay in lymphocytes obtained from patients treated with the extract; the results obtained did not show genotoxic or cytotoxic effects caused by the extract.
Clinical studies
There are some interesting data about clinical studies that involved the antidiabetic effects of C. obtusifolia. For example, the study done by Herrera-Arellano et al. (2004) carried out a double-blind design with uncontrolled diabetic patients between 30 and 60 years. Patients were provided with tea bags from C. obtusifolia leaves to prepare the infusions with an oral dose of 2.99±0.14 mg/g of CGA before each meal. The length of the treatment was 21 days and showed that glucose (15.25%), cholesterol (14.62%) and triglycerides blood levels (42%) decreased with respect to the control group. A similar study was carried out by Revilla-Monsalve et al. (2007), in which they took diabetic patients without pre-treatment with conventional oral hypoglycemic drugs, and they were treated with infusions of C. obtusifolia leaves using concentrations of CGA=2.91 mg and ISO=2.4 mg; the patients displayed a significant reduction in glycosylated hemoglobin (HbA1c) after six weeks, without finding significant changes in the patient's insulin secretion, nor in alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALKP) levels; indicating no hepatotoxicity during the treatment.
Other biological activities
In this respect, antihypertensive effects are identified; since Salas et al. (1987) reported a drop-in blood pressure (-23.5% with respect to pre-injection values) in rats with consistent hypertension, when they were administered the lyophilized aqueous extract from leaves. In the same way, analgesic and anti-inflammatory effects have been reported (Pérez-Guerrero et al., 2001). This justifies its use in different pathologies such as inflammatory, rheumatic and renal ones, which can also be associated with DM-2 symptoms and its complications.
Discussion and Conclusions
In Mexico, there is an extensive variety of treatments based on medicinal plants (phytomedicines). All these plants are part of the traditional Mexican herbalism, comprehending around 3000 species. Due to this abundant species number, Mexico is positioned as one of the world's most important countries regarding the number of registered and used medicinal plants (Barragán, 2006). In both developed and developing countries, the use and commercialization of phytomedicines and natural products for medicinal purposes has grown in recent years. In addition, it is important to note that due to the immense diversity of possible bioactive molecules present in plants, there is currently an increase of patent protected allopathic medicines which are from plant origin. Due to this, the production of secondary metabolites using plant cell cultures, the generation of knowledge about the biosynthetic pathways and the enzymes involved, their functions in the host and the influence of environmental conditions in their synthesis are only some of the niches of opportunities for investigative work.
In the particular case of C. obtusifolia, despite its relevance and potential at the pharmacological level, there are no genetic or genomic data for this species and, for example, it is unknown whether orthologous genes already reported in other plant species are also responsible for the synthesis of interesting metabolites such as chlorogenic acid and isoorientin. In addition, the questions are how different metabolic pathways modulate the synthesis of active molecules and how these affect the synthesis of these molecules remain unanswered (Wang et al., 2009). Most of the specialized metabolites of interest are produced in non-model plants for which genomic/transcriptomic sequence information is not yet available. Fortunately, NGS (Next Generation Sequences) technologies can be implemented to sequence plant transcriptomes from medicinal plants (Morozova et al., 2009; Fonseca et al., 2016). The massive gene lists that can be generated thus represent a seemingly unlimited catalog of enzymes, transporters, or regulators to be used in synthetic biology programs (Ozsolak and Milos, 2011). This wealth of sequences has shifted the paradigm in gene discovery quests, which no longer focus on the isolation of candidate plant gene sequences, but rather on how to find the right one in the immensity of duplicated and neofunctionalized genes, which through evolution have resulted in hundreds of thousands of different specialized metabolites that can be found in the plant kingdom (Saito and Matsuda, 2010). If sequence assembly is complemented with extensive in silico analysis, a (tentative) functional annotation can be assigned and then this information can be used to reconstruct metabolic pathways and discover missing enzymes in the pathways (Metzker, 2010; Garber et al., 2011; Fonseca et al., 2016).
Besides gene sequences, metabolic pathways construction will heavily depend on metabolomics for validation (Saito and Matsuda, 2010). Plants are challenging in this regard as a single plant (cell) can harbor thousands of metabolites, many of whose structure is yet unknown. Plant metabolomics will require both targeted and non targeted analysis, within which high resolution mass spectrometry based techniques, along with nuclear magnetic resonance spectroscopy, will be most suitable because of the remaining need for the annotation of unknown compounds (Saito and Matsuda, 2010; Soda et al., 2015; Mochida and Shinozaki, 2017). Here, we propose C. obtusifolia as a “non-model” plant species which should be used for, by the systematic integration of transcriptomics and metabolomics, investigating the molecular basis of the synthesis and function of some metabolites, mainly from those which have potential to treat important diseases.
Author contributions
JCZ and EIL contributed to the writing of the work and critical review of its intellectual manuscript. PNT and JGA substantially contributed to the conception and revision. All authors contributed to the design and writing, revision, and approval of the final manuscript. Consent is given for its publication. All authors of the manuscript have read and agreed to its content and are accountable for all aspects of the accuracy and integrity of the manuscript. The authors declare that they have no competing interests.











 text new page (beta)
text new page (beta)



