Introduction
Coral reefs are critical providers of food, coastal protection, recreational opportunities, economic opportunities, and cultural value (Barbier et al. 2011, Reguero et al. 2021). Coral bleaching events in the past decade have inflicted severe mortality to reefs worldwide (Stuart-Smith et al. 2018, Raymundo et al. 2019, Raj et al. 2021). Chronic stress from land-based sources of pollution and sedimentation threaten the ability of reefs to continue providing these services (Bruno et al. 2003, Fabricius 2005). Increasingly frequent coral bleaching events represent an acute stressor overlaying chronic issues (Heron et al. 2016). As major disturbances become more frequent due to climate change, the probability of long-term ecosystem shifts increases, which may disrupt the provision of reef-based services to island communities (Norström et al. 2009, Pratchett et al. 2009).
Strategies that promote climate resilience are receiving attention as the consequences of bleaching events increasingly affect vast swaths of the ocean (Comte and Pendleton 2018). By focusing on resilience, defined as the ability to either resist shifting to a different ecosystem state or to recover quickly to the original state following disturbance-induced change (Holling 1973, Kenneth et al. 2015), managers seek to maintain ecological processes that allow coral survival, growth, recovery, and recruitment. Resilience-based management (RBM) strategies include coral outplanting in climate refuges, conserving reefs with a history of low mortality during bleaching events, and maintaining healthy herbivore populations to reduce algal overgrowth (Graham et al. 2013, Maynard et al. 2015, Chung et al. 2019, Mcleod et al. 2019).
Resilience-based management is more likely to succeed when implemented under unstressful conditions or in stress-tolerant populations. Areas not subject to climate-related stress will become increasingly rare in the next century. Contrasting gradients in projected heat stress and acidification suggest that few locations will be true climate refuges maintaining twentieth-century conditions (van Hooidonk et al. 2014). Strategies that manage and mitigate local stressors increase the ability of corals to withstand the consequences of global stressors. For example, reducing sediment and nutrient pollution from coastal watersheds is important for ensuring clear water and reducing algal overgrowth in coral reefs, particularly in the wake of damage from bleaching events or storms (Smith et al. 2010, Risk and Edinger 2011).
Coral species that experience high temperature variability or that are regularly exposed to high temperatures can evolve to increase their thermal tolerance, highlighting the importance of corals that survive under sub-optimal environmental conditions (Safaie et al. 2018, Schoepf et al. 2020). Likewise, coral and endosymbiont taxa differ in their tolerance to nutrient pollution and contaminants, resulting in variable spatial distributions of corals capable of adapting or acclimating to multiple stressors (Fox et al. 2021, Nalley et al. 2021). Thus, coral characteristics influence which RBM strategies are appropriate for each coral species or population. Indeed, populations exhibiting tolerance to stressful conditions over time may be good candidates for additional conservation measures and can provide genetic material to restore corals elsewhere (Oliver and Palumbi 2011, Safaie et al. 2018). In addition, sites not subject to stressful or extreme environmental conditions may be good locations for restoration measures (Foo and Asner 2020; Table 1).
Table 1 Conceptual categorization of example resilience-based management strategies. The examples are arranged along 2 axes: (1) Coral resilience and (2) environmental favorability. The 4 resulting quadrants may be subject to different reef management strategies.
| Environmental favorability | Coral resilience | |
| Low or positive change, high stability | Negative change, low stability | |
| Favorable environment | Conserve or restore | Restore coral |
| Unfavorable environment | Conserve coral
Genetic material Mitigate stressors |
Neither |
Geographic assessments that identify either resilient coral populations or favorable environmental conditions are used to inform management recommendations throughout the Pacific Islands region, including those in Guam, Hawaii, and the Commonwealth of the Northern Mariana Islands (Maynard et al. 2015, 2018; Chung et al. 2019a, b). Thus, the objective of this study was to identify locations in Guam and American Samoa where reef managers could implement RBM strategies under present-day conditions and in future climate scenarios.
Materials and methods
Study areas
Guam (13.4255° N, 144.7471° E) and American Samoa (14.3191° S, 170.7586° W) are volcanic islands surrounded by nearshore coral reefs. Both locations experienced multiple coral bleaching events over the last decade, sometimes in consecutive years. The decline in coral cover following a bleaching event has not occurred uniformly around the islands (Raymundo et al. 2017, 2019; NOAA 2018). Both locations also experience water quality problems due to land management practices and land-based sources of pollution (Houk et al. 2005, Shuler and Comeros-Raynal 2020). Geologically, Guam is comprised of limestone in the north and steep volcanic hillsides in the south that burn seasonally, generating large amounts of sediment runoff. Development and land clearing are also associated with high sedimentation rates (Kottermair 2012, Prouty et al. 2014). In the volcanic archipelago of American Samoa, most human activity occurs on the island of Tutuila, whereas the Manuʻa Islands (Ofu, Olosega, and Ta‘ū) to the east are lightly inhabited and are largely protected as part of the National Park of American Samoa. The remote Swains Island and Rose Atoll of American Samoa were not considered in this study due to data limitations.
Scoring environmental favorability
We assessed geographic patterns in environmental conditions to identify areas favorable for coral growth and survival. We gathered spatial data layers with sufficient resolution (≤10 km) to evaluate environmental variation around the islands (Table S1). We divided environmental conditions into 2 categories, “managed” and “globally influenced,” to differentiate conditions either under management or outside its control. Managed environmental conditions were those influenced by typical management levers up to the spatial scale of island, including chlorophyll concentrations (a proxy for land-based nutrient runoff), fish biomass, herbivore biomass, turbidity (a proxy for land-based sediment runoff), ocean-based pollution, and macroalgal cover. Globally influenced environmental conditions were those outside the control of managers at the island scale. These conditions either require global cooperation to affect substantial change or cannot be controlled and include calcite concentrations (a proxy for ocean acidity), ocean surface irradiance, thermal stress (degree heating weeks), and wave energy (Table S1). We excluded the ocean-based pollution layer for American Samoa from the analysis because it exhibited no spatial variability and did not contribute to our goal of discerning spatial differences in environmental conditions.
Following the approach of Maynard et al. (2018), we scaled each environmental variable linearly from 0 to 1 in ArcMap 10.7 (Environmental Systems Research Institute, Inc.), with 0 representing conditions least favorable to coral growth and survival and 1 representing the most favorable conditions (Fig. 1), scaling each jurisdiction separately. Each raster cell represented one data point with many environmental attributes (NGuam = 299, NAS = 7,766). We controlled for covariation between variables with a principal component analysis (PCA) using the ‘FactoMineR’ package (Lê et al. 2008) in R v. 4.1 (R Core Team 2021). We performed separate PCAs for the managed and globally influenced conditions (Supplementary material, Tables S2, S3). We linearly rescaled the principal components (PCs) from 0 to 1 based on the minimum and maximum values, with 1 representing the most favorable conditions. We averaged these 2 PCs to obtain an overall score of environmental favorability (Fig. 1). The values with environmental favorability scores in the top 20th percentile served as a heuristic for “highly favorable environmental conditions”.
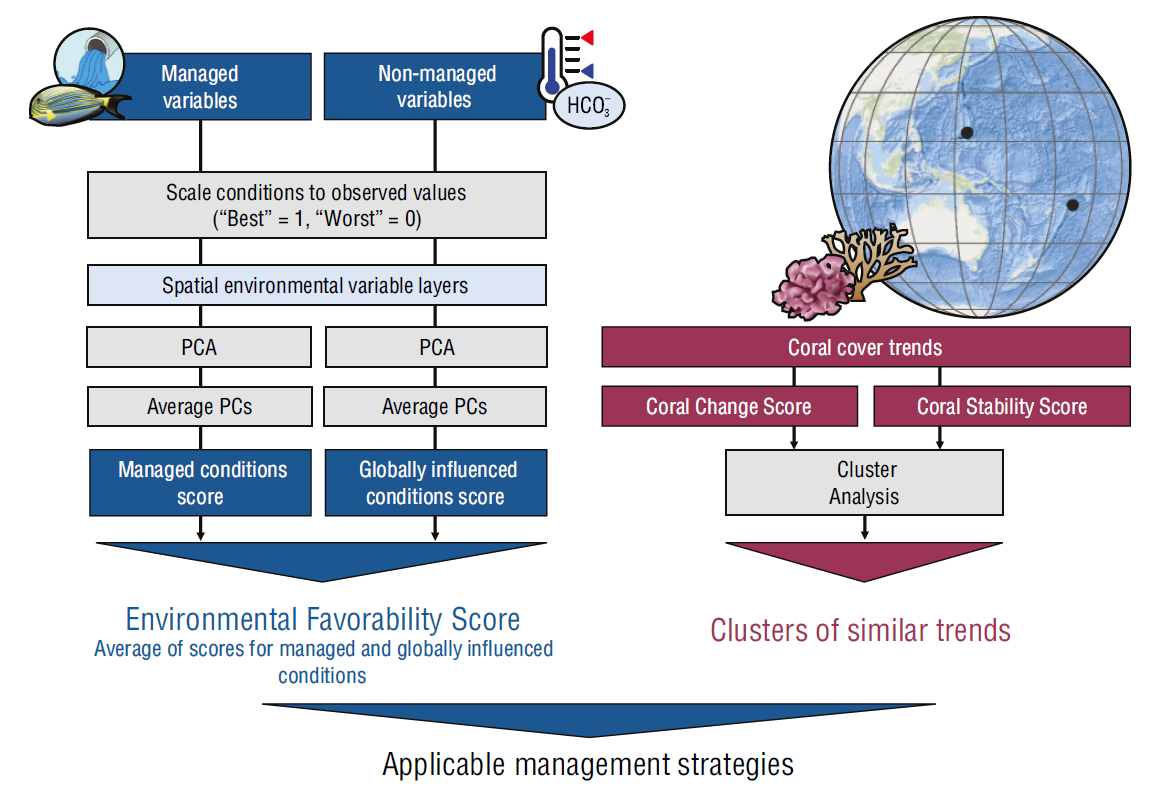
Figure 1 Overview of methods used to assess spatial patterns of environmental favorability and coral resilience. This process was repeated for each climate scenario. Black points on the globe represent study the locations, Guam (left) and American Samoa (right). PCA stands for Principal Component Analysis and PC stands for Principal Component.
We assumed all input variables carried equal weight when contributing to the scores for managed and globally influenced conditions. Thus, we weighted the influence of these 2 sets of conditions on coral growth and survival equally. The degree to which environmental stressors synergistically affect coral reef ecosystems is location-specific (Ateweberhan et al. 2013, Houk et al. 2014), making variable weighting difficult to apply in our study areas. However, prior coral reef resilience assessments have also weighted all environmental conditions equally, including climatic and anthropogenic stressors (Maynard et al. 2015, 2018). We sought to account for potential correlation (Tables S4-S7) between environmental variables through the PCA, reducing the number of dimensions based on variables that increased or decreased in a coordinated manner (Bastazini et al. 2007, Jales-Cavalcanti et al. 2015, Moritsch 2018).
In addition to examining patterns under present-day climate conditions, we repeated this process for 2 future climate scenarios from global climate models representing conditions in 2100. Scenario 1, the intermediate emissions scenario, assumed greenhouse gas emissions stabilized mid-century, corresponding to Representative Concentration Pathway (RCP) 4.5. Scenario 2, the worst-case emissions scenario, assumed greenhouse gas emissions continued unabated, corresponding to RCP 8.5 (Riahi et al. 2011, Thomson et al. 2011). For the future climate scenarios, we replaced the present-day thermal stress layer with a layer of the projected timing of annual severe bleaching under RCP 4.5 or RCP 8.5 as a proxy for future thermal stress (van Hooidonk et al. 2016, UNEP 2017). We added soil erosion due to sea-level rise (SLR) as another future condition that could negatively impact corals by either reducing light availability for symbiont photosynthesis or increasing physiologically damaging sedimentation (Field et al. 2011, Storlazzi et al. 2011, Weber et al. 2012, Bessell-Browne et al. 2017). We measured the proximity to easily erodible soils, which SLR would inundate under RCP 4.5 and RCP 8.5, as a proxy for the severity of the effects of SLR (Supplementary material, erosion estimates related to SLR). Projections of the other globally influenced conditions (calcite concentrations, irradiance, and wave energy) were not available in a spatial resolution fine enough to discern geographic patterns within the jurisdictions, so we used the present-day climate scores for these 3 conditions for both future climate scenarios. We also assumed the managed conditions would remain the same across all scenarios because robust projections for how these conditions may change were not available.
Characterizing coral resilience
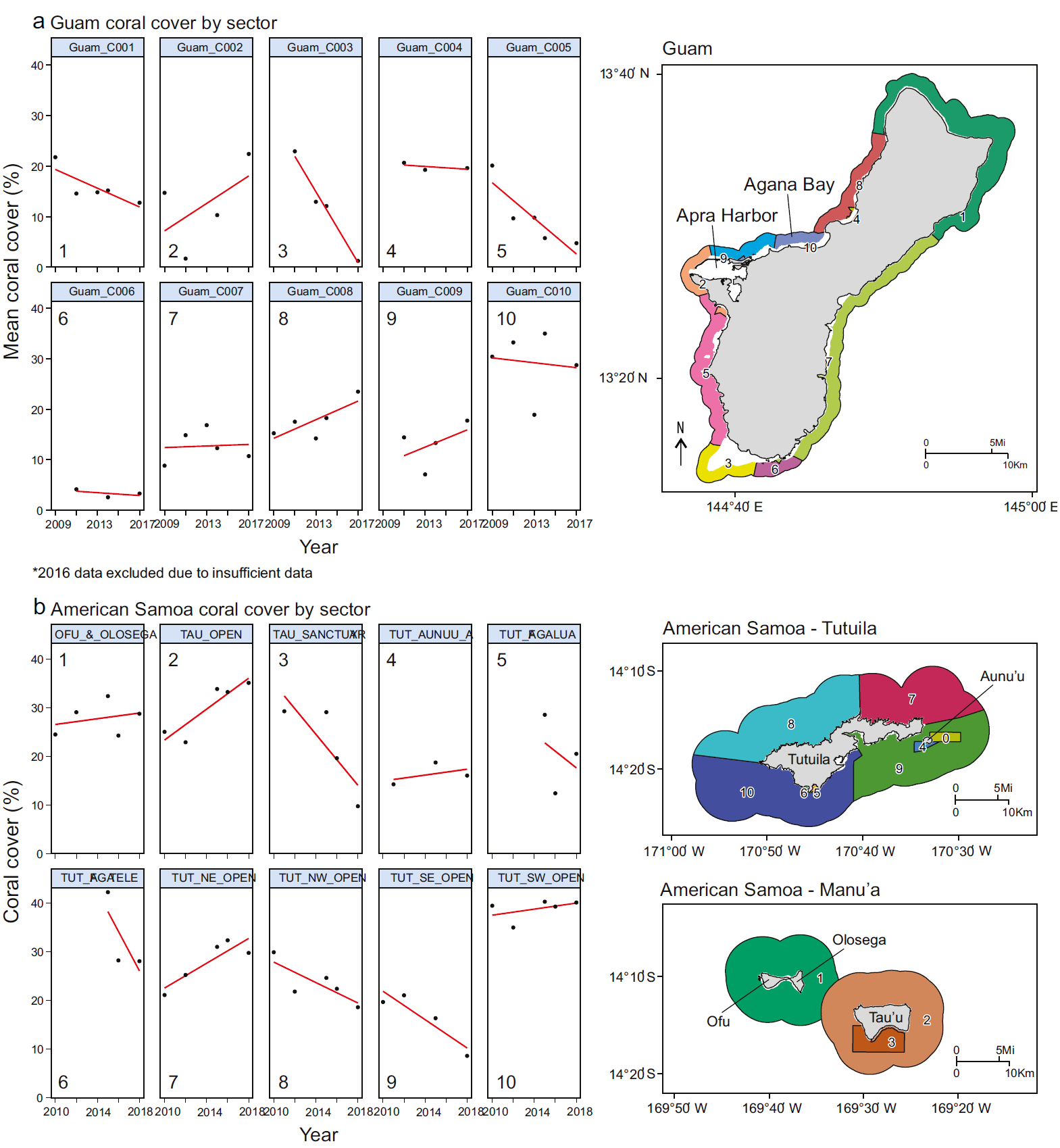
We used existing coral cover data from the National Oceanographic and Atmospheric Administration National Coral Reef Monitoring Program (NOAA NCRMP) to measure 2 aspects of coral resilience: the ability to recover coral cover quickly and the ability to resist change in coral cover over time. The NCRMP diver surveys recorded coral cover with multiple methods and sampling sites. These surveys excluded the reef flat zone, resulting in an underrepresentation of the complexity of coral cover dynamics across reef sites. For Guam, surveys from 2009 to 2017 were aggregated into 10 sectors of variable size that accounted for ecological similarities in substrate, coral communities, and benthic cover (Oliver et al. 2020a; Fig. 2a). We excluded data from 2016, when major storm events prevented adequate sampling for several sectors. For American Samoa, we used 11 coastal management sectors defined by NOAA (NOAA 2018) as units for measuring coral cover trends (Fig. 2b, c). Boundaries of the National Marine Sanctuary of American Samoa influenced sector boundaries, wherein geographically separated areas of the sanctuary were made into their own sectors. All sector names followed NOAA’s established naming conventions. We excluded the sector Tutuila Aunu‘u B due to insufficient data, leaving a total of 10 sectors. Annual sector-level averages, which were required due to the spatially variable sampling design of the NCRMP, were not conducive to identifying resilient coral populations at sub-kilometer scales.
*Tutuila Aunnu B data excluded due to insufficient data
Figure 2 Mean percent coral cover by sector and map of sectors for (a) Guam and (b) American Samoa based on yearly aggregated data from the National Oceanographic and Atmospheric Administration National Coral Reef Monitoring Program. Trendlines are represented in red. Guam sectors are unnamed and are referred to by arbitrarily assigned numbers. Surveys from Guam in 2016 and from American Samoa sector Tutuila Aunu‘u B (labeled sector 0) in all years were excluded from analysis due to insufficient data. Map colors represent unique sectors. American Samoa sector names are: (1) Ofu and Olosega, (2) Ta‘ū Open, (3) Ta‘ū Sanctuary, (4) Tutuila Aunu‘u A, (5) Tutuila Fagalua, (6) Tutuila Fagatele, (7) Tutuila Northeast Open, (8) Tutuila Northwest Open, (9) Tutuila Southeast Open, (10) Tutuila Southwest Open. Reef flat, dredged, lagoon, back reef and intertidal areas are excluded from view.
We quantified whether coral cover was increasing, a potential indicator of post-disturbance recovery, or remaining stable, a potential indicator of resistance to disturbance, by calculating a coral change score and coral stability score for every sector (Fig. 1). Without detailed information on the timing of stressors, we could not definitively determine whether coral cover demonstrated resilience. The coral change and coral stability scores did not account for species composition or biodiversity by sector, as this information was not available. These scores were calculated based on coral cover data alone; no environmental conditions were considered in calculating this score.
The coral change score (Equation 1) characterizes sectors according to their ability to rapidly increase coral cover without major fluctuations.
Equation 1. Coral change score
By using the slope of the coral cover trendline over time, rapid increases (positive slope) in coral cover resulted in higher positive scores, whereas rapid declines (negative slope) resulted in lower negative scores. Several sectors exhibited trendlines with non-significant slopes (P > 0.05), although these still suggested divergent trajectories for coral cover. Rather than remove these sectors due to their lack of significance, we incorporated root mean square error (RMSE) as a term to reflect the limited confidence in trend direction in these sectors. We divided the slope of the sector trendline by its RMSE so that sectors with high variability in coral cover received scores closer to zero than sectors with low variability, indicating weaker positive or negative trajectories. We square root-transformed the output to minimize the impact of outliers in the scores, such that the range of coral change scores was similar for Guam and American Samoa.
For the coral stability score (Equation 2), we characterized sectors according to their ability to maintain steady levels of coral cover over time without major fluctuations.
Equation 2. Coral stability score
Sectors with higher mean coral cover over the entire sampling period received higher scores than sectors with lower mean coral cover. By dividing by the slope and RMSE, rapid increases or decreases in coral cover or high variability around the trendline resulted in low scores. We natural log-transformed the output to minimize the impact of outliers in the scores.
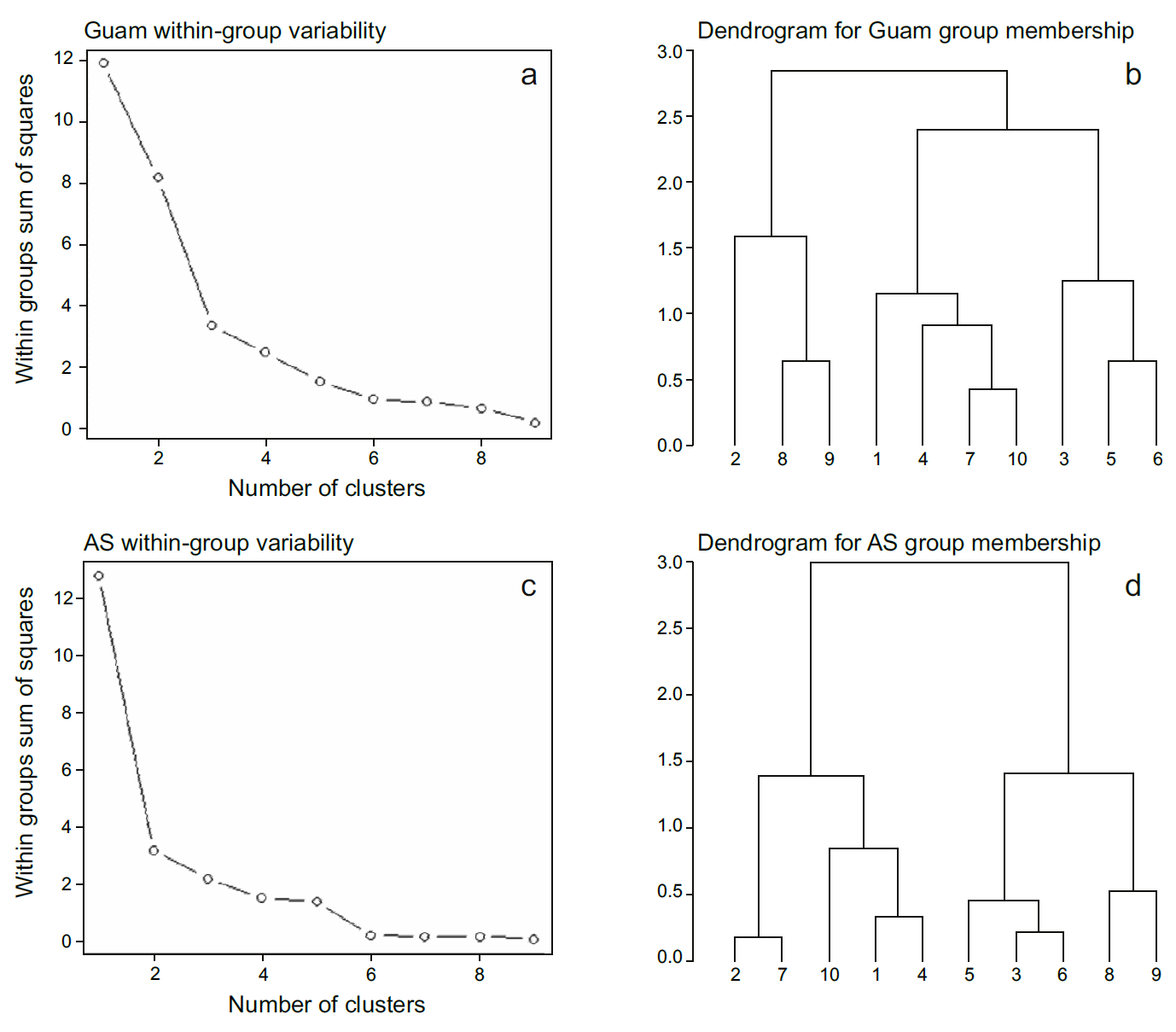
To facilitate comparisons among environmental favorability scores between sectors with similar dynamics in coral cover, we performed a cluster analysis using the coral change and stability scores of each sector to identify groups with statistically similar scores. Using the kmeans function of the ‘cluster’ package (Maechler et al. 2023) in R, we selected the optimal number of groups based on precipitous drops in the within-group sum of squares (Fig. S1). This function also assessed group membership for each sector (Fig. S1).
Identifying areas of overlap between coral resilience and favorable environmental conditions
We calculated the area of overlap between highly favorable environmental conditions and groups of coral sectors with similar cover trends using the cell center method to convert all raster grid cells to squares of the same area. We identified the spatial intersection between the resulting polygons representing environmental favorability scores ≥0.80 and sector polygons in ArcMap 10.7 (ESRI). We also identified the intersections between sectors with environmental favorability scores <0.80. Based on this overlap, we found the area in each quadrant corresponding to the potential management actions listed in Table 1 to determine the proportion of each jurisdiction that could be subject to different RBM strategies. We used a chi-squared test to determine whether these proportions were significantly different than expected at random.
Results
Environmental favorability
Under present-day climate conditions, the northeastern side of Guam exhibited the highest environmental favorability. This area had intermediate to high environmental favorability scores for managed conditions (0.50 to 0.80) and high environmental favorability scores for globally influenced conditions (>0.75). The lowest scores for managed conditions (0.22 to 0.35) were concentrated on the western side of the island around Apra Harbor. In contrast, the lowest scores for globally influenced conditions (≤0.30) were located in the southwestern quadrant of the island. High environmental favorability (≥0.70) scores were found in the northern half of Guam, and low scores (0.10 to 0.35) were present along the southwestern side (Fig. 3a).
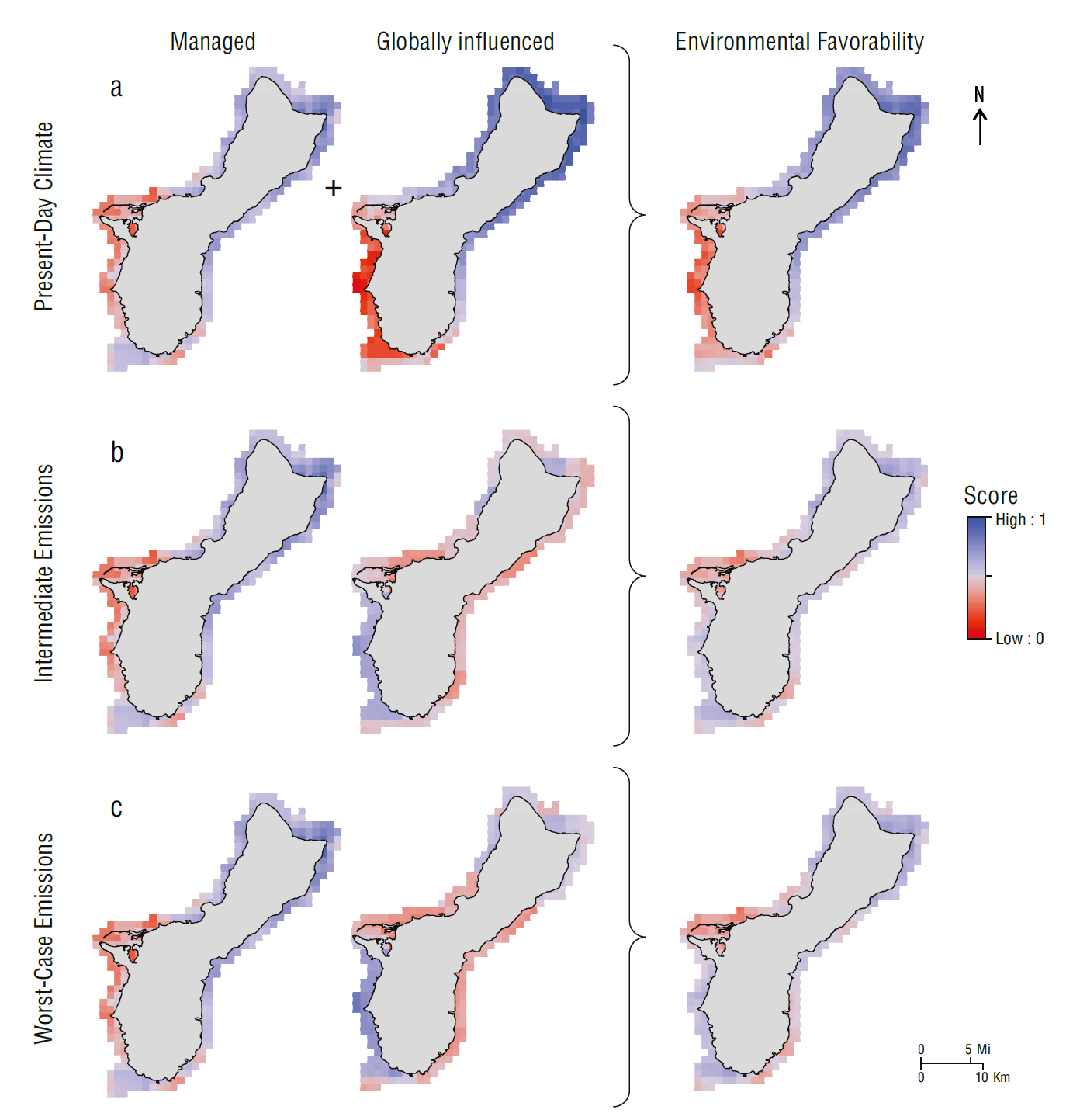
Figure 3 Scores for managed conditions, globally influenced conditions, and environmental favorability in Guam for the (a) present-day climate, (b) intermediate emissions scenario, and (c) worst-case emissions scenario. Scores for managed conditions remained the same across future climate scenarios. Red colors correspond to lower favorability and worse conditions for coral. Blue colors correspond to higher favorability and better conditions for coral.
In the intermediate emissions and worst-case emissions scenarios, this pattern was reversed. The globally influenced conditions were more favorable along the southwestern side, largely because this portion of the island is projected to experience thermal stress capable of severe annual bleaching farther into the future than the northern side. The highest relative environmental favorability scores occurred in the southwest and north, although the differences between the best- and worst-scoring locations were not as pronounced as those under the present-day climate (Fig. 3b, c).
Under present-day climate conditions in American Samoa, the environmental favorability scores for managed conditions were lowest (0.00 to 0.40) along the southeastern side of Tutuila, while intermediate scores (0.35 to 0.65) were found around the southwestern side, and the highest scores were detected around the northwestern side (>0.65). The Manuʻa Islands had relatively high (>0.65) scores for managed conditions, with the exception of a small pocket at the southwestern corner of Ofu (Fig. 4a). This pattern reflected a confluence of relatively benign conditions for multiple contributing environmental variables, with no single condition determining the locations of high scores. In contrast, the highest scores for globally influenced conditions (>0.70) were concentrated around the southwestern side of Tutuila. The lowest scores (0.10 to 0.40) were found along the eastern tip of Tutuila and were accentuated by high thermal stress in the vicinity. The Manuʻa Islands exhibited a mixture of high and low scores for globally influenced conditions. The resulting environmental favorability scores were moderately low (≤0.40) around the eastern tip of Tutuila and moderately high (>0.65) everywhere else (Fig. 4a). These patterns were similar for the intermediate emissions and worst-case emissions scenarios (Fig. 4b,c).
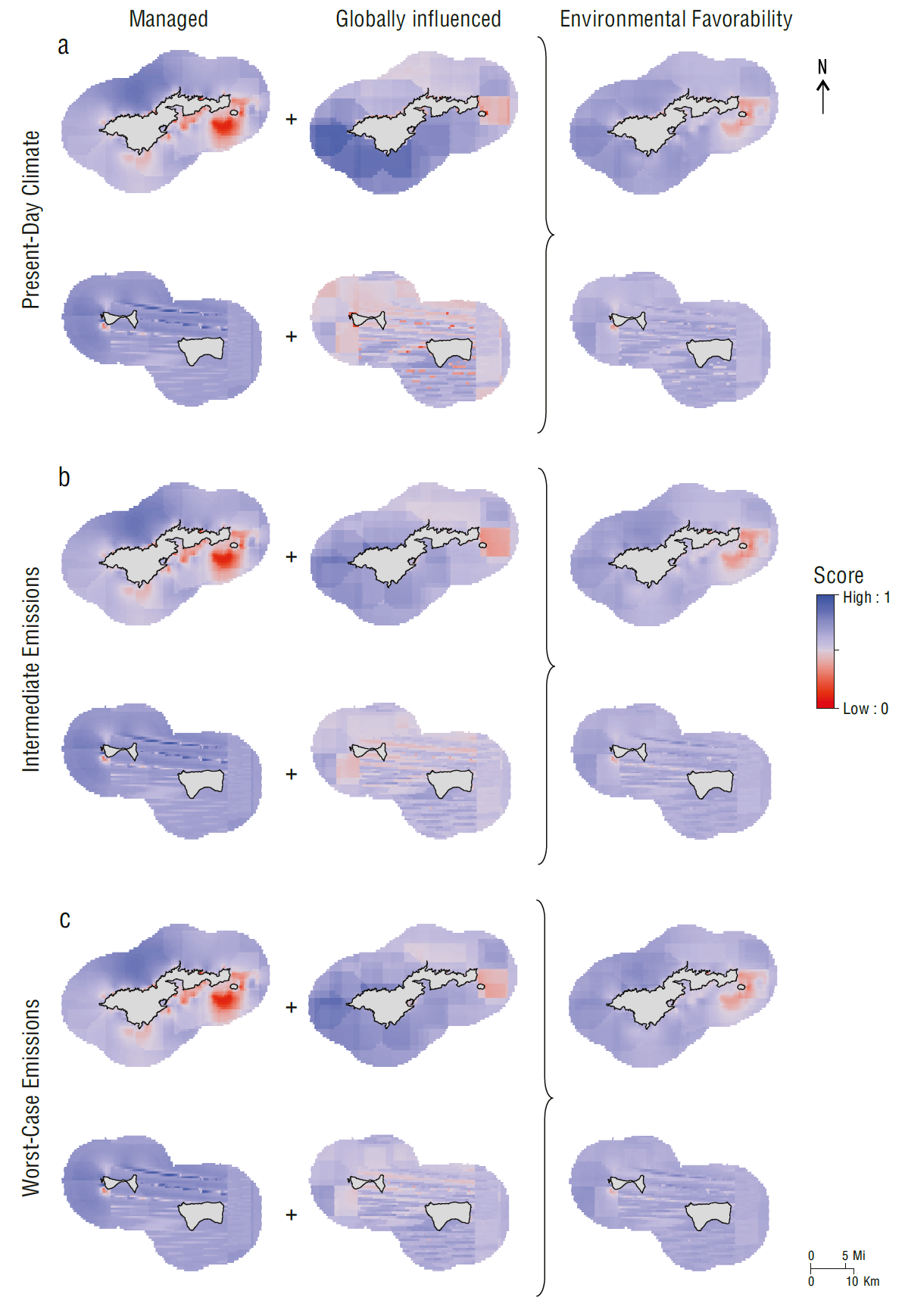
Figure 4 Scores for managed conditions, globally influenced conditions, and environmental favorability in American Samoa for the (a) present-day climate, (b) intermediate emissions scenario, and (c) worst-case emissions scenario. Scores for managed conditions remained the same across future climate scenarios. Red colors correspond to lower favorability and worse conditions for coral. Blue colors correspond to higher favorability and better conditions for coral.
Coral change and stability
In Guam, the most negative coral change scores were located around the southern tip of the island. The most positive coral change scores were identified on the southeastern and northwestern sides, reflecting gradual gains in coral cover since 2009 (Figs. 2a, 5a). The southwestern side had the lowest coral stability scores, reflecting steep declines in coral cover approaching zero. The highest coral stability scores were observed along the southeastern side and near Agana Bay (Fig. 5b) where some of the highest percentages of coral cover persisted over time (Fig. 2). The sectors formed 3 groups with distinct trends in cover: a stable group around the northern and eastern sides of the island; a group with increasing cover on the northwestern side, which exhibited positive coral change scores and low coral stability scores; and a declining cover group on the southwestern side, which had negative coral change scores and low coral stability scores (Fig. 6a). The coral change and stability scores explained 72.0% of the variation between groups (between-group sum of squares: 8.567; total sum of squares: 11.901).
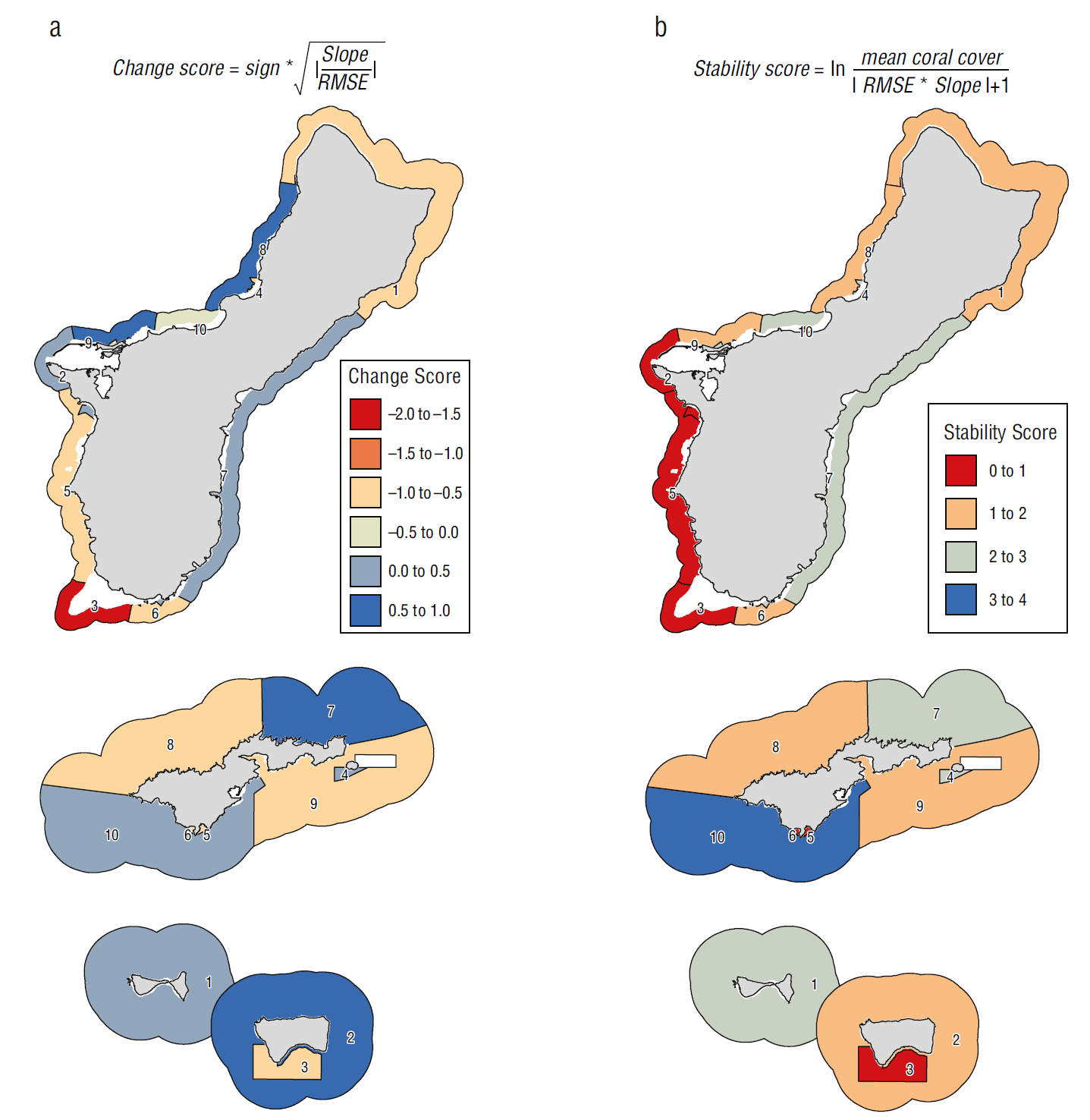
Figure 5 (a) Coral change score and (b) coral stability score by sector. Reds correspond to lower values and blues correspond to higher values. Guam sectors are unnamed and referred to by arbitrarily assigned numbers. American Samoa sector names are: (1) Ofu and Olosega, (2) Ta‘ū Open, (3) Ta‘ū Sanctuary, (4) Tutuila Aunu‘u A, (5) Tutuila Fagalua, (6) Tutuila Fagatele, (7) Tutuila Northeast Open, (8) Tutuila Northwest Open, (9) Tutuila Southeast Open, (10) Tutuila Southwest Open. Reef flat, dredged, lagoon, back reef and intertidal areas are excluded from view. Coral sector boundaries for Guam based on boundaries from Oliver et al. (2020a). Colors correspond to the coral change and stability scores. Guam sectors are unnamed and referred to by arbitrarily assigned numbers. Coral sectors for American Samoa based on boundaries from the NOAA National Coral Reef Monitoring Program. Not to scale.
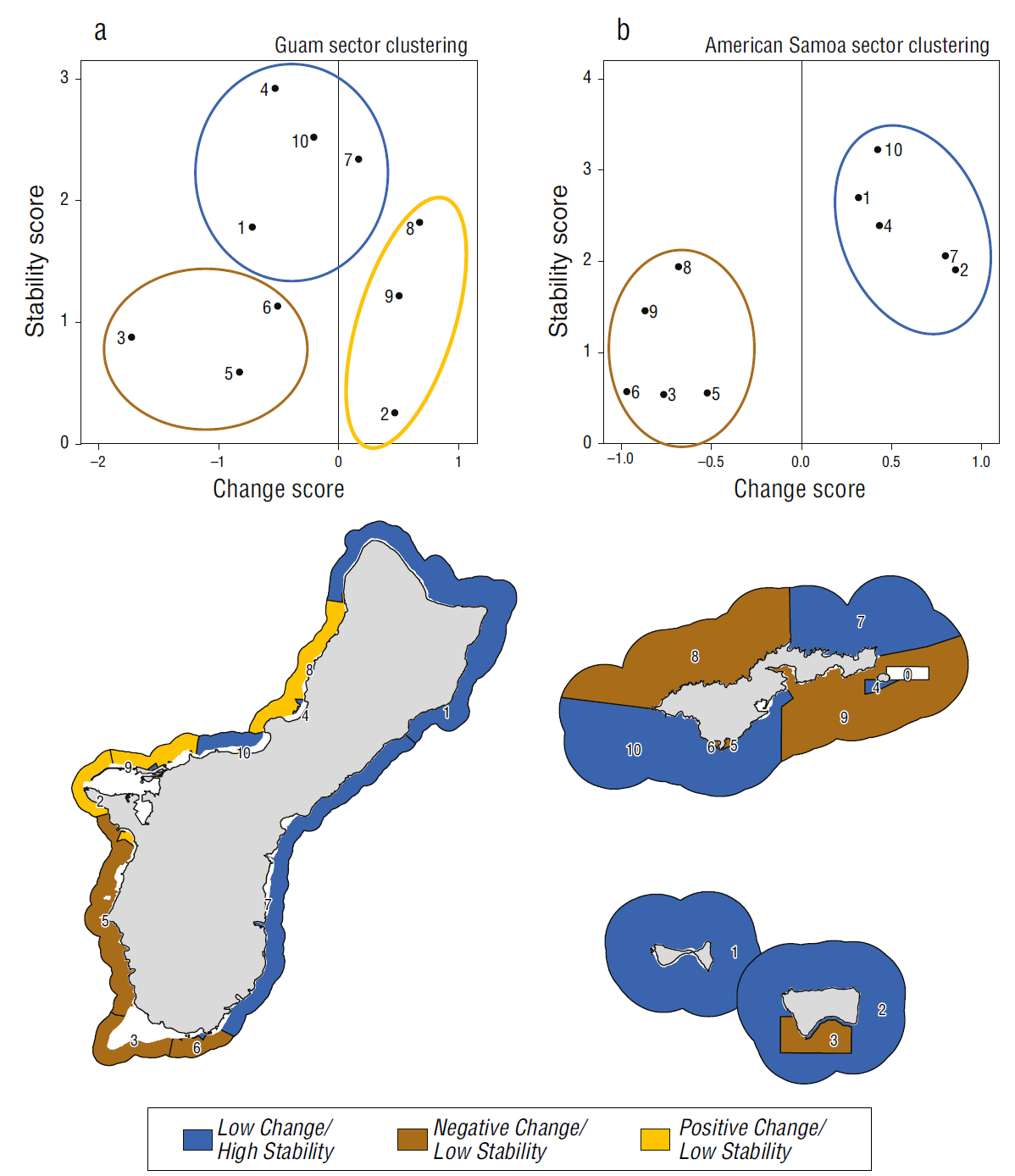
Figure 6 Sector clustering for (a) Guam and (b) American Samoa. Sectors are plotted based on change and stability scores. Cluster analysis determined group membership, shown by ellipses and corresponding colors for sector polygons on the maps. Blue indicates the low change/high stability group (high resilience). Yellow indicates the positive change/low stability group (moderate resilience). Brown indicates the negative change/low stability group (low resilience). Reef flat, dredged, lagoon, back reef and intertidal areas are excluded from view.
In American Samoa, the northeast side of Tutuila and the unprotected sector of Ta‘ū had the highest positive coral change scores due to relatively high gains in coral cover since 2010 (Fig. 5a). Southwest Tutuila, Aunu‘u, Ofu, and Olosega had low positive coral change scores, reflecting slight increases in coral cover, whereas the rest of the sectors had low to moderate negative coral change scores due to declines in coral cover (Figs. 2b, 5a). Conversely, southwest Tutuila had the highest coral stability scores, as coral cover remained the highest out of all 10 sectors. Aunu‘u, Ofu, and Olosega had moderately high coral stability scores, maintaining high coral cover at lower levels or with more variability (Figs. 2b, 5a). Northwest Tutuila, southeast Tutuila, and the unprotected sector of Ta‘ū had moderately low coral stability scores, whereas the low coral stability scores in the 3 protected sectors (Ta‘ū Sanctuary, Fagalua, and Fagatele) resulted from relatively steep declines or high variability in coral cover (Figs. 2b, 5a). Here, sectors formed 2 distinct groups: a stable group with low coral change scores and high coral stability scores around southwest Tutuila, northeast Tutuila, Aunu‘u, and most of the Manuʻa Islands and a declining cover group with negative coral change scores and low coral stability scores in northwest Tutuila, southeast Tutuila, southern Ta‘ū, Fagalua Bay, and Fagatele Bay (Fig. 6b). The change and stability scores explained 75.3% of the variation between groups (between-group sum of squares: 9.608; total sum of squares: 12.759; Fig. 6).
Synthesis of coral resilience and environmental favorability for the implementation of RBM strategies
In both locations, the overlap of highly favorable environmental conditions and stable sectors declined in future climate scenarios. The northern half of Guam exhibited the most overlap under present-day climate conditions and in both future climate scenarios (Fig. 7). The overlap decreased from 54.1 km2 under present-day climate conditions to 29.6 and 30.5 km2 in the intermediate emissions and worst-case emissions scenarios, respectively (Fig. 7b,c). The overlap between sectors with increasing cover and highly favorable conditions was considerably smaller than that of the stable sectors and limited to <8.0 km2 on the northwestern side of the island for all climates considered (Fig. 7). Declining sectors did not overlap with highly favorable conditions under present-day climate conditions, but this increased to 4.0 and 4.9 km2 near the southern end of the island in the intermediate and worst-case emissions scenarios, respectively (Fig. 7).
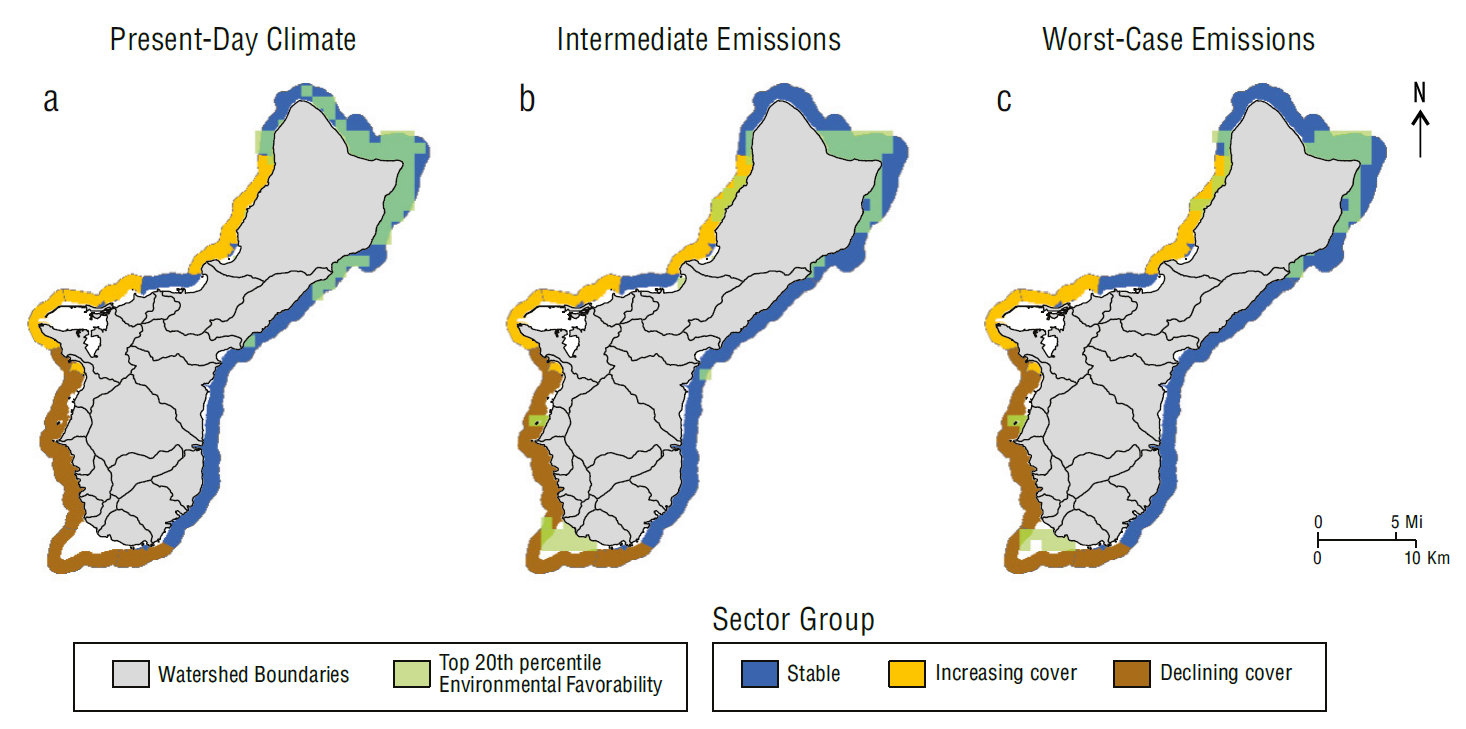
Figure 7 Map of overlap in Guam’s areas of high environmental favorability (light green) and high coral resilience (dark blue) for (a) present-day climate, (b) intermediate emissions scenario, and (c) worst-case emissions scenario. Watershed boundaries included for reference (WERI 2021). Reef flat, dredged, lagoon, back reef and intertidal areas are excluded from view.
In American Samoa, stable sectors and highly favorable conditions overlapped around the south and southwest sides of Tutuila and the western side of Ofu (Fig. 8a) under present-day climate conditions. In both future climate scenarios, this overlap shifted to include less area from the south side of Tutuila and more area from western Ofu. The overlap declined from 207.5 km2 under present-day climate conditions to 179.2 and 190.1 km2 in the intermediate emissions and worst-case emissions scenarios, respectively (Fig. 8b,c). Stable sectors outside of highly favorable conditions made up the majority of the area of the waters of American Samoa across all climate scenarios (839.9 to 868.3 km2). Declining sectors overlapped with 134.1 km2 of highly favorable conditions under present-day climate conditions, concentrated around the northwestern sector of Tutuila (Fig. 8a). This area increased in future climate scenarios, remaining concentrated in northwestern Tutuila (Fig. 8b,c).
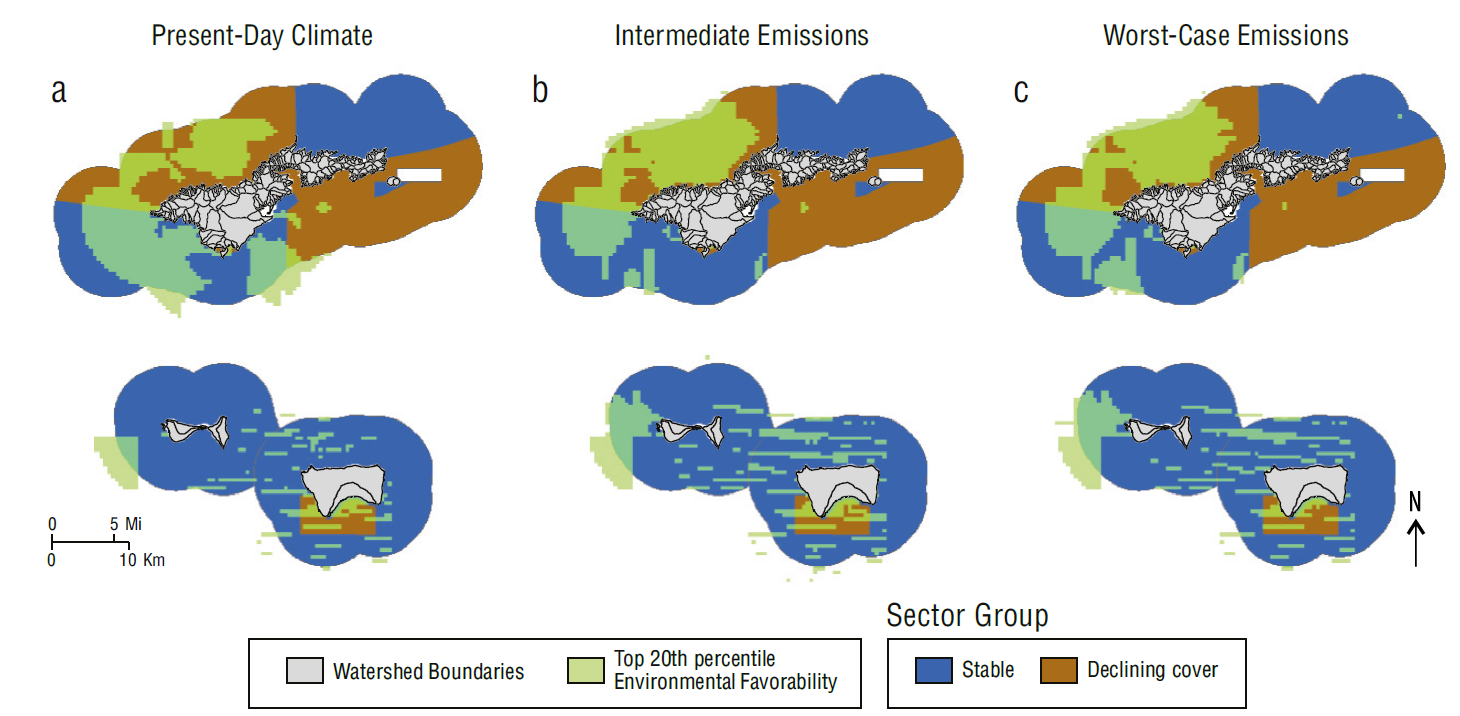
Figure 8 Map of overlap in American Samoa’s areas of high environmental favorability (light green) and high coral resilience (dark blue) for (a) present-day climate, (b) intermediate emissions scenario, and (c) worst-case emissions scenario. Watershed boundaries included for reference (Wright 2016a, 2016b). Reef flat, dredged, lagoon, back reef and intertidal areas are excluded from view.
In Guam, the area suited for the conservation of stable or increasing coral cover declined in future climate scenarios. Under present-day climate conditions, this area comprised 23% of the 238.7 km2 of the Guam study area and was spatially overrepresented compared to what was expected at random (χ2 < 0.0001, d.f. = 1, P = 0.0049). In both future climate scenarios, the area for the representation of this strategy declined to 15.1% to 15.4% of the study area (Fig. 9a) and was not significantly different from what was expected at random (intermediate emissions: χ2 = 0.2209, d.f. = 1, P = 0.3616; worst-case emissions: χ2 = 0.1455, d.f. = 1, P = 0.2971). A restoration strategy for declining coral cover was represented by a very small proportion of the study area (<3%) under all climate scenarios. Strategies for conserving genetic material or mitigating stressors could be applied to the highest proportion of Guam (52% to 60% of the study area; Fig. 9a).
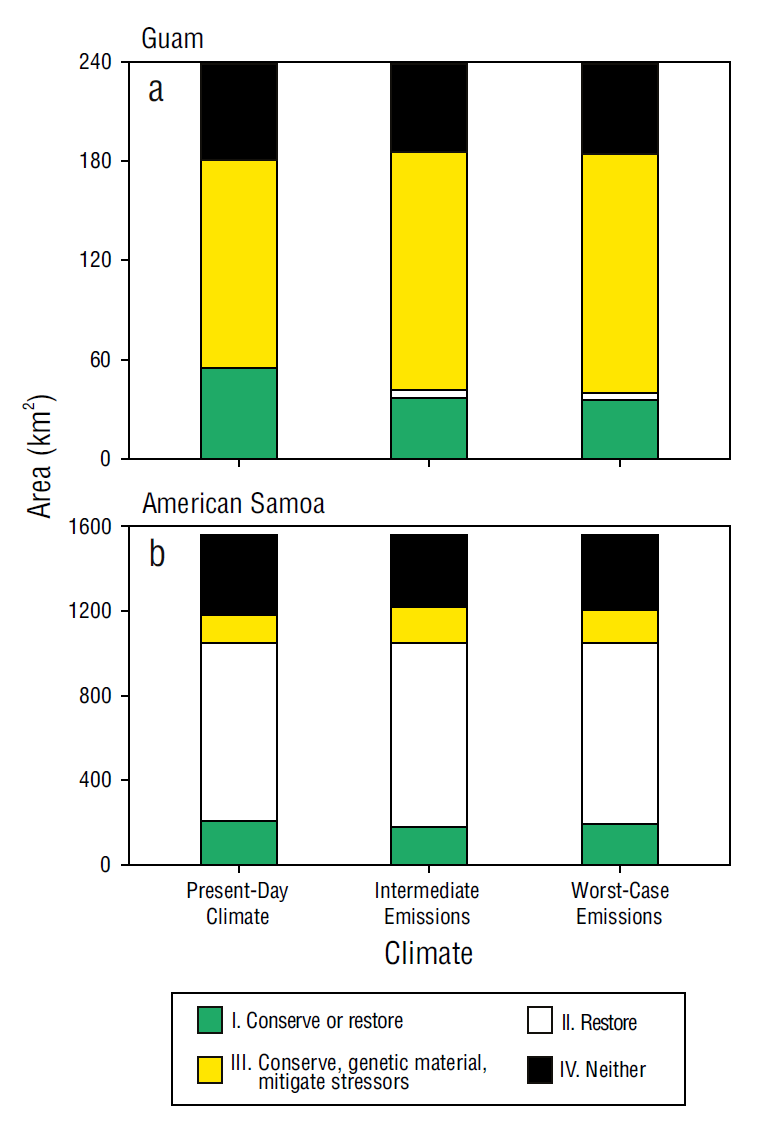
Figure 9 Area of sectors for (a) Guam and (b) American Samoa that correspond to management strategies outlined in Table 1 based on trends in coral cover and environmental favorability scores.
In American Samoa, the proportions of the areas in which each applicable set of RBM strategies (Table 1) could be applied were similar (varying by <3%) across climate scenarios (Fig. 9b). The areas for applying the strategies of conserving stable coral populations, conserving genetic material, and mitigating stressors for declining coral cover under favorable conditions were similar, each occupying 9% to 13% of the study area (1,556.9 km2). The management strategy of restoring declining coral cover could be applied in the largest area, comprising 54% to 56% of the study area. This strategy was consistently overrepresented, whereas conserving genetic material and mitigating stressors were underrepresented (9% to 11%; present-day climate: χ2 < 0.0001, d.f. = 1, P < 0.0001; intermediate emissions: χ2 < 0.0001, d.f. = 1, P < 0.0001; worst-case emissions: χ2 < 0.0001, d.f. = 1, P < 0.0001; Fig. 9b). Despite exhibiting consistent proportions among strategy areas, American Samoa showed greater geographic shifts regarding where these strategies could be applied in future climate scenarios compared to those of Guam (Figs. 7-9).
Discussion
Our results highlight areas where corals showing 2 potential characteristics of resilience, positive change and stability, co-occur with favorable environmental conditions both now and in multiple future climate scenarios. Prior resilience assessments in the Pacific Islands region partially agree with the geographic patterns identified in this study. In Guam, the environmental favorability scores were highest around the northern half of the island (Fig. 3), which aligned with the highly resilient sites that Maynard et al. (2018) identified based on scaled scores for coral cover, coral diversity, coral recruitment, bleaching resistance, macroalgae cover, herbivorous fish biomass, and temperature variability. The northwestern coast of Guam is also a center of importance for marine biodiversity, suggesting that RBM strategies in this area could contribute additional benefits to taxa other than corals (Dobson et al. 2021). The stable coral sectors in American Samoa partially aligned with the results of a prior resilience assessment that incorporated coral diversity and coral performance during past bleaching events (Oliver et al. 2020a), particularly around the Manuʻa Islands (Fig. 6). However, around Tutuila, the stable sectors in this study were different than those identified by Oliver et al. (2020a), who found that only the northwestern sector of Tutuila exhibited medium to high resilience based on average scaled scores of coral diversity, herbivorous fish biomass, macroalgae cover, juvenile coral diversity, fishing depletion, bleaching resistance, coral disease, and temperature variability. This divergence illustrates that examining climate-related changes in community structure and ecosystem functions could complement our species-indifferent approach, yielding a more comprehensive picture of ecosystem resilience, as coral stress tolerance varies by taxon and life history stage (McCowan et al. 2012, Nalley et al. 2021).
The large differences in the relative proportions of the applicable RBM strategies between the 2 jurisdictions (Fig. 9) could have been driven by the greater overlap of highly favorable conditions with declining coral sectors in American Samoa, creating a larger area potentially suitable for restoration (Figs. 7, 8), whereas restoration did not emerge as a widespread strategy for Guam (Fig. 9). The principles of RBM suggest that restoration is more successful in areas with low environmental stress compared to the surrounding areas (Mcleod et al. 2019). In contrast, the presence of the large area of Guam suitable for the conservation of genetic material or stressor mitigation suggests that it contains several sectors resilient to challenging conditions. Repeated exposure to environmental variability and stress has been documented to increase positive outcomes after severe disturbance events. For example, corals exposed to variable water temperatures maintain higher photosynthetic efficiency and exhibit higher survival rates during heat stress events than those of corals living in less variable water temperatures. These differences can be attributed to symbiont clades and to the corals themselves (McClanahan et al. 2007, Oliver and Palumbi 2011).
Strengths and limitations of this approach
The sector-wide averages of the aggregated NOAA NCRMP data did not allow us to explicitly incorporate coral community structure into our approach. The sector boundaries in American Samoa were based on NOAA management units and were largely influenced by protected area boundaries. The disagreement between our results and those of Oliver et al. (2020a) regarding which sectors of American Samoa showed signs of resilience could stem from the fact that breaks in community structure were not closely aligned with sector boundaries. In contrast, coral community structure informed the boundaries of the Guam sectors, possibly explaining the comparatively better agreement between our results and those of Maynard et al. (2018). This suggests that setting the boundaries of aggregation units based on similarities in community structure can improve the use of coral cover metrics in resilience assessments in which repeated surveys of community composition are spatially limited or absent.
One strength of our approach is that it utilizes aggregated data such as those reported by government monitoring agencies. The collection and interpretation of coral community data, while offering valuable insights into ecosystem dynamics, requires more time and expertise than analyzing coral cover without regard to species. Coral change and coral stability scores provide metrics for comparisons among coral cover trends over time and are easily repeatable. Furthermore, it is easy to spatially overlay these metrics with environmental variables to reveal alignments in potential drivers. However, these scores do not capture nuances of community change post-disturbance. For example, in the wake of repeated bleaching events, a low-diversity assemblage of fast-growing species may recolonize a formerly diverse assemblage. Alternatively, an assemblage of slower-growing, heat-tolerant species may be all that survives (van Woesik et al. 2011, Guest et al. 2012). Implications for fish and invertebrate communities are also not captured in these scores. We do not suggest that this approach should replace community surveys. Rather, our approach complements these labor-intensive surveys by providing a rapid way to assess sector-wide trends.
Implications for management
When selecting sites to implement RBM, many managers prefer to target areas with desirable conditions for specific taxa in multiple climate scenarios. These preferences are based on the assumption that positive outcomes, in terms of ecosystem function and biodiversity, are more likely in these locations, regardless of the uncertainty surrounding the severity of climate change impacts (Moilanen et al. 2006, Polasky et al. 2011, Neuman et al. 2019). In this respect, Guam may exhibit more geographic consistency for applying RBM strategies than American Samoa as the climate continues to change. In contrast, the proportions of applicable RBM strategies remained similar in American Samoa, although larger geographic shifts regarding where they should be implemented were present (Figs. 7-9). Regardless, for both jurisdictions, the RBM strategies outlined in this study (Table 1) could be suitable in >75% of their sectors (Fig. 9).
A lack of fine-scale climate projections poses a challenge for evaluating the potential impacts of future environmental conditions on coral growth and survival. For example, sea surface temperature projections from the Coupled Model Intercomparison Project (CMIP6) were only available at 0.5-degree resolution (Krasting et al. 2018) at the time of analysis, representing small island territories with only a few cells. Future projections for only 2 globally influenced variables, thermal stress and SLR-related erosion, were available at sufficient resolution to be included in the analysis of future climate scenarios. Notably, the projected onset of annual severe bleaching differed by less than a decade between the intermediate emissions and worst-case emissions scenarios, likely contributing to the similarity in environmental favorability scores. In the Pacific Islands region, many marine management actions are considered at the scale of single watersheds, which typically have <10 km of coastline (Comeros-Raynal et al. 2019), demonstrating a mismatch between the spatial scales of the available climate projections and the detail needed for local management decisions. As the resolution of downscaled climate projections improves for more variables, this assessment could be repeated to provide greater local-scale nuance.
We have highlighted areas that may be suitable for conserving existing robust coral populations, restoring corals, and mitigating environmental stressors. These ecological considerations can be added to socioeconomic and logistical considerations to determine locally appropriate RBM strategies (Oliver et al. 2020c), assisting reef managers and local stakeholders in making decisions that facilitate adaptive responses to climate change.











 text in
text in