INTRODUCTION
The Kellet’s whelk, Kelletia kelletii (Forbes, 1850), is a mostly subtidal buccinid gastropod endemic to the Eastern Pacific with a range that spans from Monterey Bay, California (USA), to Baja California Sur (Mexico) (McLean 1978, Herrlinger 1981). It is a conspicuous snail with a bright yellow to orange body and fairly large, high-spired shell often covered in pink and green algae. This species is dioe cious and its mating aggregations can include 200-300 indi viduals (Rosenthal 1970, Morris et al. 1980, Lonhart 2001). Its biogeography, life history, and many of its larval charac teristics have been described by MacGinitie and MacGinitie (1949), Rosenthal (1970), Morris et al. (1980), Lonhart (2001), Zacherl et al. (2003), Zacherl (2005), Romero et al. (2012), Rodriguez (2017), and Wilson (2017). Herein, additional aspects of this species’ larval biology are docu mented, including pre- and post-hatching endogenous yolk reserves, veliger velar lobe morphology, particle capture and ingestion, asymmetrical development, and larval shell mor phology. Kelletia kelletii snails were observed in aquaria and egg capsules were selected, dissected, and examined at dif ferent stages of larval development to chronicle the charac teristics listed above. Larval shells and veligers were also examined using scanning electron microscopy (SEM) at early to late veliger stages.
In the more than 700 species within the gastropod family Buccinidae, egg capsules are laid in clutches, masses, towers, or capsule strings on benthic substrates that sometimes include the shells of conspecifics (e.g., Solenosteira, Neptunea, and Kelletia) (Power and Keegan 2001). Depending on the species, egg capsules can house a single embryo to more than 1,000 (Thorson 1950). Intra capsular embryos develop into either non-planktonic larvae that crawl out of their capsule as metamorphosed juveniles (direct development) (Romero et al. 2004) or planktonic veliger larvae that swim out of their capsule, may or may not be obligated to feed, and then metamorphose into ben thic adults (planktonic development) (Pearce and Thorson 1967). Developmental mode is not known to vary within buccinid species (Miloslavich and Penchaszadeh 1994, Tan and Morton 1998).
During intracapsular development, buccinid larvae derive nourishment from their own yolk, intracapsular albumen (protein-rich fluid that fills the capsule), and/or by ingesting eggs (oophagy), young embryos (nurse eggs), and/or sibling larvae (adelphophagy) within their capsule. Unlike most buc cinids in the North Pacific, K. kelletii has a planktonic devel opmental mode (Rosenthal 1970, Pechenik 1979, Romero et al. 2012) wherein early development, including the tro chophore, occurs inside an egg capsule without nutritive intracapsular eggs or embryos (Rosenthal 1970). At excap sulation, larvae emerge from capsules as feeding veligers (Buckland-Nicks et al. 2002).
With few exceptions, documentation of larval develop ment, and particularly larval morphology, in buccinid gas tropods is lacking, especially for taxa with planktonic larvae. Accounts of detailed larval characteristics across this diverse family could inform studies of larval development, larval ecology, and developmental evolution (Collin and Moran 2018). Thus, this study is intended to complement the work that has documented much of early ontogeny in K. kelletii and provide additional information for comparative studies of development within and across neogastropod clades.
MATERIALS AND METHODS
Voucher material including adult shells, preserved egg capsules, and mounted larval shells are deposited in the Uni versity of California (UC) Museum of Paleontology (UCMP) extant shell collections and wet collections.
Kelletia kelletii specimen collection and maintenance
Twelve adult specimens of K. kelletii were collected in California by hand using scuba from subtidal kelp forests near Monterey (n = 7: 4 females, 3 males) by T. Herrlinger (UC Berkeley) in January 2006 and Santa Barbara (n = 5: 2 females, 3 males) by S. Anderson (UC Santa Barbara) in June 2007.
Snails were maintained in the Valley Life Sciences Building on the UC Berkeley campus in a climate- and light-controlled room in a re-circulating and aerated 10-gallon saltwater aquarium. Automatic overhead fluores cent lights were kept on a 12-h light/day-dark/night cycle. Water temperature remained at 13-16 ºC and was measured every other day using a standard glass aquarium thermom eter mounted to the inside of the aquarium. Salinity was mea sured weekly using a salinity refractometer and maintained at 34-35.
One third of the tank water was replaced with artificial or natural seawater once per month. Artificial seawater was made with Instant Ocean Sea Salt (Instant Ocean, Blacks burg, Virginia) to 34 salinity following the manufacturer’s instructions. Natural, filtered seawater was acquired from Hopkins Marine Station, Pacific Grove, California. Adult snails were fed shrimp or bivalves every 3 to 4 weeks.
Behavioral, capsule, and larval observations
Mating and oviposition behavior was checked for at least 4 d per week and observed from January 2006 through October 2009. To chronicle the sequence of developmental changes in early ontogeny, 65 egg capsules were removed randomly from within the same clutches as well as from dif ferent clutches. Only egg capsules for which the date of ovi position was known were used for data collection.
Embryos and larvae were excapsulated by hand (with forceps and dissecting scissors) to allow different stages of development to be sampled, drawn, and photographed. A random sample of 5 egg capsules from different clutches was used to estimate egg/embryo number per capsule, and 15 cap sules were measured from their base to their escape aper ture to determine their typical dimensions. The onset of velar structures, organs, larval pigment, and larval shell mineral ization were observed and recorded. After observations were made, the contents of capsules were sacrificed and preserved.
Intracapsular behavior was observed through transparent capsules laid by one female collected from Santa Barbara. Capsules, eggs, larvae, and swimming veligers were observed under magnification with a Wild Heerbrugg M-series ste reomicroscope. Digital photographs and digital videos were taken through this microscope using an optical coupler (Optem) and Coolpix (Nikon) 995 camera. The total number of eggs, embryos, and larvae examined was not counted but would be 32,500 if there were 500 eggs, embryos, or larvae in each of the 65 examined capsules.
Larval feeding
In 5 trials, at least 50 veliger larvae that naturally emerged from their capsules and at least 50 that were excapsulated by hand were transferred by glass pipettes to Syracuse watch glasses filled with micro-filtered seawater. To test for feeding ability, a solution of 100 mL seawater was mixed with approximately 1 teaspoon of red or blue non-toxic micro-particles (DayGlo fluorescent pigment; DayGlo Color Corporation, Cleveland, Ohio), which was introduced into the water via a pipette. Veligers were left undisturbed in this particle-rich seawater for 10 min before being checked for ingested particles, which would be visible in their gut through their translucent larval shell. If after 10 min none had particles visible within their gut, they were considered unable to feed; if particles were visible, they were consid ered planktotrophic.
Larval shells: preparation for scanning electron microscopy
Ontogenetic series of larval shells (of at least 3 specimens each) were prepared from 35 samples of both intracapsular and emerged veligers. Isolated larval shells were prepared for SEM analysis following a modified protocol of Pedersen and Page (2000): veligers were gently removed from beakers using a pipette, anaesthetized in MgCl2 for 2 h, then bathed in a 3:10 (bleach: water) solution for up to 18 h. Larval shells were rinsed with deionized water, allowed to dry on lint-free paper, then transferred by a fine sable brush into glass vials filled with acetone prior to mounting.
Cleaned larval shells were mounted with a sable brush onto conductive carbon adhesive pads on aluminum SEM stubs. Shells were repositioned when necessary using a paintbrush hair mounted on a wooden stirring stick. Mounted specimens were sputter coated with iridium on a Medo20 Sputter Coater to a thickness of 0.014 kÅ (kiloangstrums) and visualized with a Philips XL-30 ESEM. Specimens were viewed at 10-15 kV accelerating voltage and 15 mm working distance. Digital micrographs were taken using Philips XL-30 imaging software, and contrast and brightness were adjusted in Adobe Photoshop CS4. From these images, characteristics of larval shell ornamentation, microstructure, and shape were observed and recorded. Measurements were made using ImageJ digitizing and imaging software (v.1.36b, National Institutes of Health; Image J 2004).
Larvae: preparation for scanning electron microscopy
Veliger larvae were relaxed in calcium-free seawater (Louise Page, pers. comm.), fixed in a 2% glutaraldehyde solution, rinsed in an osmium tetroxide and sodium caco dylate buffer, and dehydrated in a 7-step ethanol series from 35% to 99% ethanol. Fixed specimens were then critical point dried and visualized uncoated using a Hitachi TM-1000 SEM at an accelerating voltage between 15 and 20 kV.
RESULTS
Shell size, mating, and oviposition
Mean maximum length (ML) (from apex to siphonal canal tip) of shells collected in Santa Barbara snails (n = 5) was greater than that in Monterey-collected snails (n = 6) at ML = 92.5 mm, SD = 8.5 mm vs. ML = 85.2 mm, SD = 7.8 mm. The same was true for mean maximum width (MW) (i.e., Santa Barbara-collected specimens: MW = 51.6 mm, SD = 2.9 mm; Monterey-collected specimens: MW = 44.3 mm, SD = 4.7 mm). Males and females from Santa Barbara were not significantly different in shell length or width. Although the sample size is small, in Monterey-collected snails, the mean ML of shells was larger in females than in males: for females (n = 4) ML = 86.4 mm, SD = 8.9 mm, MW = 45.6 mm, SD = 4.7 mm; for males (n = 3) ML = 81.9 mm, SD = 5.0 mm, MW = 42.2 mm, SD = 3.6 mm.
In aquaria, Santa Barbara-collected snails (n = 5) mated from January-February, and Monterey-collected snails (n = 6) mated from May-June. Females tended to lay egg capsules next to clutches already laid by other females on the glass sides of the aquarium, on rocks, and in one case, on the shell of another adult (Fig. 1a). Oviposition while mating was observed twice.
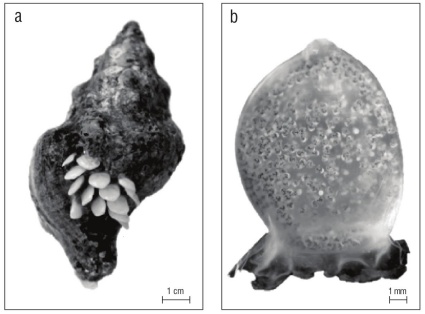
Figure 1 Kelletia kelletii egg capsules. (a) Living K. kelletii with egg capsules oviposited on its shell. (b) Single egg capsule with larvae nearly ready to emerge; capsule’s escape aperture is at the top.
Sixteen instances of egg laying (oviposition) were recorded between May and October from 2006 to 2008: 3 in May, 6 in June, 3 in July, and 4 in August. The identity of the female that was ovipositing could be determined in 8 instances, and of these, the same Monterey-collected female oviposited from May-July, and 2 Santa Barbara-collected females oviposited from July-August.
Egg capsules and intracapsular eggs and embryos
Egg capsules were laid in benthic clutches of 3 to 82 cap sules per clutch (n = 15, mean = 25.1, SD = 23.3). In most capsules, the escape aperture and capsular plug were at the capsule top (Fig. 1b), although in one clutch the escape aper ture was on the side of all capsules. Capsule walls were mostly translucent white to rarely transparent, and in the specimens examined here, rarely enclosed zero eggs or embryos. Egg/ embryo number varied between 477 and 966 per capsule (n = 5, mean = 887.4, SD = 183.8). Capsules averaged 8.09 mm in height from their base to escape aperture (n = 15, SD = 1.1) and 6.60 mm in maximum width (n = 15, SD = 0.34). Almost all were lingulate, slightly convex/concave, and without sutures. Capsule thickness was not measured.
Larval development
Development in 13-16 ºC seawater was observed from uncleaved fertilized eggs, to trochophores, to excapsulated swimming veligers. Eggs and trochophores were bright yellow to orange. Black pigment developed on veligers prior to their emergence and this color change made the translu cent capsules appear gray (Fig. 1b). The onset and sequence of larval traits from the day of oviposition are summarized below and in Figure 2.
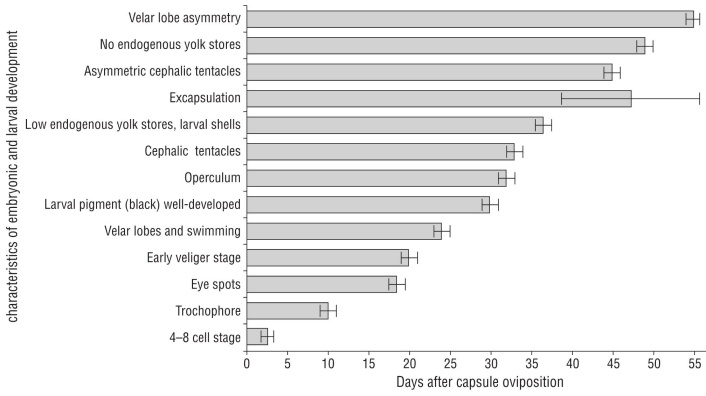
Figure 2 Characteristics of embryonic and larval development in Kelletia kelletii in aquaria at 13-16 ºC beginning at oviposition (day 0). Error bars indicate mean ± SD. Graph design inspired by Horwitz et al. 2017.
At the day of oviposition, zygotes were observed as uncleaved eggs (Fig. 3a). Cell differentiation into 4-8 cells was evident at 2-3 d (Fig. 2, 3b). The post-gastrula to tro chophore developed by 9-11 d (Fig. 3c). At the late tro chophore stage, embryos slowly rotated inside capsules. At 18-19 d, eye spots were present and some larvae had black pigment. Early veligers were evident at 19-21 d and they were closely packed and had a larval shell, velum, and red-colored larval kidney. After 21 d, veligers showed cil iary movement and black pigment had become a prominent ring around the shell margin. At 23-25 d after oviposition, veligers actively swam inside the capsule with small velar lobes. At 27 d, if excapsulated, veligers were phototaxic and competent swimmers with 2 endogenous yolk balls in their shell (Fig. 3d). By 30 d veligers had well-developed black pigment. Their operculum was obvious by 32 d. At 33 d, cephalic tentacles were present and veligers released a grainy exudate. By 36-37 d, yolk reserved were small to absent, larval shells had holes and were not fully mineral ized (Fig. 3e, f), and many veligers were nearly breaking through the capsular plug. If excapsulated at this time, veli gers did not ingest micro-particles. At 38-55 d, veligers emerged from their capsules as phototaxic planktonic veli gers, some with small endogenous yolk reserves in their shells, some with holes in their shells, and all with black pigment on their foot. By day 45 swimming veligers had asymmetrical cephalic tentacles. At 49 d, there were no endogenous yolk reserves within the shells of most veli gers. At 53-54 d, velar lobes were noticeably asymmetrical. At 69 d, veligers could ingest micro-particles by trapping them in their velar lobes and bringing them toward the mouth. At 82 d after egg capsule deposition, few swimming veligers were alive without a food source.
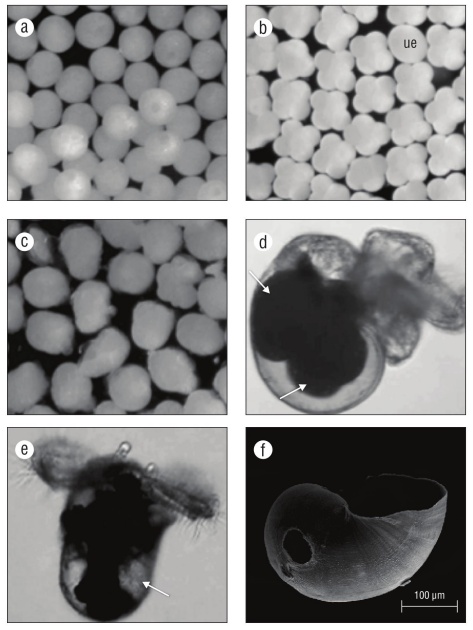
Figure 3 Kelletia kelletii embryonic and larval stages. (a) Zygote; (b) 4-8 cell stage (ue: egg or undeveloped embryo); (c) post-gastrula to trochophore stage; (d) encapsulated veliger (pre-hatching), with arrows indicating large endogenous yolk reserves; (e) excapsulated veliger (post-hatching), with arrow indicating low endogenous yolk reserve; and (f) SEM micrograph of late pre-hatchling larval shell with brittle edges and hole due to poor mineralization.
Velar form and function
Feeding experiments indicate that opposed band cilia (the prototroch and metatroch) of velar lobes of K. kelletii are capable of particle capture and transport in addition to swimming. Before veligers naturally hatched from their cap sules, they used the velum to swim within the albumen of the capsule. All veligers that could swim outside of the capsule whether naturally hatched or excapsulated by hand were pho totaxic. Before and after natural excapsulation, while yolk reserves were present, veligers could pull micro-particles from the water into the food groove of either velar lobe, but the particles would not be ingested; they would travel toward the mouth (Fig. 4) and be expelled down the foot.
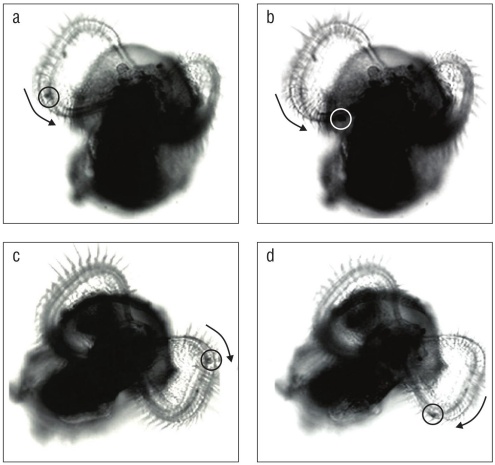
Figure 4 Particle capture and transport through the velar food groove in Kelletia kelletii veligers. Images were isolated from digital videos. Arrows indicate direction of particle movement. (a, b) Particle capture and counterclockwise movement in the right velar lobe of a post-hatched veliger. (c, d) Particle capture and clockwise movement in the left velar lobe of a post-hatched veliger.
Asymmetrical development
Prior to hatching, veliger velar lobes were of equal size but cephalic tentacles were not (Fig. 5a, b). In all observed veligers, the right tentacle was longer than its counterpart on the left. By at least 50 d old or 2.5 weeks in the plankton, depending on the date of excapsulation, velar lobes were also asymmetrical, with the larva’s right lobe larger than its left (Fig. 5c).
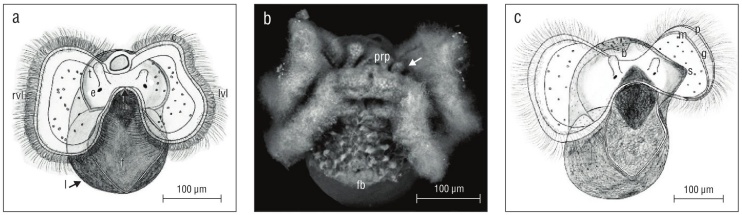
Figure 5 Kelletia kelletii veliger larvae at 2 weeks prior to hatching (a, b) and 2 weeks after hatching (c). (a) Veliger showing symmetrical velar lobes and asymmetrical cephalic tentacles; abbreviations: c, velar cilia; rvl, right velar lobe; lvl, left velar lobe; t, cephalic tentacle; e, eye; m, mouth; f, ciliated foot; l, larval shell. (b) SEM micrograph of veliger. Arrow indicates smaller left cephalic tentacle; abbreviations: prp, post-oral ciliary patch; fb, foot bristles. (c) Veliger showing asymmetrical velar lobes and asymmetrical cephalic tentacles; abbreviations: b, larval shell beak; g, food grove; s, proto-siphonal canal; p, prototroch; m, metatroch.
Undeveloped eggs, yolk reserves, and particle ingestion
Undeveloped or developmentally arrested embryos com prised 2.5%-38% of intracapsular individuals. In all dissected capsules (n = 65) there was a proportion of eggs/embryos that did not match the developmental stage of the majority of individuals in the capsule. This was true as early as the 4-cell stage as well as at veliger excapsulation. Undeveloped eggs were neither damaged nor were they in any stage of disinte gration in all capsules examined, indicating that they had not been damaged or eaten by developing larvae. Observations through transparent capsules confirmed that trochophore and veliger larvae did not eat or disturb undeveloped eggs and embryos as they would have if these were nutritive mate rial such as nurse eggs. Intracapsular embryos and veligers were fed by endogenous egg stores and possibly ingested protein-rich albumen.
Prior to hatching, bright yellow endogenous yolk reserves filled most of the veliger larval shell (Fig. 3d). Yolk was distributed into 1 or 2 ball-shaped reserves visible through the translucent shell. If veligers were excapsulated while yolk reserves were abundant, they would not ingest micro-particles. If micro-particles were introduced to an excapsulated pre-hatchling veliger, the pigment particles would get caught in the velar lobes, settle in the food groove, and move toward the mouth where they would be expelled down the ciliated foot. Veligers did not ingest particles unless their yolk stores were depleted (e.g., in some cases at 18 d in the plankton). Ingestion of micro-particles was confirmed by the bright color of the DayGlo micro-particles visible within the gut.
Larval shell morphology
Shells of late pre-hatching intracapsular veligers were thin, brittle, and often had holes indicating poor shell miner alization (Fig. 3f). Shells of emergent veligers were similar to those of pre-hatchlings but were slightly more mineralized and not as brittle. Between day 13 and 15 as a veliger, larval shells were fully mineralized and growth lines were evident (Fig. 6a, b, c). Late stage veligers at or near 20 d as excap sulated swimming veligers had a larval shell apertural beak and the beginnings of a siphonal canal (proto-siphonal canal) (Fig. 5c, 6c). In shells of veligers of different ages, bead-like micro-protuberances were evident up to the apertural margin (Fig. 6d, e, f). The maximum diameter of K. kelletii larval shells was 0.407 mm (n = 10) in apical view. How ever, overall size varied within capsules and cohorts were not always at the same stage of shell development and calcifi cation, especially as pre-hatchlings and early-stage emerged veligers.
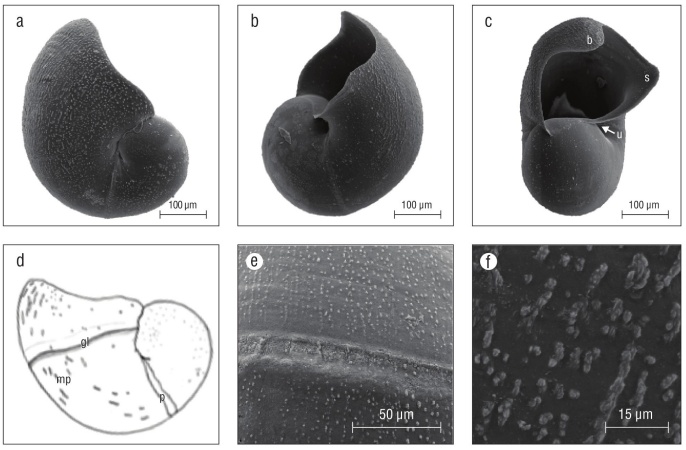
Figure 6 Kelletia kelletii larval shells. (a-c) SEM micrographs of larval shells of veliger larvae 2 weeks after hatching, (d) line drawing of late-stage veliger shells with labeled features, (e) SEM micrograph of protoconch 1 boundary, and (f) SEM micrograph of larval shell micro-protuberances. Abbreviations: b, apertural beak; u, umbilicus; s, proto-siphonal canal; gl, shell boundary indicating shell size at hatching; mp, bead-like micro-protuberances; p: boundary/break in growth of protoconch 1.
DISCUSSION
Many observations of K. kelletii reported here are consis tent with those documented elsewhere (e.g., by MacGinitie and MacGinitie 1949, Rosenthal 1970, Morris et al. 1980, Zacherl et al. 2003, Zacherl 2005, Romero et al. 2012, Rodriguez 2017, Wilson 2017). For example, larvae are non-feeding within their egg capsules, excapsulated larvae swim and feed in the plankton as veligers, and excapsulated larvae are phototaxic. Notably, several observations of larval mor phology and development are reported here for the first time: all examined egg capsules included developmentally arrested or non-fertilized eggs; after emergence from their capsule, veligers fed on micro-particles only after their endogenous yolk stores were depleted; early larval shells were not fully mineralized; and excapsulated veligers had asymmetrical cephalic tentacles and velar lobes.
The substantial range in excapsulation timing in this study, from 39 to 55 d at 13-16 ºC compared to 30-34 d at 14.5-17.5 ºC in other studies (Rosenthal 1970, Zacherl et al. 2003), could be due to the cooler water temperatures main tained in this study as well as the minimal water disturbance in parts of the aquarium, which seemed to allow plugging of the capsule escape aperture with masses of undeveloped eggs. In areas of the aquarium that were better aerated and had greater water disturbance, veliger excapsulation was assisted by the water’s agitation of the capsules. In still sea water, e.g., in a glass beaker, it was not uncommon for veli gers to hatch after 45 d or more and for many intracapsular veligers to die inside of the capsule.
The asymmetrical development of velar lobes in late-stage K. kelletii veligers may counterbalance asymmetrical shell development, which, at that time, includes a proto-siphonal canal. This shell extension is opposite the larger right velar lobe, and when this feature is absent (as in younger veli gers), the velar lobes are equal in size. Unequal velar lobes in swimming veligers with asymmetrical shell develop ment has also been observed in other gastropods, including Echinolittorina hawaiiensis (Rosewater & Kadolsky, 1981) [formerly Littorina picta] (Struhsaker and Costlow 1968). In veligers, right cephalic tentacles that are larger than the left during some stage of larval development have also been observed in other marine snails (e.g., Thais chocolate (Duclos, 1832) (Romero et al. 2004) and Amphissa versicolor Dall, 1871 (Page and Parries 2000).
Dense taxon sampling is necessary within the Buccinidae, and other gastropod groups, for phylogenetic analyses that can elucidate the evolution and distribution of larval devel opmental mode (Collin and Moran 2018). Of course, such studies are only as good as the larval data examined with the phylogeny. Therefore, detailed data for this species, and others, of larval developmental timing, morphology, nutri tive eggs, and intracapsular and extracapsular feeding mode are essential to inferring homology, understanding ancestral versus derived character states (Collin and Moran 2018), and making novel insights about evolutionary patterns in larval evolution.











 text new page (beta)
text new page (beta)


