Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Ciencias marinas
Print version ISSN 0185-3880
Cienc. mar vol.31 n.3 Ensenada Sep. 2005
Artículos
Diet and weaning age affect the growth and condition of Dover sole (Solea solea L.)
La dieta y la edad de destete afectan el crecimiento y la condición del sol común (Solea solea L.)
Rebeca A. Rueda-Jasso1*, Luis E.C. Conceição2, Wim De Coen3, Jean Francois Rees4 and Patrick Sorgeloos1
1 Laboratory of Aquaculture and Artemia Reference Center, Ghent University Rozier, 44 B 9000 Gent, Belgium. * E-mail: Rebeca_Aneli@yahoo.com
2 CCMAR Centre of Marine Sciences, University of Algarve, Campus de Gambelas 8000-117 Faro, Portugal.
3 Laboratory for Ecophysiology, Biochemistry and Toxicology, University of Antwerp, B 2020 Antwerp, Belgium.
4 Animal Biology Unit, Catholic University of Louvain, B 1348 Louvain-la-Neuve, Belgium.
Recibido en julio de 2004;
aceptado en abril 2005.
Abstract
The effect of diet type (frozen Artemia biomass and two inert diets: micro-bound [MB] and micro-extruded [ME]) and two weaning ages (early weaning and late weaning, 50 and 64 days after hatching, respectively) were studied in Solea solea larvae. The experiment lasted 56 and 42 days for early and late weaning, respectively. The mortality results showed the highest values for late weaning (39%) in the Artemia treatment. No significant differences in mortality were observed between the inert diets. The final dry weight values were higher for late weaning than for early weaning. At both weaning ages, fish receiving the same treatments had similar tendencies for dry weight and standard length. Fish fed with MB presented significantly higher dry weight and standard length, followed by ME, while the lowest values at both weaning ages were recorded for the Artemia treatment. Similar amounts of highly unsaturated fatty acid fractions among the inert diets were reflected by the absence of significant differences in the susceptibility to oxidation (thiobarbituric acid reactive substances testing); however, significant differences were found in carbohydrate, protein and lipid contents of whole-body homogenates for both early and late weaning. At the end of the experiment no significant differences in biochemical contents were observed between the two inert diets. The results of this study suggest that weaning starting on day 50 (early weaning), using a good quality inert diet, leads to higher survival, growth and fish condition.
Key words: Dover sole, larvae, weaning, inert diets, Artemia.
Resumen
Se estudiaron los efectos del tipo de dieta (biomasa de Artemia congelada y dos dietas inertes: una micro-compactada [MB] y una micro-extruida [ME]) y dos edades de destete (temprana y tardía, a 50 y 64 días de edad después de la eclosión, respectivamente) en larvas de Solea solea. El experimento duró 56 y 42 días para el destete temprano y tardío, respectivamente. Los mayores valores de mortalidad se presentaron con el tratamiento Artemia en destete tardío (39%). No se observaron diferencias significativas en la mortalidad entre dietas inertes. Los valores finales de peso seco fueron mayores para el destete tardío que para el temprano. Independientemente de la edad de destete, los peces que recibieron la misma dieta mostraron tendencias similares en peso seco y longitud estándar. Los peces alimentados con MB presentaron peso seco y longitud estándar significativamente mayores, seguidos por los alimentados con ME, en tanto que el tratamiento con Artemia alcanzó los valores más bajos. El hecho de que las dietas inertes presentaran fracciones similares de ácidos grasos altamente insaturados quedó reflejado en la ausencia de diferencias significativas en la susceptibilidad a la oxidación (prueba de substancias reactivas al ácido tiobarbitúrico). Sin embargo, sí se encontraron diferencias significativas en los contenidos de carbohidratos, proteinas y lípidos medidos en los homogeneizados de pescado completo tanto para el destete temprano como para el tardío. Al final del experimento no se observaron diferencias significativas en los contenidos bioquímicos de los organismos sometidos a ambas dietas inertes. Los resultados de este estudio sugieren que un destete temprano (día 50), usando una dieta inerte de buena calidad, conduce a mayores tasas de supervivencia, crecimiento y condición del pez.
Palabras clave: sol común, larva, destete, dietas inertes, Artemia.
Introduction
The Dover sole Solea solea L. is one of the most consistently high-priced fish throughout most of Europe. Metamorphosis and weaning of sole larvae are critical periods for the cultivation of this species, and are normally accompanied by increased mortality. Various types of diets and methods have been tested for weaning Dover sole. Some include the utilization of live prey such as Artemia nauplii, which increases production costs. Others have been mainly based on providing fresh ingredients in wet pastes and/or pellets containing attractive compounds (Metailler et al., 1981). Weaning can be either progressive (co-feeding) or short-term. Early weaning, starting immediately after metamorphosis, has resulted in higher mortality and a slow growth (Person-Le Ruyet, 1984). A successful weaning diet should take into consideration the larva's mouth size, feeding behaviour and nutritional requirements, and food palatability and stability in water.
The physiological response of an organism can be affected by nutritional, genetic or physico-chemical factors. It is known that nutritional components can influence the oxidation levels and antioxidant defences in animal tissues (Tocher et al., 2002a, b). Lipid and polyunsaturated fatty acids (PUFA) are implicated in peroxidation, while dietary micronutrients such as vitamin E act as antioxidant protection. Under normal physiological conditions animal cells produce reactive oxygen species (ROS). At the same time, antioxidant defences are produced and their function is to cope with ROS; however, an unbalance between the antioxidant defences and ROS can occur, producing oxidative stress (Tocher et al., 2002b). The ROS can oxidize most cellular constituents such as DNA, proteins and lipids (Janssens et al., 2000), causing damage, cross-linking and the decline of the cellular integrity by reducing enzyme activities. Fish tissues, especially bio-membranes and fish diets, are typically rich in n-3 PUFA, which are highly susceptible to oxidation.
The susceptibility to oxidation of fish tissues should be considered when designing appropriate diets for the initial larval stages and weaning. This is especially important given that the source of dietary energy (either digestible protein, lipids or carbohydrates) can affect lipid oxidation, as shown in muscle homogenates of rainbow trout and European seabass (Alvarez et al., 1998, 1999; López-Bote et al., 2001).
The purpose of this study was to evaluate the effect of the diet (two inert diets and frozen Artemia) and the weaning start age (early and late weaning) on growth, survival, condition and oxidative status, in order to improve the weaning of Dover sole under culture conditions.
Materials and methods
Egg and larval culture conditions
The broodstock were maintained at the laboratory of the Fisheries Department in Oostende, Belgium (temperature of 9 ± 2°C, 200 lumen m-2 continuous illumination measured at the water surface and bottom aeration). The fish were fed fresh mussels on a daily basis. When spawning occurred, the eggs were collected and transported to the Laboratory of Aquaculture and Artemia Reference Center, where the eggs were placed in 70-L cylindro-conical tanks and incubated at 15°C in an open flow-through system with a water renewal rate of 80-100% per hour.
The start of hatching was considered day zero. The chorion, unfertilized eggs and dead larvae that sank were carefully removed by siphoning to avoid water quality deterioration. On day 3 after hatching, larvae were first fed rotifers at a density of 2 ind mL-1, gradually increasing to 8 ind mL-1. On day 7 the larvae started to feed on enriched Artemia metanauplii, from an initial density of 0.5 to 7 metanauplii mL-1 on day 22. Larvae were fed twice a day, in the morning and in the evening. Artemia metanauplii were enriched for 24 h with an experimental Selco emulsion (Selco DHA/EPA = 4; INVE Aquaculture, N.V Belgium), containing 10% vitamin C. Before each feeding, three 10-mL samples were taken from the rearing tanks and uneaten rotifer and nauplii were evaluated and the density was re-established. From day 28 after hatching to the beginning of the experiment, the larvae were fed ad libitum with on-grown Artemia. The NH4+, NO2- and NO3- concentrations in the water of the rearing tanks were evaluated three times per week.
Experimental design
Three treatments consisting of two inert diets and frozen Artemia biomass were tested in three replicates at two weaning ages, 50 and 64 days after hatching (DAH). The two experiments at different weaning ages were conducted independently. One of the diets was of the micro-bound (MB) type (SSF, Norwegian Herring and Oil Res. Industry, Norway), while the other was a micro-extruded (ME) diet (an experimental product of INVE Aquaculture, N.V. Belgium). The analyses of ash, moisture and crude protein for the three diets (table 1) were done following the AOAC (1984) procedures, and the total lipid content and highly unsaturated fatty acid (HUFA) profiles (table 1) were determined using standard analytical methods. Lipids were extracted according to Folch et al. (1957), following the modification of Ways and Hanahan (1964). Fatty acid methyl ester (FAME) composition was verified by gas chromatography according to Coutteau and Sorgeloos (1995), and identified by a Chrompack CP9001 gas chromatograph equipped with TPOCI (temperature programmable on-column injector). The injections were performed on a polar 50-m capillary column BPX70 (SGE Australia), with a diameter of 0.32 mm and layer thickness of 0.25 ^m, connected to a 2.5-m methyl deactivated pre-column.

At the beginning of metamorphosis (28 DAH), the larvae were transferred to 35-L flat tanks at a density of 16 larvae L-1. Each tank was connected to an individual biofilter, in a recircu-lation system, with a water renewal of around 180% per day. Photoperiod was 12L:12D, temperature 16 ± 1.5°C and salinity 35 g L-1. Remaining food and faeces were siphoned out of the system on a daily basis. Dead animals were counted and removed. The fish were fed manually three to four times per day. For the inert diets, the particle sizes were changed according to the size of the fish's mouth: a pellet size of 200 to 400 |am was used from the beginning to 70 DAH, of 400 to 600 |am from 71 to 90 DAH, and of 600 |am to 1 mm from 91 DAH until the end of the experiment (106 DAH). The change to the experimental diets was gradually introduced during three days of co-feeding with Artemia.
A sample of 10 fish per tank was taken at the beginning of the experiment and each week. The individuals were anaesthetized with 0.3-ppm 2-phenoxyethanol and measured for total and standard length, and dry weight. The daily rations of the different diets were calculated as 10% of body weight per day. The weekly average wet weight data per tank were used to calculate the amount of diet to be added. A preliminary evaluation of the equivalence of 1 g of frozen Artemia to dry weight was done to allow calculation of the frozen Artemia daily ration.
Relative growth rate (RGR) (% d-1) was calculated according to the formula: RGR = (eg-1) x 100, where g = (Ln W2 - Ln W1)/(t2 - t1). At the end of the experiment, four fish per tank were anaesthetized and homogenized for thiobar-bituric reactive substances (TBARS) testing and four other fish for biochemical contents (carbohydrates, proteins and lipids).
Homogenate preparation
Samples of 12 fish per treatment were prepared as homoge-nates for biochemical contents and TBARS tests. The fishes (whole-body) were weighed and their standard length was measured. They were then cut into small pieces and homoge-nated. The preparation of the homogenates was made using an ice Bain Marie and a four up-and-down strokes Polytron PTA 10S homogenizer. A measured volume of buffer (depending on the size of the sample) (0.1 M Tris-HCl pH 8.5, 15% (w/v) Poly Vinyl Pyrrolidone, 153 µM MgSO4 and 0.2% (w/v) Triton X-100) was added to blend the sample. The volume of buffer used to prepare the homogenates depended on the size of the larvae, varying from 1 to 5 mL per fish. Homogenated samples were stored at -80°C.
TBARS test
The susceptibility of the whole-body homogenates to induced lipid oxidation was performed by TBARS testing (Janssens et al., 2000). The homogenates were centrifuged and 400 |aL of 15% trichloroacetic acid (TCA) and 800 |aL of thiobarbituric acid (TBA) (0.67% diluted in NaOH solution 0.3N) were added to 500 µL of supernatant. After heating (95°C) for 20 min and cooling down, an extraction of pigment was induced with the addition of 3 mL of butanol and centrifu-gation (2000 rpm, 4°C, 5 min). Supernatants were recovered and analyzed in a fluorescence spectrophotometer (Spectramax 190, Molecular Devices, Sunnyvale CA 94089, USA) (Em 555 and Ex 515). The TBARS levels were calculated from a standard curve constructed with malonaldehyde dimethyl acetate (MDA). Results are expressed as moles of MDA mg-1 protein. Protein measurements were carried out applying the methodology of Lowry et al. (1951) and using albumin as standard.
Biochemical analyses
The measurements of carbohydrates and proteins were made in 200 µL of homogenates (Holland and Gabbot, 1971; Bradford, 1976). After the addition of 15% TCA and centrifii-gation of the mixture, the pellets were washed with 5% TCA and both supernatant fractions were combined. Supernatant fractions were used for the carbohydrate determinations, while pellet fractions were used for protein measurements. Fifty µL of phenol (5%) and 200 µL of concentrated H2SO4 were added to the supernatant fractions (50 µL). After 30 min incubation (room temperature) the absorbances were measured at 492 nm using glucose as standard. The remaining pellets were diluted in 500 µL of NaOH 1.0 N, incubated at 60°C for 30 min and neutralized with 300 µL of HCl 1.67M. Total protein content was determined using Bradford's reagent (Bradford, 1976). The absorbance was measured at 590 nm using bovine serum albumin as standard.
Total lipids were extracted according to Bligh and Dyer (1959). Chloroform (500 µL) and methanol (500 µL) (spectophometric grade) and 400 µL distillate water were added to the homogenates (100 µL). After centrifugation, the top phase was separated (100 µL) and 500 µL of H2SO4 were added to the extract and charred 15 min at 200°C. The remaining fractions were diluted in 1 mL distillate water and from these dilutions, 250 µL were used for total lipid content spectrophotometrical determination, at 370 nm of absorbance using tripalmitin (Glyceryl Tripalmitate, Sigma) as standard.
The energy value of the macronutrients was calculated from the contents of the different biochemical fractions and transformed into energetic equivalents using the enthalpy combustion constants (17500 J g-1 carbohydrates, 24000 J g-1 protein and 39500 J g-1 lipids) (De Coen et al., 1995). Given the small size of the fish, the energetic values were calculated per fish.
Statistical analyses
Statistical analyses were performed using one-way analysis of variance (ANOVA) and when differences were found, Tukey's honest significant differences test (HSD) was used. The homogeneity of the variances of means was checked by Cochran's univariate test. The level of confidence considered for all the statistical analysis was a = 0.05.
Results
Growth, condition and oxidative status
The biochemical composition of the three diets tested (ash, moisture, protein and lipids), as well as the HUFA fractions in ME and MB are shown in table 1. The cumulative mortality results at two weaning ages (50 and 64 DAH) showed significantly higher values for late weaning in the Artemia treatment (39% for the whole experimental period). The other treatments (including frozen Artemia at early weaning) had similar values (around 20-24%) (fig. 1a, b). No significant differences were observed in the cumulative mortality of the MB and ME diets.
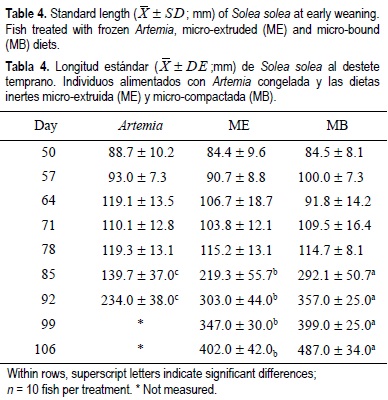
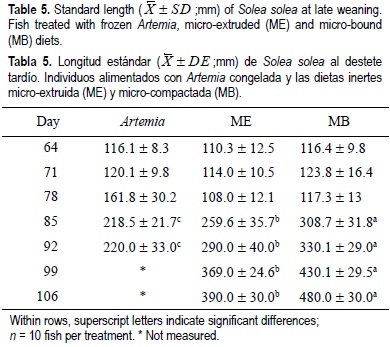
For all the experimental period, the dry weight and standard length results showed similar tendencies for the same treatments regardless of weaning age (tables 2, 3, 4, 5). From the beginning of the experiment until days 71 and 78, the dry weight and standard length values were not significantly different between treatments; however, in the following weeks, fish growth (dry weight and standard length) was significantly lower in the Artemia treatment when compared with the inert diets (P < 0.05) (tables 2, 3, 4, 5). After 85 DAH, the fish fed the MB diet displayed a significantly greater growth (dry weight and standard length) performance, followed by ME and finally Artemia for both weaning ages, except for the standard length measures on day 92 for both weaning ages. At this moment no significant differences were observed between the two inert diets (tables 4, 5). At the end of the experimental period (106 DAH) (the data were analyzed as point values), no significant differences in growth (dry weight and standard length) were detected between fish treated with the same diet at different weaning ages (P < 0.05). Because of the high mortality and the low dry weight and standard length increases obtained, the Artemia-fed group was stopped 92 and 99 DAH for early and late weaning, respectively.

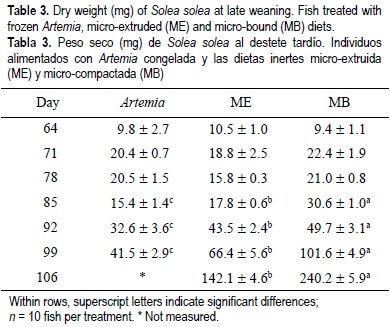
The relative growth rate (RGR) showed significant differences among the diets. Fish fed Artemia reached a significantly higher RGR in the second week and in the first two weeks on early and late weaning, respectively; however, in subsequent weeks the RGR decreased to almost imperceptible, except for early weaning on day 92. In the case of early weaning, from day 71 to the end of the experiment, the highest weekly RGR alternated between MB and ME without a clear tendency. For late weaning, fish treated with the MB diet showed better performance in terms of RGR (fig. 1c, d). No significant differences were found for the condition factor between the two weaning ages (P < 0.05) (table 6); however, higher condition factor values was observed for early weaning. There were no significant differences in the results of the susceptibility to oxidation test (TBARS) between treatments (P < 0.05) (table 6).
Biochemical contents
The macromolecule contents (carbohydrates, proteins and lipids) determined every 14 days for early and late weaning are presented in tables 7 and 8. In the first early weaning sampling on day 64 (table 7), carbohydrates and lipids were significantly higher in fish fed frozen Artemia (P < 0.05), but on day 78 they presented similar values in the MB and Artemia diets. On day 92, lipids were higher in fish fed the MB diet, but carbohydrates were significantly higher in fish fed Artemia, while proteins were significantly higher in the Artemia and ME treatments. For early weaning on day 106, no significant differences were found between the two inert diets, except for the protein contents (table 7).
In the case of late weaning, higher values were observed in nearly all the biochemical compounds was evident for MB. However, in fish fed with inert diets, significantly higher lipid contents were recorded on days 78 and 92, and on day 106 they were significantly higher for MB (table 8).
Discussion
The mortality rates observed during the present experiment (20-24% and 39% for Artemia early weaning) were lower than the values found by Gatesoupe and Luquet (1981), who weaned sole larvae 70 DAH, obtaining mortalities of 55-70%. These authors obtained a lower mortality (20.5%) when Dover sole larvae were reared using frozen Artemia nauplii from 10 to 15 DAH.
Metailler et al. (1981) reported a survival rate of 30-40% for fish weaned with different diets (mixed dry pellets and fresh ingredients as attractants), compared with the 100% obtained for larvae fed with live Artemia. The same authors found that dry pellets induced greater growth of sole larvae (90 DAH), comparable with that obtained feeding with live Artemia. In the present experiment, frozen Artemia was well accepted by the fish only the first 30 min after the addition, and the remaining fraction was ignored (producing deterioration of water quality until it was siphoned out from the tank). The lowest mortality rates of the present study were also comparable to the best results obtained by Day et al. (1997), although this latter study was performed with sole over 170 DAH.
Metailler et al. (1981) reported the highest weight (751 ± 102 mg) of Dover sole cultured until 90 DAH, when fish were fed with dry pellets, but with a low survival of 38%. This result was lower when compared with our results (76-80% of survival with inert diets and 61% for the Artemia early weaning treatment). The average length (32 mm after 60 days of culture) recorded in Dendrinos and Thorpe's (1987) experiment, was lower compared with the results of the present work. During the first three weeks of the experiment, dry weight and standard length did not increase markedly and this could be considered a long adaptation period to the new food; however, after this period the growth pattern changed radically for all treatments, particularly for Artemia. During the first two weeks of the experiment, the results obtained in the frozen Artemia treatment were satisfactory in terms of dry weight, standard length and biochemical composition (tables 2, 3, 4, 5, 7, 8).
Gatesoupe and Luquet (1981) and Fuchs (1982) successfully weaned S. solea using frozen Artemia nauplii. Nevertheless, for the later stages of cultivation, as opposed to the inert diets, frozen Artemia does not seem to meet the nutritional requirements of sole (tables 7, 8). Person-LeRuyet et al. (1990) recommended the use of frozen adult Artemia to aid the transition of the weaning. The results of dry weight, standard length, condition factor, relative growth rate 57 DAH and biochemical composition at early weaning on day 64 for fish fed frozen Artemia support this suggestion; however, for later stages, frozen Artemia was not an appropriate feed. It should be considered that weaning success depends not only on the quality of the diet, but also on the physiological state, developmental stage and age of the larvae. Furthermore, the present results suggest that the first four weeks of weaning are a critical period, with a tendency towards growth diminution and depletion of the energy reserves. A depletion of the lipid stores seems particularly important when fish are weaned early (table 7).
Alvarez et al. (1998, 1999) suggested that diets should be evaluated in terms of fatty acid composition and the susceptibility to oxidation of the fish tissue. Lipid in the diet is also considered to be important in the lipid oxidation process in muscle homogenates of rainbow trout and European seabass (López-Bote et al., 2001). In this case, the similar amounts of HUFA in the inert diets were reflected in the absence of significant differences in the levels of TBARS.
The effect of diets on larvae has commonly been evaluated based on growth, survival and condition factor, but these parameters cannot offer a full image of the physiological condition. The acute salinity stress test proposed by Dhert et al. (1992) was applied in order to expose the fish to extreme conditions and to test their resistance capacity (results not presented). Sole larvae showed a tolerance to high salinities and no clear effect of the diet was evidenced. Yin and Blaxter (1987) also observed an excellent low salinity tolerance of euryhaline marine fish larvae (flounder), but there are no references dealing with tolerance to high salinities. Future experiments should consider the acute ammonia stress test. Cavalli (2000), Hernández (2001) and Palacios et al. (2002) found that it was a feasible and sensitive criterion for quality determination of crustacean larvae of Macrobrachium rosembergii, Peneaus vannamei and Litopeneaus vannamei.
According to the results found in the present work, early weaning starting on day 50 is suitable for S. solea larvae. Of the diets tested, the MB diet proved to be the most appropriate for weaning Dover sole, producing higher survival, growth and energy reserves.
Acknowledgements
We thank Peter Bossier for critically reviewing the manuscript. Rebeca Rueda and Luis Conceição acknowledge support from CONACYT (Mexico) and grant SFRH/BPD/ 7149/2001 (FCT, Portugal), respectively. The technical assistance of Cecile Marchand and Geert Van De Wielde is greatly appreciated.
References
Alvarez, M.J., López-Bote, C.J., Diez, A., Corraze, G., Arzel, J., Días, J., Kaushik, S.J. and Bautista, J.M. (1998). Dietary fish oil and digestible protein modify susceptibility to lipid peroxidation in the muscle of rainbow trout (Oncorhynchus mykiss) and sea bass (Dicentrarchus labrax). Brit. J. Nutr., 80: 281-289. [ Links ]
Alvarez, M.J., López-Bote, C.J., Diez, A., Corraze, G., Arzel, J., Días, J., Kaushik, S.J. and Bautista, J.M. (1999). The partial substitution of digestible protein with gelatinized starch as an energy source reduces susceptibility to lipid oxidation in rainbow trout (Oncorhynchus mykiss) and sea bass (Dicentrarchus labrax) muscle. J. Anim. Sci., 77: 3322-3329. [ Links ]
AOAC (Association of Official Analytical Chemist) (1984). Official Methods of Analysis. 14th ed. AOAC, Arlington, VA, 1141 pp. [ Links ]
Bligh, E.G. and Dyer, W.J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol., 37: 911-917. [ Links ]
Bradford, M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle dye binding. Anal. Biochem., 72: 248-254. [ Links ]
Cavalli, R., Menschaert, G., Lavens, P. and Sorgeloos, P. (2000). Maturation performance, offspring quality and lipid composition of Macrobrachium rosenbergii females fed increasing levels of dietary phospholipids. Aquacult. Int., 8: 41-58. [ Links ]
Coutteau, P. and Sorgeloos, P. (1995). Intercalibration exercise on the qualitative and quantitative analysis of fatty acids in Artemia and marine samples. ICES Coop. Res. Rep., 211, 30 pp. [ Links ]
Day, O.J., Howell, B.R. and Jones, D.A. (1997). The effect of dietary hydrolysed fish protein concentrate on the survival and growth of juvenile Dover sole, Solea solea (L.), during and after weaning. Aquacult. Resour., 28: 911-921. [ Links ]
DeCoen, W.M., Janssens, C.R. and Persoone, G. (1995). Biochemical assessment of Cellular Energy Allocation in Daphnia magna exposed to toxic stress as an alternative to the conventional "Scope for Growth" methodology. ANPP Int. Symp. Biological Markers of Pollution. Chinon, France, 21-22 Sept., 1995. [ Links ]
Dendrinos, P. and Thorpe, J.P. (1987). Experiments on the artificial regulation of the amino acid and fatty acid contents of food organisms to meet the assessed nutritional requirements of larval, post-larval and juvenile Dover sole (Solea solea (L.)). Aquaculture, 61: 121-154. [ Links ]
Dhert, P., Lavens, P. and Sorgeloos, P. (1992). Stress evaluation: A tool for quality control of hatchery-produced shrimp and fish fry. Aquacult. Eur., 17(2): 23-125. [ Links ]
Folch, J., Lees, M. and Staley, G.H.S. (1957). A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem., 266: 497-509. [ Links ]
Fuchs, J. (1982). Production de juveniles de sole (Solea solea) en conditions intensives. I. Le premier mois d élevage. Aquaculture, 26: 321-339. [ Links ]
Gatesoupe, F.J. and Luquet, P. (1981). Weaning of the sole (Solea solea) before metamorphosis. Aquaculture, 26: 358-368. [ Links ]
Hernández, R. (2001). Biochemical and physiological indicators of larval and postlarval quality of white shrimp Litopenaeus vannamei (in Spanish). Centro de Investigaciones Biológicas del Noroeste, S.C., México, La Paz, México, 91 pp. [ Links ]
Holland, D. and Gabot, P. (1971). Micro-analytical scheme for determination of proteins, carbohydrate, lipid and RNA levels in marine invertebrate larvae. J. Mar. Biol. Assays UK, 51: 659-668. [ Links ]
Janssens, B.J., Childress, J.J., Baguet, F. and Rees, J.F. (2000). Reduced enzymatic antioxidative defense in deep-sea fish. J. Exp. Biol., 203: 3717-3725. [ Links ]
López-Bote, C.J., Diez, A., Corraze, G., Arzel, J., Alvarez, M.J., Días, J., Kaushik, S.J. and Bautista, J.M. (2001). Dietary protein source affects the susceptibility to lipid peroxidation of rainbow trout (Oncorhynchus mykiss) and sea bass (Dicentrarchus labrax) muscle. Anim. Sci., 73: 443-449. [ Links ]
Lowry, O.H., Rosebrough, N.L. and Randall, R.I. (1951). Protein measurement with Folin phenol reagent. J. Biol. Chem., 193: 265-275. [ Links ]
Metallier, R., Menu, B. and Moriniere, P. (1981). Weaning of Dover sole (Solea vulgaris) using artificial diets. J. World Maricult. Soc., 12(2): 111-116. [ Links ]
Palacios, E., Bonilla, A., Pérez, A. and Racotta, I.S. (2002). Effect of different levels of HUFA on osmoregulatory mechanisms in Penaeus vannamei postlarvae. Abstracts, Annual Meeting of the World Aquaculture Society. China, 23-27 April, 2002. [ Links ]
Person-Le Ruyet, J. (1984). Production of sole and turbot juveniles at IFREMER. Acts of the Norwegian-French Workshop on Aquaculture, pp. 157-177. [ Links ]
Person-LeRuyet, J., Lahayane, J., Deniel, C., Metallier, R., Devauchelle, N., Menu, B., Noel, T. and Baudin-Laurencin, F. (1990). Sole and turbot culture. In: G. Barnabé (ed.), Aquaculture. Vol. 2. Ellis Horwood, London, pp. 687-734. [ Links ]
Tocher, D.R., Mourente, G., Van Der Eecken, A., Evjemo, J.O., Diaz, E., Bell, J.G., Geurden, I. and Olsen, Y. (2002a). Effects of dietary vitamin E on antioxidant defence mechanisms of juvenile turbot (Scophthalmus maximus L.), halibut (Hippoglossus hippoglossus L.) and sea bream (Sparus aurata L.). Aquacult. Nutr., 8(3): 195-203. [ Links ]
Tocher, D.R., Mourente, G., Van Der Eecken, A., Evjemo, J.O., Diaz, E., Wille, M., Bell, J.G. and Olsen, Y. (2002b). Comparative study of antioxidant defence mechanism in marine fish fed variable levels of oxidised oil and vitamin E. Aquacult. Int., 11(1-2): 196-216. [ Links ]
Ways, P. and Hanahan, DJ. (1964). Characterization and quantification of red cell lipids in normal man. J. Lipids Res., 5: 318-328. [ Links ]
Yin, M.C. and Blaxter, H.S. (1987). Temperature, salinity tolerance, and buoyancy during early development and starvation of Clyde and North Sea herring cod and flounder larvae. J. Exp. Mar. Biol. Ecol., 10: 279-290. [ Links ]














