Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Ciencias marinas
Print version ISSN 0185-3880
Cienc. mar vol.29 n.4 Ensenada Oct. 2003
Artículos
Development and optimization of a digestion method for heavy metal determination in scleractinian corals by Atomic Absorption Spectrometry (AAS)
Desarrollo y optimización de un método de digestión para la determinación de metales pesados en corales escleractíneos por Espectrometría de Absorción Atómica (EAA)
M.D. Galindo-Riaño1*, L. Castellón1, K. González2 and M. García-Vargas1
1 Departamento de Química Analítica, Facultad de Ciencias, Universidad de Cádiz, 11510 Puerto Real, Cádiz, España. *E-mail: dolores.galindo@uca.es
2 Instituto Superior de Ciencias y Tecnología Nucleares, Ave. Salvador Allende Esq. Luaces, Quinta de los Molinos, Plaza, Ciudad de La Habana, Cuba.
Recibido en noviembre de 2000;
aceptado en septiembre de 2002.
Abstract
In the last decade a great number of studies have been directed especially to the evaluation of the environmental impact of heavy metal concentration levels in coral barriers. Related to studies in scleractinian corals, many chemical techniques have been proposed to determine heavy metals but Atomic Absorption Spectrometry (AAS) is the most widely used; however, regarding sample pretreatment methods there is not a unique criterion on the requirements to obtain the precise and accurate results needed in this analysis. So, in this paper the application of several widely used pretreatment methods for digestion of corals and sediments were studied in which HNO3, HCl and HClO4 are used in different conditions (concentration, temperature, etc.), with the aim of comparing their efficiency. The method in which a combination of HNO3 and HClO4 was used resulted the most effective one. A 22 experimental design was carried out to study the influence of the HNO3 volume as well as to optimize the HNO3:HClO4 mixture. Finally the method was validated using reference certificate material (SOIL-7) using the NIST accuracy test and applying it in the analyses of different coral samples which produced results with appropriate analytical quality.
Key words: sample pretreatment, scleractinian coral, heavy metal, marine pollution.
Resumen
En la última década un gran número de estudios se han enfocado especialmente a la evaluación del impacto ambiental de los niveles de concentración de metales pesados en las barreras coralinas. En relación al estudio de metales pesados en corales escleractíneos, la técnica de análisis más utilizada ha sido la Espectrometría de Absorción Atómica (EAA); sin embargo, en cuanto al pretratamiento químico de digestión de las muestras no existe un criterio único sobre el método más adecuado que proporcione los resultados con la precisión y exactitud requeridos en este tipo de análisis. Por ello en este trabajo se estudia la aplicación de algunos de los tratamientos más utilizados en la digestión de corales y sedimentos, mediante el empleo de HNO3, HCl y HClO4 en diferentes condiciones (concentración, temperatura, etc.) con objeto de comparar la eficacia de los mismos. El método de disolución de la muestra con HNO3 y HClO4 resultó ser el más adecuado. Para realizar la optimización de dicho tratamiento se realizó un diseño experimental 22 con el fin de estudiar la influencia del volumen de HNO3, así como el sinergismo de la mezcla HNO3:HClO4. Finalmente, el método fue validado con la utilización de material de referencia certificado (SOIL-7), utilizando el test de exactitud NIST que es aplicado al análisis de diferentes muestras de corales obteniendo resultados con la calidad analítica adecuada.
Palabras clave: pretratamiento de la muestra, corales escleractíneos, metales pesados, contaminación marina.
Introduction
Coral reefs are biological communities born in the marine floor consisting of a solid structure of calcareous stone constituted by CaCO3. Their major constituents are corals which are characterized by a low resistance to human impacts and slow recovery rates.
The incorporation of heavy metals in the coralline skeleton is dealt in biological and physiological studies that interpret the analysis of their metal concentrations considering two fundamental incorporation routes: first, the incorporation of dissolved metals in the system into the aragonite structure of the calcareous skeleton; and second, the incorporation by adsorption as particulate or suspended material (Howrad and Brown, 1984; Barnes and Chalker, 1990).
Scleractinian corals are so important in the reef development that they can also be considered, according to many authors, as indicative of heavy metal pollution levels in marine ecosystems. Therefore, when heavy metal pollution assesments are required, in first place it is necessary to count on an analytical tool that allows to establish their total content in this kind of samples. Is in this field that the application of the Atomic Absorption Spectrometry (AAS; Price, 1979) to studies related to the environment has found their wider and most diversified repercussion. The AAS is an analytical technique able to determine the immense majority of elements of ecological interest, as heavy metals. Heavy metals feature two characteristics that make them highly dangerous: first, they can not be eliminated from the environment by natural processes and, second, because through complex processes they can even get incorporated into the nutritional chains, being able to indirectly affect humans.
Concentrations of these elements of environmental interest can fluctuate within a wide interval from mg kg-1 to ng kg-1 or ng L-1, depending on the element and the sample studied. It is therefore an attribution of the analysts to evaluate which of the different analytical procedures available to employ, in order to select a method that combines the best sensibility, precision and accuracy requirements (Valcárcel and Ríos, 1992).
The use of AAS combines the high selectivity of the spectral atomic methods with the use of wet methods that oftenly require a previous digestion step in which solid or liquid samples with suspensed material are converted into an homogeneous liquid phase; therefore, during the chemical digestion or dissolution it is important for the sample to be completely dissolved and, thus, the most adequate solvent and the simplest procedure must be used.
The wide variety of acid digestion methods, or acid extractions, can be classified, according to their strength, in: total, strong, moderate and weak. Total treatments release all the metal present in the sample but imply the use of HF. Thus, many analysts prefer using HNO3, HCl, HClO4 and H2SO4, separatedly, or in different combinations and conditions (concentration, temperature, etc), attaining different digestion efficacies.
Several comparative studies and international intercalibra-tion exercises (OIEA, 1985; 1987) show contradictory results regarding the acid digestion methods in coral samples. This results consider the HNO3:HCl mixture as the most widely used method and its strong digestion comparable to total digestion. In relation to organism digestions two opposing criteria exist in relation to the HClO4 use, some opposing it, due to the explosion risk, and some other promoting it because of its organic matter dehydrating capacity. Some other authors use this acid mainly with HNO3, since this way its dehydrating capacities are decreased, avoiding such explosive reactions and increasing the oxidizing properties of the mixture (González, 1989).
In a pollution study carried out at the marine station in Moa Bay, Cuba, coral samples were subjected to digestion using a mixture of concentrated HNO3 and concentrated HCl, and the Co, Cu, Fe, Mn, Ni, and Zn contents were quantified by AAS, yielding results that can be reproduced consistently, but their accuracy was not specified (Martínez et al., 1989).
In works carried out in the Great Barrier Reef, a variety of octocorals and scleractinian corals were collected to determine their metal concentrations. The octocorals samples were digested using concentrated HNO3 and a 1:1 mixture of HNO3:HClO4. The scleractinian coral samples were previously subjected to an extraction and concentration process in order to separate the trace elements from the Ca matrix interference. The samples were digested with 20 mL of HNO3 (1 mol L-1), and the pH was adjusted between 4 and 5 with NH4OH and/or HNO3 solution. The extraction was carried out with APDC and AMK. In this study, heavy metals as Zn, Cu, Cd, Ni and Pb were determined (Denton and Burdon-Jones, 1986). These proposed methods are seldom used since they are technically hard procedures requiring a lot of time (72 hours) and there are no criteria regarding their accuracy.
Another kind of digestion was used in studies carried out on corals from the Galapagos Islands. The samples were dissolved in HNO3 (2 mol L-1) and trace metals were separated by co-precipitation using Co as a collector and APDC (Shen and Boyle, 1988) as a complex agent. This procedure isolated the trace metals from the calcareous matrix facilitating their graphite-oven analysis.
Other authors have used a combination of HNO3 and H2O2 for digestion, preparing the calibration curve standards with CaCO3, in order to correct possible Ca interferences (Glynn, 1989) and to achieve an easy sample manipulation.
Finally, considering the chemical treatment of the coral samples, it can be deduced that there is not a unique criterion to treat the samples and, among the digestion methods used, there are no data regarding their efficiency and/or accuracy and whether the total digestion can be or not substituted by them (Schneider and Smith, 1982; Brown, 1987; Linn et al., 1990; Luoma, 1990; Hanna and Muir, 1990; D'Elia et al., 1991; Páez-Osuna and Ruíz Fernández, 1995; Esslemont, 1997).
In order to obtain good quality analytical results and, therefore achieve a correct evaluation of the heavy metal concentration levels and their environmental impact to the marine ecosystem we proposed the selection of the most reliable procedure among the most widely used and simple methods, to find the most suitable experimental conditions and those that generate the most accurate and precise results in coral samples.
Material and methods
With the aim to establish a methodology for the determination of heavy metals in scleractinian corals, the influence of the previous treatment to the sample in the quality of the results was studied, emphasizing the need to quantify the actual and total value of the metal concentrations with the smallest variability.
Treatments are based on the use of HNO3 with different proportions and concentrations, as well as its combined effect with HCl and HClO4.
Among the characteristics of HNO3 that make it the most widely used are its strength, extreme corrosiveness, oxidizing power and its inability to form insoluble salts with any metal.
Since the use of H2SO4 would not have allowed the sample to totally dissolve due to the matrix (CaCO3) precipitation with some of the metals of interest (such as Pb), it was not used.
Collection and preparation of samples
For this study Colpophyllia natans was selected during all the methodological process and the methology developed was applied to Dichocoemia stokesi, Porites porites, Porites astreoides and Micetophyllia lamarkiana, all of them sclerac-tinian corals of the Cuban insular platform.
Corals were taken by autonomous diving at depths of 5 and 10 m in the Jaimanitas zone coastal reef, north of the City of Havana (Cuba). They remained in freshwater for 72 hours and then were cleaned with abundant water with pressure and sunk in nitric acid (1%), where they were preserved for 24 hours. All this process guarantees all organic matter to be eliminated from the coral surface, just remaining the calcareous skeleton. Finally they are cleaned with abundant distilled water and dried in a heater at 105°C during 24 hours (González, 1989).
The specimens were sectioned with a diamond-top saw in ~5-mm thick. They were cleaned with HNO3 (1%) and distilled water to eliminate possible superficial pollution and dried again in a heater at 105°C. Samples were pulverized during 15 min in an automatic agate mortar, after a previous crushing in a porcelain mortar, until obtaining a fine homogeneous powder with a grain size of 50-100 µm (Gómez et al., 1995).
Finally the samples were wrapped in vegetable paper envelopes and put in a drier until their processing.
Digestion methods
Four different types of digestions were used in 1-g samples. The samples were dissolved by triplicate, with two blank solutions for each method. Each portion was measured three times and the statistic processing of the results was performed with the Babxel (1995) software.
The heavy metal concentrations in the dissolved samples were measured in an atomic absorption spectrometer Unicam model 929 Solaar System coupled to a PC for the control of the equipment and the acquisition of data, using for all cases air-acetylene flame and deuterium lamp background correction.
The calibration curves were prepared for the acid concentration in samples and standards to remain constant. For the Fe determination, the compensation adjustment to the calibration curve was used, adding to standards a 35% of Ca to offset the matrix effect (Brown, 1987; Shen et al., 1987).
Digestion I (weak)
In a 250-ml polyethylene (PE) screw-top bottle, 25 mL of 8 mol L-1 HNO3 were added to a 1-gr sample, with stirring, and put all together in a 90°C hot water bath during 30 min. The resulting disolution was then filtered cold using Whatman 41 filter paper and poured into a 25-mL volumetric flask, where it was finally leveled up with 1% HNO3 (Carmody et al., 1983).
Digestion II (strong)
This treatment was based on the use of HNO3 conc. and HCl conc.; 1 gram of sample was put into a 250-mL Erlenmeyer glass flask with 25 mL of deionized water. Shaking the emulsion, 1mL of HNO3 conc. and 10 mL of HCl conc. were added, heating the solution with reflux for 3 hours in a heating plate at 100°C until almost dry. The filtered solution was poured into a 25-mL volumetric flask where it was finally leveled up with 1% HNO3 (Martínez et al., 1989).
Digestion III (strong)
This treatment was considered a strong one since it was based on the use of concentrated 65% HNO3: One gram of sample was put into a 250-mL Erlenmeyer glass flask to which 3mL of concentrated HNO3 were added slowly, drop by drop, with stirring. The solution was almost dried in a heating plate at 100°C for 1 hour. After cooling it down, 1mL of acid was added, drying again the solution with the same procedure. This operation was repeated until completing 6 mL of HNO3 conc. Finally the residues were rinsed with 10 mL of deionized water, the solution left to cool and vacuum-filtered, and finally poured into a 25-mL volumetric flask, where it was leveled up with deionized water (Shen et al., 1987).
Digestion IV (strong)
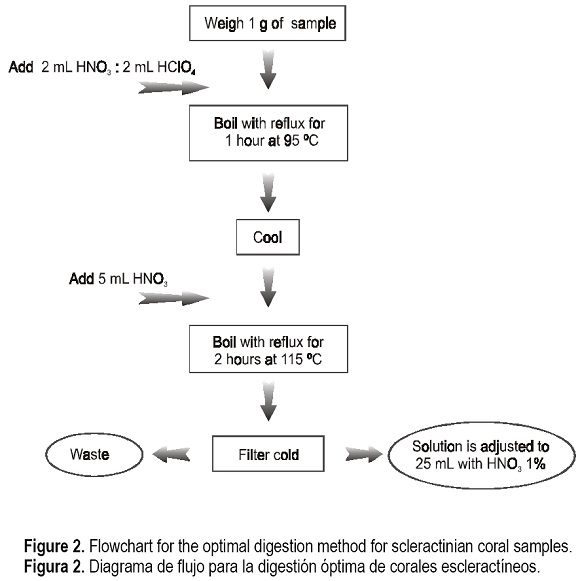
The strong digestion was carried out with concentrated HNO3 and HClO4: A mixture HNO3:HClO4 (4:2) was added to 1 gram of sample in a 250-mL Erlenmeyer glass flask, heating with reflux during 1 hour at 95°C, until the sample was almost dry. Afterwards, this was cooled down and, then, 10 mL of concentrated HNO3 were added, heating again to almost total dryness with reflux during 2 hours at 115°C. Finally, the sample was rinsed with 10 mL of deionized water, cooled down and filtered, and the filtered solution was poured into a 25-mL volumetric flask, where it was leveled up with deionized water (Denton and Burdon-Jones, 1986).
Results and discussion
In order to study the significant differences among the digestion methods, the elements Fe, Cu and Zn were selected. These metals were selected since, in previous analyses, Fe was the most abundant element after Ca, and it is representative of the elements whose measurements are affected by the calcium matrix effect when using air-acetylene flame, meanwhile Zn and Cu are elements not showing interferences by the matrix effect. The results obtained from using of the four different digestion methods are shown in table 1.
From these results we performed a mean's comparison test, based on the t-Student criterion, where our null hypothesis, [H0]: 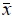 1 =
1 = 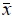 2 , consisted on proving whether there are or not significant differences among the concentration values obtained by means of the different treatments and, as an alternative hypothesis [H1]:
2 , consisted on proving whether there are or not significant differences among the concentration values obtained by means of the different treatments and, as an alternative hypothesis [H1]: 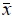 1 ≠
1 ≠ 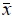 2 and, in this case, if
2 and, in this case, if 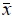 1 >
1 > 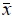 2 , then
2 , then 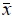 1 is the value accepted as truth, and the method used is the procedure capable of extracting the greatest concentration of the coralline matrix.
1 is the value accepted as truth, and the method used is the procedure capable of extracting the greatest concentration of the coralline matrix.
From the Student's t-test significant statistical differences among the digestion methods used were found at 95% level, and digestion IV using HNO3:HClO4 turned out to be the most efficient procedure for dissolving the samples.
Experimental Design 22
In order to characterize the parameters of the digestion IV procedure, chosen as the best, an experimental design 22 was used to obtain a mathematical model for this dissolution process, in a zone determined by a factorial space.
In this system, the influence of different synergic mixtures of HNO3:HClO4 and the volume of HNO3 in the second step were studied, over the recovery of the metal concentrations obtained .
In the mixture HNO3:HClO4 we have decided to maintain and fix 2 mL of HClO4, because it is considered as the minimal quantity used to digest the organic matter that could be present in the calcareous skeleton of the coral (González, 1989) and in this way, modifying the relationship of the mixture in function of the volume of HNO3.
The mathematical model was described as a linear function (Y) of two independent variables: Z1 and Z2, where Z1 is the HNO3 volume in the initial mixture of HNO3 and HClO4, Z2 is the HNO3 volume added in the second step and Y is the metal concentration (µg g-1) (fig. 1).
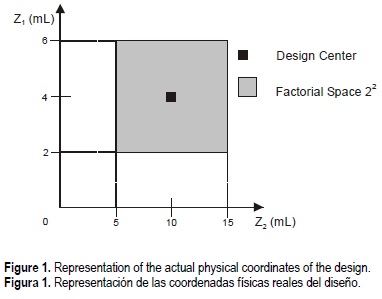
The factorial space chosen was designed based on the center of the plane. Optimization of the digestion IV procedure aimed (1) to extract the actual and greatest quantity of metal in the sample and (2) to minimize the quantities of acid used.
The mathematical model that describes factorial designs 22 is a linear model:
Y = b0 + b1 X1 + b2 X2 + b12 X1 X2,
where X1 and X2 are the codified variables Z1 and Z2 for this study.
In table 2 are shown the conditions of the experiments, as well as the results of those for each element studied (Fe, Cu, Zn). In all cases, the experiments were done by triplicate with three determinations for each replicate with total nine determinations in parallel.
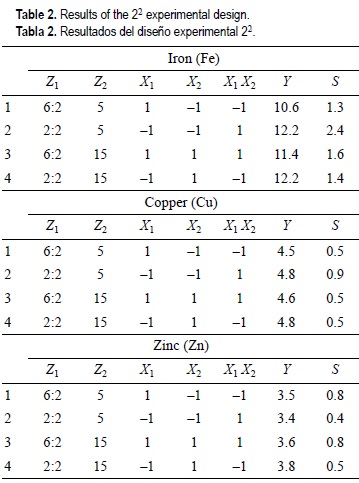
After checking the reproducibility of the experiments, the coefficients of the model proposed for each metal were calculated using the software Statistica (1997). A Fisher's Significance Test was applied for each coefficient calculated in which the variance of the whole design and the variances of each coefficient were compared, as well as the adequate fitting of the model (table 3).
From the results in table 3, the mathematical model is reduced to a constant function, Y = b0, for all cases. So, when the suitability of the regression equation is verified, it is possible to establish that the equation obtained properly describes the experimental results for α = 0.05 and, threrefore it can be used in around the factorial space in which the experiments were carried out.
In any point of the factorial space the metal concentration is constant, then optimization is achieved without affecting the quality of the results, by taking as optimum digestion that in the factorial space with the lowest coordinate values both for Z1 (HNO3 volume in the HNO3:HClO4 mixture) and for Z2 (HNO3 volume added in the second step) (fig. 2).
Accuracy test
The accuracy test of a method is directly related to the validation methods of its analytical procedures, since the accuracy or bias is related to the concordance between the concentration measured and the actual or known concentration (Valcárcel and Ríos, 1992). The origins of the bias stem on the random and systematical errors that occur during the preparation of samples, as well as on the operational and instrumental errors.
There are three ways to validate an analytical procedure and therefore evaluate the accuracy of the methodology established: the analysis of certified reference material, the recovery/dilution tests, and the comparison with the results obtained by other validated methods.
Aiming to validate the proposed sample digestion procedure labeled as digestion IV, the analysis of a certified reference material was chosen, since it is considered the best way to evaluate accuracy. Since there are not currently certified reference materials for metal analysis in corals, we used for this validation the certified soil sample SOIL-7, a reference standard supplied by the OIEA (1985).
The selection of this standard was based on two fundamental criteria: (a) to use a material with a high Ca content (17%), having an alkaline or alkaline-earthen matrix similar to that of the coral samples, and (b) to use a similar mineral structure, or perhaps a more complex one than that of the samples studied, since, if the digestion procedure selected is optimum for a soil reference sample, with a more complex mineral structure, this should also be adequate for a coral.
The accuracy test consisted on using the proposed methodology on the reference standard strictly following the recommendations of the procedure and the analytical flowchart. Then, these results were analyzed according to the recommendations of the National Institute of Standard Technology (NIST, 1992).
In the test, the absence of systematic deviation requires the difference (Δ) between the average of the measurements (µ1) and the true or actual value (µo) to be less than the critical value of deviation (Δc), where Δ = (µ1 -µo) is the difference between the measured value and the actual value;

is the critical difference considering t1-α/2, a two-tailed t-Student distribution; S is the standard deviation of the measurements; n is the number of repetitions; U are the uncertainty levels assigned to the actual values, which are assumed symmetrical.
When Δ < Δc the test is positive, therefore validating the results of the method validates granting its trazability and quality.
For this case we prepared 10 replicates and the simultaneous preparation of two blanks. The results of this test are shown in table 4.
According to the results obtained in the test, no systematical errors in respect to the certified values were detected, so the mean value of the measurements was accepted as real.
Application of the methodology
Once the optimum sample digestion methodology was established, it was applied to five kinds of corals. The results obtained are shown in table 5. Also, a transversal analysis of Dichocoenia stokesi and Porites astreoides was accomplished, dividing up the colony in three parts: the lower part (attached to marine floor and close to the sediment), the middle part and the upper part (the most recent superficial part and close to water).
Results in table 5 show the following conclusions: in general, the increasing order of metal concentrations is Ca > Fe > Zn > Mn > Cu > Ni, except for Cu in Colpophyllia natans with an outstanding value, D. stokesi and P. astreoides showed higher values owing to the compact form of their colonies and their greater superficial area. For the sectioned corals, the higher concentrations were obtained in the lower part, near the sediment that produces higher influence on the metal levels of these corals; only Ni showed higher concentration in the middle part, probably influenced by its concentration in the water and the metabolism of this metal in the coral.
Conclusions
We have established the optimum digestion conditions for heavy metal analysis in coral samples by AAS, according to the flowchart in figure 2, using a simple procedure. The digestion and dissolution method of the sample based on the use of HNO3 and HClO4 turned out to be the most reliable in the analysis among the common methods evaluated. The results obtained about the efficiency of the optimum digestion are assured by the accuracy test and so the quality of the analytical results based on the application of this methodology is guaranteed.
References
American Public Health Association (1998). Standard Methods for Examination of Water and Waste Water, 17 ed., City Press, Baltimore, 786 pp . [ Links ]
Babxel, Arcal IV (1995). rla/002/3.
Barnes, D.J. and Chalker, B.E. (1990) Calcification and photosynthesis in reef-building corals and algae. In: Z. Dubinsky (ed.), Coral Reefs. Elsevier, Amsterdam, pp. 109-131. [ Links ]
Brown, B.E. (1987). Fate of metals in biota and biological interactions in the tropical coastal zone. In: U. Seelinger, L.D. de Lacerta et al. (eds.) Metals in Coastal Environments of Latin America. Singer-Vertag, 297 pp. [ Links ]
Carmody, D.J. et al. (1983). Chemical methods for use in marine monitoring. UNESCO 12, report, 53 pp. [ Links ]
D'Elia, C.F., Burddemeier, R.W. and Smith, S.V. (1991). Work-shop on coral bleaching, coral reef ecosystems and global change: report or proceedings. Maryland Sea Grant College publication number UM-SG-TS-91-03, University of Maryland, College Park, 49 pp. [ Links ]
Denton, G.R.W. and Burdon-Jones, C. (1986). Trace metals in corals from the Great Barrier Reef. Mar. Poll. Bull., 17(5): 209-213. [ Links ]
Esslemont, G. (1997). Heavy metals in scleractinian corals and marine sediments from Darwin Harbour, Australia. In: J.R. Hanley, G. Caswell, G. Megirian and H.K. Larson (eds.), Proceedings of the Sixth International Marine Biological Workshop. The Marine Flora and Fauna of Darwin Harbour. Northern Territory, Australia, Museums and Art Galleries of the Northern Territory, Darwin. pp. 399-410. [ Links ]
Glynn, P.W. (1989). Condition of coral reef cnidarians from the Northern Florida Reef Tract. Mar. Poll. Bull., 20(11): 568-576. [ Links ]
Gómez, M. et al. (1995). Preparación de muestras de corales escleractíneos para análisis espectrométricos, Reporte de Investigación, Instituto de Oceanología, Cuba. [ Links ]
González, H. (1989). Estudio de la contaminación marina por metales pesados en algunas áreas cubanas. Tesis de Doctorado, Revista CENIC, Cuba. [ Links ]
Hanna, R.G. and Muir, G.L. (1990) Red Sea coral as biomonitors of trace metals pollution. Environ. Monit. Asses., 14: 211-222. [ Links ]
Howrad, L.S. and Brown, B.E. (1984). Heavy metals and reef corals. Ocean. Mar. Biol. Ann. Rev., 22: 195-210. [ Links ]
Linn, L.J., Delaney, M.L. and Druffel, E.R.M. (1990). Trace metals in contemporary and seventeenth century Galapagos coral-records of seasonal and annual variations. Geochim. Cosmochim. Acta, 54: 387-394. [ Links ]
Luoma, S.N. (1990). Processes affecting metal concentrations in estuarine and coastal marine sediments. In: R.W. Furness and P.S. Rainbow (eds.), Heavy Metals in the Marine Environment, CRC Press, Boca Raton, Florida pp. 51-66. [ Links ]
Martínez, N., Martínez, M. et al. (1989). Metales pesados en corales escleractíneos de la plataforma marina de Moa-Cuba. Reporte de Investigación, Instituto de Oceanología, Cuba. [ Links ]
NIST (1992). Use of NIST standard reference materials for decision on performance of analytical chemical methods and laboratories. USA Commerce Department's Technology Administration. Special Publication 829. [ Links ]
OIEA (1985, 1987) Intercalibration of Analytical Methods on Marine Environmental Samples. Trace Element Measurements on Shrimp Homogenate. Results of the Worldwide Intercomparison Run MA-A-3/TM and of the MEDPOL Exercise MA (S) MED 86//M. Laboratory of Marine Radioactivity, Monaco. Reps. no. 24 and 26 (1985), and 34 (1987). [ Links ]
Páez-Osuna, F. and Ruiz-Fernandez, A.C. (1995). Comparative bioaccumulation of trace metals in Penaeus stylirostris in estuarine and coastal environments. Estuar. Coast. Shelf Sci., 40: 35-44. [ Links ]
Price, W.J. (1979). Spectrochemical Analysis by Atomic Absorption, Heyden and Son Ltd., Belgium, 238 pp. [ Links ]
Schneider, R.C. and Smith, S.V. (1982). Skeletal Sr content and density in Porites porites in relation to environmental factors. Mar. Biol., 66: 121-130. [ Links ]
Shen, G.T., Boyle, E.A. and Lea, D.W. (1987). Cadmium in corals as a tracer of historical upwelling and industrial fallout. Nature, 328: 794-796. [ Links ]
Shen, G.T. and Boyle, E.A. (1988) Determination of lead, cadmium and other trace metals in annually-banded corals. Chem. Geol., 67: 47-62. [ Links ]
StatSoft (1997). Statistica 6.0 for Windows. Release 5.1, Copyright 1984-1998. StatSoft Inc. [ Links ]
Varcárcel, M. and Ríos, A. (1992). La Calidad en los Laboratorios Analíticos. Editorial Reverté, S.A., España, 426 pp. [ Links ]














