The bacterial spot in tomato (S. lycopersicum) and chili pepper (C. annuum) caused by Xanthomonas spp. is one of the most important and destructive diseases for these crops worldwide (Potnis et al., 2015; Sharma and Bhattarai, 2019; Bogatzevska et al., 2021; Rotondo et al., 2022). Despite this disease being found in all the tomato and chili pepper producing regions, it is most severe in subtropical and tropical regions, where the pathogen is favored by high temperatures and constant rains (Koike et al., 2007).
The initial foliar symptoms caused by Xanthomonas spp. consist of irregular aqueous spots that later turn dark maroon to black and generally measure <5 mm in diameter. As the disease progresses, the spots may coalesce and give way to large necrotic areas on the leaves. In advanced and severe infections, plants may lose leaves, particularly in their lower section. Infections of the fruits are irregular, rough, elevated brown scabs that measure 2-5 mm in diameter. The spots on the fruits are grouped near the end of the peduncle (Koike et al., 2007).
Worldwide there are four species of Xanthomonas (X. euvesicatoria, X. vesicatoria, X. perforans and X. gardneri) commonly associated to the disease known as bacterial spot in tomato and chili pepper (Jones et al., 2004). To date, five Xanthomonas spp. races have been reported for tomato: race T1 reported in X. euvesicatoria, race T2 in X. vesicatoria and X. gardneri, races T3 and T4 reported in X. perforans, as well as race T5, which was reported as a recombination between races T3 and T4 of X. perforans (Adhikari et al., 2020; Jibrin et al., 2022). Meanwhile, in the case of chili pepper, 11 races have been reported (P0, P1, P2, P3, P4, P5, P6, P7, P8, P9 and P10). The determination of the races in the Xanthomonas spp. strains is based on the hypersensitivity reaction in response to the effector proteins mediated through the type III secretion system in the host cells and with recognition from specific resistance proteins in differential tomato and chili pepper plants (Stall et al., 2009).
For tomato, there are earlier studies that determined the diversity of races and/or species of Xanthomonas in strains gathered in the U.S.A. (Bouzar et al., 1994; Rotondo et al., 2022), India, Argentina, Spain, New Zealand, France, Hungary (Bouzar et al., 1994), Taiwan (Hartman and Yang, 1990; Bouzar et al., 1994; Lue et al., 2010), Mexico (Bouzar et al., 1994; Bouzar et al., 1996), Caribbean Islands (O’Garro, 1998), Brazil (Bouzar et al., 1994; Quezado-Duval and Camargo, 2004), Canada (Bouzar et al., 1994; Abbasi et al., 2015), Australia (Bouzar et al., 1994; Roach et al., 2018), Thailand (Sitthitanasin et al., 2020), Bulgaria (Bogatzevska et al., 2021) and Africa (Jibrin et al., 2022).
For chili pepper, Xanthomonas races and species have been identified with strains from Argentina, Brazil, Hungary, Senegal, Spain, Tongo (Bouzar et al., 1994), U.S.A. (Bouzar et al., 1994; Kousik and Ritchie 1995; Rotondo et al., 2022), Taiwan (Hartman and Yang, 1990; Bouzar et al., 1994; Lue et al., 2010), Mexico (Bouzar et al., 1994; 1996; Carrillo-Fasio et al., 2001), Caribbean Islands (Bouzar et al., 1994; O’Garro, 1998), Australia (Bouzar et al., 1994; Roach et al., 2018), Thailand (Sitthitanasin et al., 2020), Bulgaria (Bogatzevska et al., 2021) and Africa (Jibrin et al., 2022).
In Sinaloa, the main tomato and chili pepper producing state in Mexico, the bacterial spot is widely distributed and it continuously appears as an epidemic, causing important losses in the production of both crops (Carrillo-Fasio et al., 2001). In this sense, some previous studies of the diversity of Xanthomonas races in these crops were based on strains isolated from fields in Sinaloa and Sonora (Bouzar et al., 1994; 1996; Carrillo-Fasio et al., 2001). Nevertheless, the information of the distribution and presence of Xanthomonas spp. races in the tomato and chili pepper crops must be updated, based on studies complemented with molecular data. Therefore, the aim of this study was to identify the races and species of Xanthomonas that infect tomato and chili pepper crops in the state of Sinaloa, Mexico with the combination of morphological, pathogenic, biochemical, physiological and molecular tests.
Materials and methods
Samples collection. Between October, 2016 and March, 2017, leaves were taken from tomato and chili pepper plants with typical symptoms of bacterial spot in 47 commercial fields distributed among the municipal areas of Ahome (8), Guasave (7), Navolato (7), Culiacán (9), Elota (5) and Escuinapa (11), in Sinaloa, Mexico. The crops were found in different stages of development, from the initial stage to the stage of production.
Isolation of bacteria. The leaves gathered were disinfested superficially in 70% alcohol for 30 seconds, cut into small pieces from the edges of the lesions and ground in sterile distilled water. The suspension was planted in grooves on Petri dishes with a nutrient agar (NA) culture and a yeast-dextrose-calcium carbonate (YDC) medium. The dishes were incubated at 28 °C for 48 h in constant darkness, and the round, convex, yellow cultures with a mucous-like consistency were then selected and purified. Pure cultures were kept in tubes with water at 4 °C for short-term storage, and in 20% glycerin at -20 °C for their long-term storage (Schaad et al., 2001).
Hypersensitivity reaction. In order to verify that the isolated strains were pathogenic, a hypersensitivity test was carried out using two-month-old tobacco plants (Nicotiana tabacum). A bacterial solution was prepared with a concentration of 3 ´ 108 UFC mL-1 according to McFarland´s scale (Kyraly et al., 1974); using a hypodermic needle, the solution was infiltrated into the reverse side of the leaf, identifying each infiltrated area with the data or the register of the strain. The plants were placed in greenhouse conditions and monitored for the first 48 h (Király et al., 1974). The strains that induced a positive hypersensitivity reaction were chosen for further studies.
Biochemical and physiological tests. Out of the 93 isolated strains, only 47 (39 for tomato and 8 for chili pepper) displayed the phenotypical characteristics reported for Xanthomonas spp., and with these, the biochemical and physiological tests (Gram stain, oxidative/fermentative metabolism of carbohydrates, oxidase and starch) described by Schaad et al. (2001) were performed. All tests were carried out three times.
DNA extraction and amplification by PCR. Bacterial cell lysis was carried out by thermal shock from young cultures, planted for 2 days in YDC medium. To do this, a pure culture was placed in a 1.5 mL microcentrifuge tube with 100 µL of injectable water and the solution was homogenized. It was then placed for 15 min at 95 °C in a thermoblock (Labnet) and immediately transferred to a container with ice for 7 min. Once the time was over, it was centrifuged at 10 000 x g for 15 min, the supernatant was poured and the tablet was resuspended in 50 µL of injectable water.
Table 1 Specific primers for the identification of Xanthomonas species in strains obtained from tomato and chili pepper in Sinaloa, Mexico.
| Species | Primer name | Gene sequence | Amplicon (pb) |
|---|---|---|---|
| Xanthomonas spp. | RST 65 | 5’-GTCGTCGTTACGGCAAGGTGGTCG-3’ | 420 |
| RST 69 | 3’-TCGCCCAGCGTCATCAGGCCATC-5’ | ||
| X. euvesicatoria | Bs-XeF | 5′CATGAAGAACTCGGCGTATCG-3′ | 173 |
| Bs-XeR | 3′-GTCGGACATAGTGGACACATAC-5′ | ||
| X. vesicatoria | Bs-XvF | 5′-CCATGTGCCGTTGAAATACTTG-3’ | 138 |
| Bs-XvR | 3′-ACAAGAGATGTTGCTATGATTTGC-5´ | ||
| X. perforans | Bs-XpF | 5′-GTC GTG TTG ATG GAG CGT TC-3′ | 197 |
| Bs-XpR | 3′-GTGCGAGTCAATTATCAGAATGTGG-5′ | ||
| X. gardneri | Bs-XgF | 5′-TCAGTGCTTAGTTCCTCATTGTC-3’ | 154 |
| Bs-XgR | 3′-TGACCGATAAAGACTGCGAAAG-5′ |
The PCR amplification was carried out with 5 pairs of primers (Sigma) that flanked the HrpB2 gene, specified in Table 1 (Obradovic et al., 2004), and a reaction mixture shown in Table 2. The reagents were from the kit GoTaq® PCR Core System I. The amplification conditions were: an initial denaturalization at 95 ºC for 5 min, 35 cycles at 94 ºC for 1 min, 56 ºC for 1 min, 75 ºC for 1 min; and a final extension at 75 ºC for 10 min. The reactions took place in a Proflex PCR system thermocycler (Applied Biosystem).
Table 2 Reaction mixture for PCR for the identification of Xanthomonas species.
| Reactive (Initial concentration) | Final Volume (µL) | Final Concentration |
|---|---|---|
| Buffer (10X) | 2.5 | 1 X |
| MgCl2 (50mm) | 1.2 | 2.5 mM |
| Taq Pol (5 u/mL) | 0.125 | 0.25 U |
| Primer RST65 (10 mM) | 1.25 | 0.5 mM |
| Primer RST 69 (10 mM) | 1.25 | 0.5 mM |
| Primer Bs-XeF (10 mM) | 0.5 | 0.2 mM |
| Primer Bs-XeR (10 mM) | 0.5 | 0.2 mM |
| Primer Bs-XvF (10 mM) | 1.0 | 0.4 mM |
| Primer Bs-XvR (10 mM) | 1.0 | 0.4 mM |
| Primer Bs-XpF (10 mM) | 0.75 | 0.3 mM |
| Primer Bs-XpR (10 mM) | 0.75 | 0.3 mM |
| Bs-XgF (10 mM) | 0.75 | 0.3 mM |
| Bs-XgR (10 mM) | 0.75 | 0.3 mM |
| DNTP’s (10 mM) | 0.5 | 0.2 mM |
| DNA | 1 | |
| H2O cbp | Gauge to 25 |
MgCl2: Magnesium chloride, Taq Pol: polymerase DNA, dNTPs: deoxynucleotide triphosphate, Sense primer for genus Xanthomonas: RST65, antisense primer for genus Xanthomonas. RST 69, sense primer for X. euvesicatoria. Bs-XeF, antisense primer for X. euvesicatoria: Bs-XvR, sense primer for X. vesicatoria. Bs-XeF, antisense primer for X. euvesicatoria: Bs-XeR, sense primer for X. perforans: Bs-XpF, antisense primer for X. perforans: Bs-XpR; sense primer for X. gardneri. Bs-XgF, antisense primer for X. gardneri: Bs-XgR.
Amplicons were observed via electrophoresis in 1% agarose gel using an electrophoresis chamber (BioRad) and 60 V were run through them for 50 min. Later, the gels were viewed in a transluminator (Benchtop) with UV light.
Physiological races of Xanthomonas in chili pepper. The physiological races of Xanthomonas spp. were determined using the chili pepper material cv. Early California Wonder (susceptible) and a set of isogenic lines derived from Early California wonder (ECW), including ECW-10R, ECW-20R, ECW-30R, PT235047 and ECWPLUS. Lines ECW-10R, ECW-20R, ECW-30R contain resistance genes 10R, 20R and 30R from Capsicum pubescens, respectively; whereas line ECWPLUS contains genes 10R, 20R, 30R, 50R and 60R (Table 3).
The plants were cultivated in a greenhouse under conditions of controlled relative humidity (60-70%) and temperature (25 °C) for 60 days. Next came infiltration, which was carried out with a 3 ´ 108 UFC mL-1 bacterial solution, using a 5 mL syringe without a needle, taking care that the infiltration covered a great part of the area of the leaf. A group of differential plants, inoculated with distilled water, served as a control. The plants were evaluated after 48 h and after a week after inoculation to determine the reactions of hypersensitivity (RH) and susceptibility (S). The test considered RH when dark brown spots were observed. In the exclusive case of line ECWPLUS, an RH was considered as a dry yellowish spot, whereas an S reaction was considered when the leaf displayed flaccidity and wateriness. The results were checked against the information presented in Table 3 (Stall et al., 2009).
Table 3 Classification of Xanthomonas spp. races in chili pepper (Capsicum annuum) by susceptibility and hypersensitivity reactions.
| ECW | ECW10 | ECW20 | ECW30 | CP | ECW-12356 | RACE |
|---|---|---|---|---|---|---|
| S | HR | HR | HR | HR | HR | 0 |
| S | S | HR | HR | HR | HR | 1 |
| S | HR | HR | S | S | HR | 2 |
| S | S | HR | S | HR | HR | 3 |
| S | S | S | HR | HR | HR | 4 |
| S | HR | S | S | S | HR | 5 |
| S | S | S | S | HR | R | 6 |
| S | S | HR | HR | S | HR | 7 |
| S | S | HR | S | S | HR | 8 |
| S | S | S | HR | S | HR | 9 |
| S | S | S | S | S | R | 10 |
S= Susceptible.; HR= Hypersensitivity reaction.
Physiological races of Xanthomonas in tomato. To identify the physiological races of Xanthomonas spp. strains in tomato, differential lines Hawaii7998, Hawaii7891, PI114490 and LA716 (Table 4) were used, with the same methodology mentioned earlier for chili pepper. The plants were evaluated after 48 h and one week after inoculation, the reactions of hypersensitivity (RH) and susceptibility (S) were recorded. The test was considered as RH when dry and dark brown spots appeared, whereas a reaction was considered S when the leaf displayed flaccidity and wateriness. The results were checked against the information presented in Table 4 (Stall et al., 2009).
Results and discussion
The symptoms of bacterial spot were observed in the leaves of chili pepper and tomato plants when gathering the plant material in all the sites visited. The incidence of the disease was 5-10% and 15-25% in commercial fields with chili pepper and tomato, respectively.
The tests performed showed that, out of the 93 isolated strains, only 47 displayed the morphological characteristics of round, convex, yellow cultures with a mucous-like texture in a YDC medium, along with the fact that they were Gram and negative oxidase. These morphological and biochemical characteristics coincided with those reported by Schaad et al. (2001) for the case of Xanthomonas spp. It is worth pointing out that 32 out of the 47 strains registered negative amylase.
Likewise, out of the 93 isolated strains, only 47 induced RH in tobacco plants 24 h after inoculation, and based on the biochemical, physiological and pathogenicity tests, the 47 were confirmed to belong to the genus Xanthomonas.
The identification of three Xanthomonas spp. related to chili pepper and tomato was possible using the specific primers for each species (Table 5). All the endpoint PCR products were viewed in agarose gel, as in the example shown in Figure 1.
The molecular analysis in which specific primers were used confirmed that 83% of the Xanthomonas strains obtained from tomato and chili pepper plantations in Sinaloa (Table 5), corresponded to X. euvesicatoria; 10.6%, to X. perforans and 6.3%, to X. vesicatoria. Similarly, Lue et al. (2010) reported these three Xanthomonas species associated to tomato and chili pepper in Taiwan, who didn’t find X. gardneri neither.
Table 5. Species and races of Xanthomonas isolated from chili pepper and tomato from Sinaloa from November, 2016 to March, 2017.
| Strain | Municipality | Crop | HA | PCR | REA | Race P | Race T |
|---|---|---|---|---|---|---|---|
| 1 | Ahome | Jitomate | + | X. e | A | P0 | T3 |
| 2 | Culiacán | Jitomate | - | X. v | B | - | T3 |
| 3 | Elota | Chile | - | X. e | A | P8 | T1 |
| 4 | Guasave | Jitomate | + | X. e | A | - | T1 |
| 5 | Escuinapa | Jitomate | + | X. p | C | - | T1 |
| 6 | Navolato | Jitomate | + | X. e | A | - | T1 |
| 7 | Culiacán | Jitomate | + | X. e | A | - | T1 |
| 8 | Ahome | Jitomate | + | X. e | A | - | T1 |
| 9 | Guasave | Jitomate | + | X. e | A | - | T1 |
| 10 | Guasave | Jitomate | - | X. e | A | - | T1 |
| 11 | Escuinapa | Jitomate | + | X. e | A | - | T1 |
| 12 | Escuinapa | Jitomate | + | X. e | A | - | T1 |
| 13 | Culiacán | Jitomate | + | X. e | A | - | T1 |
| 14 | Ahome | Jitomate | + | X. e | A | - | T1 |
| 15 | Escuinapa | Jitomate | + | X. e | A | - | T1 |
| 16 | Navolato | Jitomate | - | X. e | A | - | T1 |
| 17 | Ahome | Jitomate | + | X. e | A | - | T1 |
| 18 | Escuinapa | Jitomate | + | X. e | A | - | T1 |
| 19 | Culiacán | Jitomate | - | X. v | B | - | T1 |
| 20 | Escuinapa | Jitomate | + | X. e | A | - | T1 |
| 21 | Guasave | Jitomate | + | X. e | A | - | T1 |
| 22 | Escuinapa | Chile | + | X. e | A | - | T1 |
| 23 | Ahome | Jitomate | + | X. e | A | - | T1 |
| 24 | Navolato | Jitomate | + | X. p | C | - | T1 |
| 25 | Elota | Jitomate | - | X. e | A | - | T1 |
| 26 | Escuinapa | Jitomate | + | X. e | A | - | T1 |
| 27 | Navolato | Chile | + | X. e | A | P0 | - |
| 28 | Escuinapa | Jitomate | + | X. e | A | P8 | T3 |
| 29 | Culiacán | Jitomate | + | X. e | A | - | T2 |
| 30 | Escuinapa | Jitomate | + | X. e | A | - | T1 |
| 31 | Guasave | Jitomate | - | X. e | A | - | T1 |
| 32 | Culiacán | Chile | + | X. p | C | P0 | - |
| 33 | Elota | Chile | + | X. p | C | P3 | T1 |
| 34 | Ahome | Chile | + | X. e | A | P6 | T1 |
| 35 | Guasave | Chile | + | X. p | C | P10 | T3 |
| 36 | Navolato | Jitomate | + | X. e | A | - | T3 |
| 37 | Culiacán | Jitomate | - | X. e | A | - | T3 |
| 38 | Guasave | Jitomate | + | X. e | A | P8 | T1 |
| 39 | Navolato | Jitomate | - | X. e | A | P8 | T1 |
| 40 | Elota | Jitomate | - | X. e | A | P8 | T1 |
| 41 | Culiacán | Chile | - | X. e | A | - | - |
| 42 | Ahome | Jitomate | - | X. e | A | - | T1 |
| 43 | Navolato | Jitomate | + | X. e | A | P8 | T3 |
| 44 | Escuinapa | Jitomate | - | X. e | A | - | T5 |
| 45 | Ahome | Jitomate | - | X. e | A | - | T5 |
| 46 | Elota | Jitomate | + | X. e | A | - | T5 |
| 47 | Culiacán | Jitomate | - | X. v | B | - | T5 |
HA= Starch hydrolysis; Raza P= Chili pepper race; Raza T= Tomato race; X. e.= Xanthomonas euvesicatoria, X. v.= Xanthomonas vesicatoria, X. p.= Xanthomonas perforans.

Figure 1 Agarose gel to detect Xanthomonas spp. species. In the first lane (M) 100 pb marker; in the second lane, primers for X. euvesicatoria (E); third, X. vesicatoria (V), fourth lane with a specific stripe for X. perforans (P); fifth, X. gardneri (G) and sixth lane with universal primers for Xanthomonas spp. (X).
Earlier studies in Mexico reported X. vesicatoria as the predominant species in tomato and chili pepper fields in the states of Sinaloa (Bouzar et al., 1996; Carrillo-Fasio et al., 2001) and Sonora (Bouzar et al., 1996). This contrasted with our study, in which X. euvesicatoria was the most frequent species in fields in Sinaloa; whereas X. perforans and X. vesicatoria were the lease frequent (Table 5). This variation can be explained by the continuous movement of contaminated seeds between countries (Abbasi et al., 2015; Jibrin et al., 2022).
For the case of the eight strains obtained from chili pepper plants in the state of Sinaloa, 62.5% corresponded to X. euvesicatoria. This coincided with studies carried out in Australia (Roach et al., 2018), Thailand (Sitthitanasin et al., 2020) and Bulgaria (Bogatzevska et al., 2021), where X. euvesicatoria was the predominant species in the chili pepper crop.
In this study, X. euvesicatoria was the most frequent species (87.1%) in the 39 strains analyzed from tomato fields in Sinaloa. Similarly, this species has been reported in tomato fields in Taiwan (Lue et al., 2010), Canada (Abbasi et al., 2015), Nigeria (Jibrin et al., 2018), Bulgaria (Bogatzevska et al., 2021) and the U.S. Midwest (Rotondo et al., 2022).
Xanthomonas perforans was the second most common species in this study, with a frequency of 10.6% in tomato and chili pepper fields in Sinaloa. This species was previously found in tomato fields in Taiwan (Lue et al., 2010), Canada (Abbasi et al., 2015), Australia (Roach et al., 2018), Thailand (Sitthitanasin et al., 2020) and the U.S. Midwest (Rotondo et al., 2022),
Xanthomonas vesicatoria was the least frequent species (6.4%) out of the strains isolated from tomato and chili pepper fields in Sinaloa, broadly coinciding with the study by Lue et al. (2010), who found this species les frequently and less distributed in tomato fields in Taiwan in comparison with X. euvesicatoria and X. perforans. On the other hand, Bogatzevska et al. (2021) pointed out that X. vesicatoria was the most frequent and widely distributed in the same crop in Bulgaria.
Out of the X. euvesicatoria strains isolated from chili pepper fields in Sinaloa, races P0, P3, P6, P8 and P10 were identified. Similarly, these races were also found in chili pepper fields in Bulgaria (Bogatzevska et al., 2021), whereas for the strains isolated from tomato, six were found to be able to attack chili pepper plants, being races P0 and P8.
Comparing the earlier results obtained by Carrillo-Fasio et al. (2001), the study of Xanthomonas spp. races in Sinaloa showed that after more than 15 years, races P1 and P2 were not found in this study, probably due to their not being present in the sampled production areas, or that the sampling date did not coincide with the presence of the pathogen. Meanwhile, races P6 and P10 were detected for the first time, whereas races P0 and P3 continue in the population of Xanthomonas spp. The predominant races in Sinaloa wereP0, P6 and P8, unlike the year 2001, in which the predominant race was P3.
In the tomato crop in the fields of Sinaloa, races TI, T2, T3 and T5 of Xanthomonas were detected. Based on strain distribution and frequency data, in northern Sinaloa (Ahome and Guasave) races T1 and T3 are found; in the center of the state (Culiacán, Navolato and Elota), races T1, T2 and T3 were found, and in the south (Escuinapa), races T1, T2, T3 and T5, being races T1 and T3 the most predominant in the entire state (Figure 2). In the case of chili pepper, the Xanthomonas spp. races were distributed as follows: in the north (Ahome and Guasave), the races found were P0, P6, P8 and P10; in the center (Culiacán, Navolato and Elota) P0, P3, P6 and P8; south (Escuinapa), P0, P6 and P8, with races P0, P6 and P8 being predominant in the entire country (Figure 2).
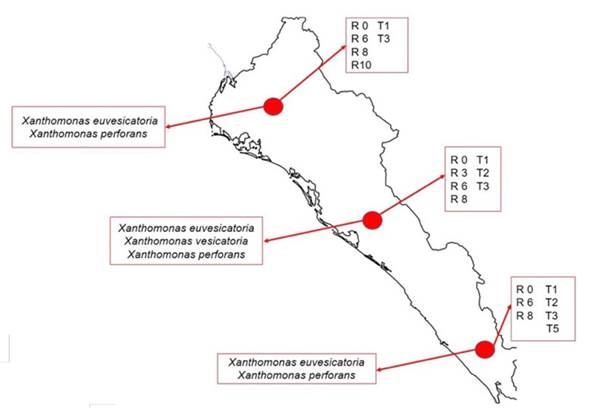
Figure 2 Distribution of species and races of Xanthomonas isolated from tomato and chili pepper in fields distributed throughout the state of Sinaloa, Mexico, during the 2016-2017 cycle.
Along with the identification of Xanthomonas spp. using specific primers, there are studies that have analyzed DNA sequences and have carried out multilocus phylogenetic analyses to distinguish the various Xanthomonas species that infect tomato and chili peppers in several countries (Abbasi et al., 2015; Schwartz et al., 2015; Timilsina et al., 2015; Roach et al., 2018). Therefore, further phylogenetic studies with the sequencing of Xanthomonas spp. DNA to obtain more infotmation on their origin and diversity in the state of Sinaloa and in other tomato and chili pepper producing states in Mexico.
Conclusions
The information produced in this study confirms that the species X. euvesicatoria, X. perforans and X. vesicatoria are found in tomato fields in the state of Sinaloa. Meanwhile, in chili pepper fields, X. euvesicatoria and X. perforans were found. In addition, the presence of races T1, T2, T3 and T5 was found in tomato, along with races P0, P3, P6, P8 and P10 in chili pepper. This is the first report of the presence of X. perforans in Mexico. Therefore, further studies are required to deposit representative sequences of each Xanthomonas species in the GenBank, understand the sensitivity of these strains to bactericides, as well as to generate data on epidemiology and the management of these phytopathogenic bacteria in Mexico. Likewise, it is important to point out that the presence of X. gardneri was not found.











 text in
text in 


